TNF-α and IL-1β Suppress N-Cadherin Expression in MC3T3-E1 Cells
Abstract
Excessive production of tumor necrosis factor (TNF) and interleukin-1 (IL-1) secondary to estrogen deficiency have been implicated as the cause of osteoporosis in postmenopausal woman. These cytokines appear to stimulate osteoclast precursor proliferation and activate mature osteoclast formation directly and possibly indirectly via osteoblasts. To investigate the other possible roles that these cytokines may play in stimulating the bone resorption process, we examined the effect of TNF-α and IL-1β on cell–cell adhesion molecules, cadherins, in osteoblastic MC3T3-E1 cells. In this study, we investigated cadherin expression and the effect of TNF-α, IL-1β, and parathyroid hormone (PTH) on the expression of cadherins in MC3T3-E1 cells. Confluent cultures of MC3T3-E1 cells were challenged with recombinant human TNF-α (1–100 U/ml), recombinant human IL-1β (1–100 ng/ml) and human PTH(1–34) (1–100 ng/ml), respectively. The results show that MC3T3-E1 cells express functional cadherin molecules, N-cadherin and OB-cadherin. TNF-α (10–100 U/ml) and IL-1β (10–100 ng/ml) suppressed N-cadherin without changing OB-cadherin expression, while PTH (1–100 ng/ml) had no effect on cadherin expression. These results raise the possibility that TNF-α and IL-1β may compromise the cell–cell adhesion of osteoblasts which cover the bone surface. The ensuing compromised cell–cell adhesion of osteoblasts may in turn facilitate the direct adhesion of osteoclasts on the calcified bone matrix surface. These results implicate an indirect role for osteoblasts in the promotion of bone resorption by TNF-α and IL-1β.
INTRODUCTION
One of the most common skeletal disorders prevalent in postmenopausal women and the elderly, osteoporosis is defined as systemic bone loss associated with compromised mechanical strength of bone and increased risk for fractures. The critical event in osteoporosis at the cellular level is the relative increase in bone resorption by osteoclasts and/or reduced bone formation by osteoblasts in the process of physiological bone remodeling. The pathophysiological mechanisms underlying the increased bone loss have been studied for many years with considerable attention being paid to the manner in which calciotropic hormones such as parathyroid hormone (PTH), 1,25-dihydroxyvitamin D3, or calcitonin and some inflammatory cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor (TNF) exhibit a capacity to promote bone resorption.1 Recently, an excessive production of immune and hematopoietic factors, such as IL-1, IL-6, TNF, macrophage colony stimulating factor, and granulocyte macrophage colony stimulating factor in bone marrow has been cited as a causative factor in the development of postmenopausal osteoporosis.2 These cytokines appear to target osteoclasts precursors (preosteoclasts) and promote differentiation of the preosteoclasts into mature osteoclasts, resulting in increased bone resorption. Of these cytokines, TNF and IL-1 seem to be particularly noteworthy in that they depress bone formation in addition to enhancing bone resorption,3, 4 a situation that would exacerbate bone loss. The extent to which osteoblasts are involved in this process awaits elucidation.
In the process of bone resorption, osteoclasts must anchor to the bone surface through adhesion molecules of the integrin family prior to resorbing bone matrix.5, 6 However, the bone surface is covered with a sheet of osteoblasts linked by intercellular adhesion. Therefore, at the beginning of bone resorption, a mechanism is needed to remove some of the osteoblasts before osteoclasts adhere to the bone matrix. Although the mechanism by which osteoclasts select a resorption site is unknown, the bone-resorbing cytokines might play a role in loosening the interosteoblastic connection by affecting the expression of adhesion molecules. To substantiate this hypothesis, we investigated the effect of the bone-resorbing cytokines, TNF-α and IL-1β, and a bone-resorbing hormone, PTH, on the expression of members of the cadherin family in the MC3T3-E1 osteoblastic cell line.7
In this study, we have addressed three basic questions: Is the osteoblastic cell–cell connection mediated by cadherins? Which members of the cadherin superfamily are expressed predominantly in osteoblasts? Is the expression of cadherins suppressed by TNF-α, IL-1β, or PTH?
