Femoral Neck Is a Sensitive Indicator of Bone Loss in Immobilized Hind Limb of Mouse
Abstract
The present study was carried out to evaluate a unilateral hind limb immobilization model in the mouse. The right legs of male mice (age 10–12 weeks) were immobilized for 3 weeks against the abdomen by an elastic bandage. Body weight decreased significantly during the immobilization. Peripheral quantitative computed tomography (