MATERIALS AND METHODS
Cell culture
The mouse osteoblastic cell line, MC3T3-E1, obtained from the Riken Cell Bank (Ibaraki, Japan), was seeded at a density of 4 × 105 cells/100-mm plastic dish (Falcon #3003; Becton Dickinson Labware, Tokyo, Japan) and cultured in alpha-minimal essential medium (α-MEM) (GIBCO BRL, Grand Island, NY, U.S.A.) containing 10% (v/v) heat-inactivated fetal calf serum (Sigma Chemical Co., St. Louis, MO., U.S.A.) and 50 μg/ml ascorbic acid at 37°C in a humidified 5% CO2 incubator. Under these culture conditions, they reached confluence within 3–4 days. After reaching confluence, the medium was replaced by α-MEM containing 1% fetal calf serum; 1 day later, the MC3T3-E1 cells were treated with various concentrations of cytokines and hormone, incubated for an additional period, and then processed for further analysis.
Reagents
Recombinant human TNF-α, recombinant human IL-1β, and human PTH(1–34) were kindly supplied by Dainippon Pharmaceutical Co. (Osaka, Japan), Otsuka Pharmaceutical Co. (Tokushima, Japan) and Asahi Chemical Co. (Tokyo, Japan), respectively. The goat polyclonal anti-pancadherin and anti–β-catenin antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). All secondary antibodies were from DAKO A/S (Glostrup, Denmark).
Cell aggregation assay
Cell aggregation was assayed with a method described in essence by Takeichi et al.8 Briefly, cells were dissociated into a single cell suspension by treatment with 0.02% trypsin either in the presence of 1 mM CaCl2 (TC treatment) or 1 mM EDTA (TE treatment) for 30 minutes at 37°C. After being washed with HEPES-buffered Ca2+, Ma2+-free Hanks' solution (HCMF), the cells were aggregated in HCMF with or without 1 mM CaCl2 and shaken for 60 minutes at 37°C at 100 cycles/minute.
Immunocytochemical staining
MC3T3-E1 cells grown on glass coverslips were fixed with acetone for 5 minutes at –20°C. After blocking in phosphate-buffered saline (PBS) containing 1% normal rabbit serum (blocking buffer) for 30 minutes at room temperature, samples were incubated with anti–β-catenin antibody (dilution ratio, 1:200) in blocking buffer overnight at 4°C. After washing with PBS, a 1:100 dilution of fluorescent-conjugated rabbit anti-goat immunoglobulin G was added for 90 minutes at room temperature. The coverslips were observed with a confocal laser scanning microscopy (LSM 410 invert Laser Scan Microscope; Carl Zeiss, Oberkochen, Germany).
Preparation of proteins
MC3T3-E1 cells were washed with ice-cold PBS and scraped off from dishes with a rubber policeman and collected by low-speed centrifugation. The cell pellet was resuspended in hypotonic buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA) containing protease inhibitor mixture (1 mM phenylmethylsulfonylfluoride, 20 μg/ml L-1-Chloro-3-(4-tosylamido)-4-phenyl-2-butanone, 1 μg/ml aprotinin, 1 μg/ml leupeptin), and homogenized on ice. The material was centrifuged at 15,000 rpm for 20 minutes and the supernatant (regard as the cytosolic fraction) and pellet were separated. The cell pellet was then extracted with NP40 buffer (0.5% NP40, 10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, protease inhibitor mixture) for 30 minutes at 4°C with occasional gentle agitation and centrifuged at 15,000 rpm for 20 minutes at 4°C. The supernatant (membrane-soluble fraction) and pellet were separated. The remaining pellet (membrane-insoluble fraction) was dissolved in SDS buffer (2% SDS, 10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, protease inhibitor mixture) to extract cadherins and catenins tightly bound to the cytoskeleton.9, 10
To obtain a whole cell extract, the cells were extracted directly with SDS buffer.
Western blotting
The protein concentration of each sample was determined using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL, U.S.A.). Aliquots of protein solution (20 μg of protein) in sample buffer (10 mM Tris-HCl, pH 7.5, 1% SDS, 2% mercaptoetanol) were boiled and loaded in each lane of SDS (7.5%) acrylamide gradient gels (ExelGel SDS Homogenous 7.5; Pharmacia Biotech, Uppsala, Sweden) and transferred to polyvinylidene difluoride (PVDF) membrane (Immobilon Transfer Membranes; Millipore, Bedford, MA, U.S.A.) according to the manufacturer's instructions.
After blocking with TBST (10 mM Tris-HCl, pH 7.4, 200 mM NaCl, 0.2% Tween 20) containing 5% nonfat dry milk (blocking buffer) for 1 h at room temperature, the membranes were incubated overnight with primary antibody (pancadherin, 1:500; β-catenin, 1:1000) in blocking buffer at 4°C. After washing with TBST, the membranes were incubated with peroxidase-conjugated rabbit anti-goat antibody (1:1000) for 1 h. Immunoblot was detected using an enhanced chemiluminescence system (ECL kit; Amersham Intl., Buckinghamshire, U.K.).
RNA isolation and reverse transcribed polymerase chain reaction
Total RNA from MC3T3-E1 cells was extracted using Isogen (Nippon Gene Co., Tokyo, Japan) according to the manufacturer's instructions. After treating with RNAse-free deoxyribonucleases I (GIBCO BRL), first-strand cDNA was synthesized using an RNA polymerase chain reaction (PCR) kit (Takara Shuzo Co., Shiga, Japan) according to the instruction manual. After an initial 10 minutes at 30°C, the reaction was adjusted to 42°C for 30 minutes, then heated (99°C, 5 minutes) and subsequently chilled to 4°C. A portion of the cDNA mixture was used as a template for PCR using primer pairs specific for E-cadherin,11 N-cadherin,12 P-cadherin,13 R-cadherin,14 VE-cadherin,15 K-cadherin,16 OB-cadherin,17 M-cadherin,18 and β-catenin19 (Table 1). P-cadherin primers were derived using the method of Munro and Blaschuk.20 Reaction mixtures were heated at 94°C for 3 minutes and then subjected to 35 cycles of PCR (30 s at 94°C, 30 s at 60–62°C, on primer pairs, and 90 s at 72°C). PCR products were separated on 2% agarose gel. All PCR products were subcloned and sequenced using DNA sequencing kit (Applied Biosystems, Warrington, U.K.). The nucleotide sequences of the cloned PCR products were compared with the EMBL and the GenBank databases.
Northern blotting
Twenty micrograms of total RNA was separated on 1.0% agarose-formaldehyde gel and blotted onto Hybond-N+ membrane (Amersham Intl.). Following prehybridization in hybridization buffer (50 mM Tris-HCl, pH 7.5, 1 mg/ml denatured salmon sperm DNA, 1% SDS, 1 M NaCl, 10 mM EDTA, 0.2% Ficol 400, 0.2% polyvinylpyrrolidone, 0.2% bovine serum albumin) for 3 h at 65°C, the membrane was hybridized overnight with [32P]-labeled cDNA probes which correspond to mouse E-cadherin, N-cadherin, OB-cadherin, and β-catenin and the loading standard glyceraldehyde-3-phosphate dehydrogenase, synthesized from MC3T3-E1 cDNA by PCR methods, in hybridization buffer at 68°C. The hybridization fragments had been labeled with [32P]dCTP using a BcaBest labeling kit (Takara Shuzo Co.). After hybridization, the membranes were washed and the signals were detected by a BioImaging Analyzer BAS-1500 (Fuji Photo Film Co., Tokyo, Japan). In reprobing, each hybridized probe was removed by boiling the membrane in 0.5% SDS and then sequentially hybridized with the respective target probes.
RESULTS
The results presented here represent at least two separate preparations of samples.
Cell aggregation assay to detect cadherin family
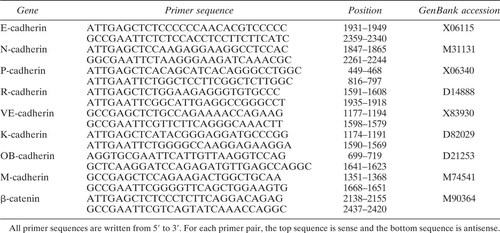
An initial experiment examined whether MC3T3-E1 cells expressed functional cadherin molecules. Cadherins are protected from proteolytic degradation by trypsine in the presence of Ca2+ (TC treatment), which degrades all other cell–cell adhesion molecules. The adhesive properties of these molecules are expressed in a Ca2+-dependent manner.8, 9 While MC3T3-E1 cells treated with TE failed to aggregate even in the presence of Ca2+ (data not shown), cells treated with TC formed cell aggregates in the presence of Ca2+, thus indicating the expression of functional cadherin molecules on the surface of the MC3T3-E1 cells in this environment (Fig. 1).
Cell aggregation assay to detect cadherin-mediated attachment. MC3T3-E1 cells were dispersed with 0.02% trypsin in the presence of 1 mM CaCl2 (TC) for 30 minutes. After washing with HCMF, the cells were aggregated for 60 minutes in the presence or absence of 1 mM CaCl2. (A) Cells cultured in the absence of 1 mM CaCl2. (B) Cells cultured in the presence of 1 mM CaCl2. Cell aggregation was observed only when cultured in the presence of Ca2+ (magnification ×100).
Immunodetection of cadherin molecules
Figure 2 shows that pancadherin antibody reacts with at least two types of cadherins in MC3T3-E1 cells and they exist in cytoplasmic membrane fractions. As a positive control for N-cadherin and E-cadherin, brain and liver extracts were immunoblotted21 and one dominant band was seen in each tissue lane.

Western blotting of cadherins in MC3T3-E1 cells. Proteins were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted. Pancadherin antibody reacted with two bands in the membrane fraction derived from the MC3T3-E1 cells. Brain and liver proteins, which were extracted with SDS buffer, were used as controls (N-cadherin and E-cadherin, respectively). Lane 1, cytosolic; lane 2, membrane fraction soluble with NP-40; lane 3, membrane fraction insoluble with NP-40.
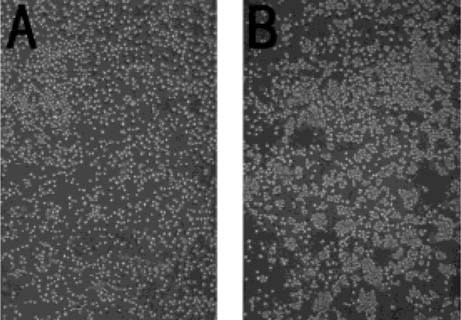
Despite the recognition of cadherins in Western blot, immunocytochemical staining of cadherin was not detected by the same antibody used in Western blot analysis, a result most probably due to the low affinity of this antibody. However, β-catenin, which binds to the cytoplasmic domain of cadherin and is stabilized by the binding of cadherin,22, 23 was detected at cellular contact sites (Fig. 3). This result might indicate the existence of a cadherin–catenin complex in MC3T3-E1 cells so that distribution of β-catenin could be a surrogate marker for cadherin.
β-catenin localization in MC3T3-E1 cells under the confocal laser scanning microscopy after TNF-α treatment. Confluent cells were treated with various concentrations of TNF-α for 3 days. (A) Control. (B) 1 U/ml of TNF-α. (C) 10 U/ml of TNF-α. (D) 100 U/ml of TNF-α. The staining of β-catenin was reduced in a dose-dependent manner.
Detection of mRNA of cadherins
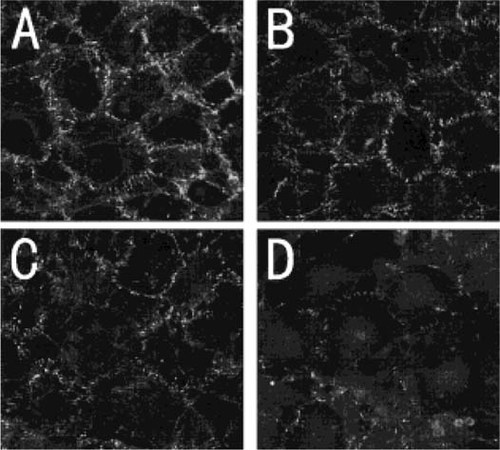
Pancadherin antibody, which is raised against a peptide corresponding to amino acids 811–829 mapping at the carboxyl terminus of the P-cadherin precursor, can react with classical cadherins. Therefore, to determine which molecules in the cadherin superfamily are expressed predominantly in MC3T3-E1 cells, an reverse transcribed (RT)-PCR approach was utilized involving primers specific to respective classical cadherins. Expression of E-cadherin, N-cadherin, and OB-cadherins were evident by RT-PCR (Fig. 4), but in Northern blot analysis, we were unable to detect E-cadherin mRNA expression (data not shown).
mRNA expression of cadherins in MC3T3-E1 cells. Total RNA was extracted from MC3T3-E1 cells, then reverse transcribed and amplified by means of PCR using specific primers. (A) The results for MC3T3-E1 cells. The cells express E-cadherin, N-cadherin, and OB-cadherin mRNA. (B) Positive controls for primers. mRNA from multiple mouse organs were extracted and subsequently subjected to RT-PCR.
Effects of TNF-α on the morphology of MC3T3-E1 cells in culture
Confluent cultures of MC3T3-E1 cells were characterized by polygonal shaped cells in close alignment. Following treatment with 100 U/ml of TNF-α for 3 days, the cell–cell contact appeared to be disrupted and the cells took on a more fibroblastic appearance (Fig. 5).
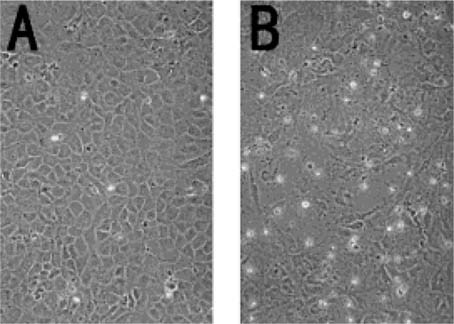
The phase contrast photographs of the morphological change observed in MC3T3-E1 cells treated with or without TNF-α (100 U/ml) for 3 days. (A) Control cells. (B) Cells treated with TNF-α. TNF-α induced morphological changes in MC3T3-E1 cells (magnification ×200).
Effects of TNF-α on cadherin and β-catenin expression in MC3T3-E1 cells
Figure 5 shows the immunostaining of β-catenin in MC3T3-E1 cells treated with TNF-α (1–100 U/ml) for 3 days. The staining of β-catenin was reduced in a dose-dependent manner. Figure 6 shows dose- (Fig. 6A) and time-dependent (Fig. 6B) changes in immunoblot analysis of whole cell extracts. The upper band of cadherin was reduced dose and time dependently without changes in the lower band and β-catenin. These results indicate that TNF-α not only suppressed one of the cadherins but also induced changes in β-catenin distribution. To investigate whether a reduction in cadherin was resulted from the modulation of its mRNA, we performed Northern blotting experiments. TNF-α suppressed N-cadherin–mRNA expression in a dose- and time-dependent manner, and this was observed at 10 U/ml TNF-α and occurred at 12 h after treatment (Fig. 7). There was no difference in the amount of OB-cadherin and β-catenin mRNA levels.
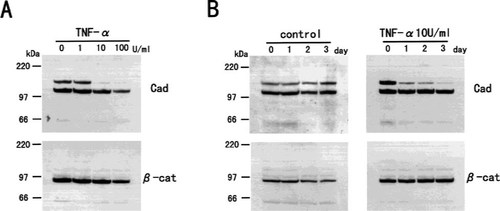
Western blotting of cadherins and β-catenin protein in MC3T3-E1 cells treated with TNF-α. (A) MC3T3-E1 cells were treated with various concentrations of TNF-α for 3 days. Whole cells were extracted with SDS buffer and immunoblotted. (B) Time course of the effects of TNF-α on the levels of cadherins and β-catenin protein. MC3T3-E1 cells were treated with 10 U/ml of TNF-α for the indicated time, and whole cells were extracted with SDS buffer and subjected to immunoblots. The upper band which reacted with pancadherin antibody was reduced dose and time dependently.

Northern blotting of the steady-state levels of N-cadherin, OB-cadherin, and β-catenin mRNA in MC3T3E cells treated with TNF-α. Twenty micrograms of total RNA was subjected to Northern blot analysis. (A) Total RNA was extracted from MC3T3-E1 cells treated with various concentration of TNF-α for 24 h. (B) Total RNA was extracted from MC3T3-E1 cells treated with 10 U/ml of TNF-α for the indicated time. N-cadherin mRNA expression was suppressed by TNF-α dose and time dependently. (G3PDH is a control for mRNA loading.)
Effects of PTH and IL-1β on cadherin expression in MC3T3-E1 cells
While PTH had no effect on cadherin/β-catenin expression (Fig. 8), IL-1β suppressed N-cadherin expression in a manner similar to that found with TNF-α (Fig. 9). However, we could not observe any morphological changes or changes in β-catenin distribution for cells treated with 100 ng/ml of IL-1β for 3 days (data not shown).
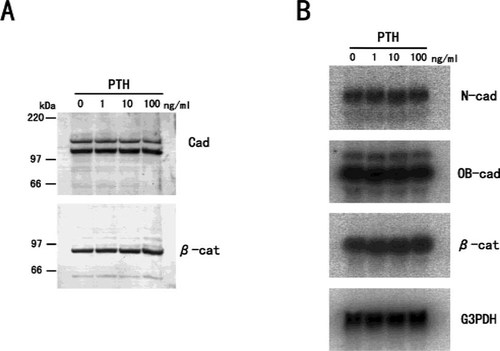
The effect of PTH on cadherins and β-catenin expression in MC3T3-E1 cells. (A) Western blotting of cadherins and β-catenin protein in MC3T3-E1cells treated with PTH. Cells were treated with various concentrations of human PTH for 3 days, and whole cells were extracted with SDS buffer, and subjected to immunoblot analysis. (B) Northern blotting of the steady-state levels of N-cadherin, OB-cadherin, and β-catenin mRNA in MC3T3-E1 cells treated with PTH. Total RNA was extracted from MC3T3-E1 cells treated with various concentrations of PTH for 24 h. No remarkable changes in the expression of cadherins and β-catenin were observed in MC3T3-E1 cells treated with PTH.
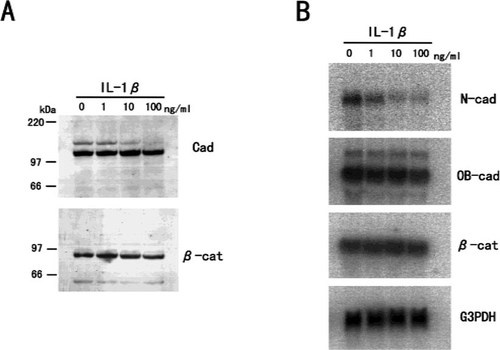
The effect of IL-1β on cadherins and β-catenin expression in MC3T3-E1 cells. (A) Western blotting of cadherins and β-catenin protein in MC3T3-E1 cells treated with IL-1β. Cells were treated with various concentrations of IL-1β for 3 days, and whole cells were extracted with SDS buffer and subjected to immunoblot analysis. (B) Northern blotting of the steady-state levels of N-cadherin, OB-cadherin, and β-catenin mRNA in MC3T3-E1 cells treated with IL-1β. Total RNA was extracted from MC3T3-E1 cells treated with various concentrations of IL-1β for 24 h. IL-1β suppressed N-cadherin expression in a dose-dependent manner.
Effects of TNF-α and IL-1β on cell aggregation in MC3T3-E1 cells
Finally, we performed cell aggregation assays for cells treated with TNF-α and IL-1β to examine the effect of these cytokines on cadherin function. One hundred units per milliliter of TNF-α was found to disturb cell aggregation, whereas cell aggregation was observed in cells treated with 100 ng/ml of IL-1β, which suppressed N-cadherin expression (Fig. 10).
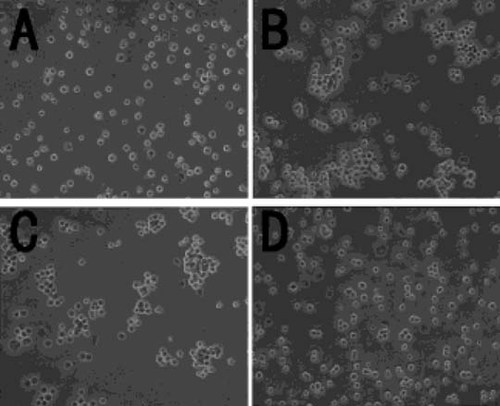
Aggregation of cells treated with TNF-α and IL-1β. Confluent cells were treated with 100 U/ml of TNF-α and 100 ng/ml of IL-1β for 3 days and then subjected to cell aggregation assays. (A) Cells treated with TE. (B) Control cells (TC). (C) Cells treated with 100 ng/ml of IL-1β (TC). (D) Cells treated with 100 U/ml of TNF-α (TC). TNF-α disturbed cell aggregation, whereas IL-1β did not (magnification ×200).
DISCUSSION
Cell–cell contacts and communications between bone cells are essential for coordinated bone development and remodeling.24 Cell–cell contacts are formed by transmembrane adhesion molecules which have been classified into several groups.25 Among these groups, the cadherin superfamily is known to play an important role in the maintenance of cell–cell adhesion. Cadherins are transmembrane glycoproteins which mediate Ca2+-dependent cell–cell adhesion26 and are concentrated at the adherens junctions.27-29 It is thought that cadherin-mediated cell–cell adhesion is involved not only in cell recognition and morphogenesis, but also in signal transduction30 and transmembrane transport.31 In bone cells, Mbalaviele et al.32 reported that E-cadherin had an important role in the formation of multinucleated osteoclasts, but the role of cadherins in osteoblasts was and remains unclear.17, 33, 34
In the present report, we have shown that MC3T3-E1 cells express functional cadherin molecules. By RT-PCR, the expression of E-cadherin, N-cadherin, and OB-cadherin mRNA in these cells was confirmed. In immunoblot analysis, we observed only two bands with a pancadherin antibody, with the lower band being quite specific to the osteoblastic cell, and TNF-α and IL-1β reduced the upper band without changes in the lower band. In combination with the results of Northern blotting, we conclude that the two dominant cadherin molecules are N-cadherin and OB-cadherin and the upper band appears to be identical to N-cadherin; the lower band is most likely OB-cadherin. These results are similar to those found with a human osteoblast-like cell line, which expresses N-cadherin and OB-cadherin.34 However, our data do not exclude the possibility that other cadherins that were not examined in this study are also expressed in this cell.
In regard to the role of cadherins, Okazaki et al.17 reported that OB-cadherin expression was specific to cells that had a potential to differentiate into osteoblasts. In MC3T3-E1 cells, the expression of the cadherins increased with progressive stages of differentiation. Additionally, Cheng et al.34 reported that a synthetic peptide containing the HAV motif present in the extracellular domain of N-cadherin abolished the differentiation of bone marrow cells induced by bone morphogenetic protein. In our report, TNF-α and IL-1β, which inhibit the alkaline phosphatase activity of osteoblasts in vitro,35, 36 suppressed N-cadherin expression. These results indicate the possibility that cadherin-mediated cell–cell adhesion is related to the differentiation of osteoblasts.
Histologically, gap and adherens junctions are observed in osteoblast cell–cell contact.37 Gap junctions that are mediated by connexin (Cx) have been well studied in osteoblasts. This junction has a role as a channel and provides a direct pathway for the exchange of the small molecules (<1 kDa) between adjacent cells.38 Among the Cx family, Cx43 is major protein in osteoblasts39 and is regulated by several hormones and cytokines.40-42 Many researchers consider that this channel synchronizes responses to hormones, cytokines, and mechanical loading in osteoblasts and osteocytes. Some reports have proposed that cadherin is also involved in the regulation of the gap junctional intercellular communication (GJIC).43, 44 Based on these reports, certain cell lines, which are deficient in GJIC but synthesized Cx43 protein, are converted to a communication-competent phenotype by transfection with E-cadherin cDNA. This suggests that cadherin-mediated cell–cell adhesion is essential for GJIC and cadherin may also regulate GJIC in osteoblasts.
During bone resorption, osteoclasts resorb bone under the influence of hormones and cytokines. The roles that osteoblasts play in these interactions are incompletely understood. For resorption to be successful, direct adhesion of osteoclasts on the bone matrix is essential.5, 6 Typically, bone surfaces are covered with osteoblastic cells which need to be released before osteoclasts can attach. Our data suggest that a key cell–cell adhesion molecule, N-cadherin, is down-regulated by TNF-α and IL-1β, which may in turn lead to disruption of intercellular communication and release of osteoblasts from the bone matrix. Because TNF and IL-1 have been implicated in the pathophysiology of postmenopausal osteoporosis, our data implicate the disruption of osteoblastic cell–cell adhesion as a key event in the bone-resorbing mechanism, especially in osteoporosis.
Several reports have suggested that the change in the cadherin-mediated adherens junction is related to morphological changes.45, 46 In our study, TNF-α and IL-1β suppressed N-cadherin, but morphological changes and changes in distribution of β-catenin were seen as a result of treatment with TNF-α but not with IL-1β. Therefore, suppression of N-cadherin expression alone may not lead to morphological changes, probably due to compromised cell–cell adhesion in this cell line. Instead, a TNF-α–specific effect on the intracellular distribution of catenin might be necessary to cause the morphological change. In addition, the results of a cell aggregation assay showed that TNF-α–treated cells did not aggregate but IL-1β–treated ones did. The function of cadherins could be detected with the assay, but the binding ability of this aggregation could not be quantitated. This cell line expresses two functional cadherins, N-cadherin and OB-cadherin, and as long as one cadherin, OB-cadherin, functions, cells may aggregate even when N-cadherin is suppressed. Even though it had no effect on the expression of OB-cadherin, TNF-α inhibited cell aggregation. This finding may also indicate a TNF-α–specific effect on the cell–cell adhesion mechanism of MC3T3-E1 cells. Previous reports have demonstrated that the cadherin–catenin complex binds to the actin cytoskeleton, resulting in a tight cell–cell adhesion.47, 48 Formation of this complex is impaired by the phosphorylation of catenins, which induces a change in catenin distribution and a loss of cadherin-mediated cell–cell adhesion.49, 50 This mechanism might also be involved in TNF-α effects. Alternatively, Tabibzadeh et al.51 reported that TNF-α induced morphological changes in endometrial epithelial cells and found evidence of disassembly of the actin filaments. Therefore, TNF-α might cause changes in the cytoskeletal organization in addition to suppressing N-cadherin expression. Further study is needed, however, to verify the different effects of TNF-α and IL-1β on the cell–cell adhesion of osteoblastic cells.
β-catenin is stabilized by binding to the cytoplasmic domain of cadherins22, 23 and, therefore, it will be reduced based on the availability of these molecules. However, in our experiments, N-cadherin expression was suppressed by TNF-α and IL-1β treatment without a corresponding change in β-catenin expression based on immunoblot analyses. One possible explanation for this discrepancy might be related to the affinity of the pancadherin antibody for N-cadherin and OB-cadherin. We considered that the amount of OB-cadherin protein might be more than that of N-cadherin and therefore a reduction in N-cadherin could not be detected as a reduction in β-catenin by immunoblotting.
In summary, N-cadherin and OB-cadherin are expressed predominantly in the osteoblastic cell line, MC3T3-E1. TNF-α and IL-1β, which act as osteoclastic cytokines in vivo, suppressed N-cadherin expression in this cell line. These results raise the possibility that TNF-α and IL-1β may compromise the cell–cell adhesion of osteoblasts which cover the bone surface, thereby facilitating the direct adhesion of osteoclasts on the calcified bone matrix surface resulting in increased bone resorption. These results also implicate osteoblasts as cell mediators in the promotion of bone resorption by TNF and IL-1.
Acknowledgements
The authors thank Dr. Dave Morris and Dr. Tominaga Shimizu for preparation of the manuscript and Dr. Kohzo Nakayama for technical assistance.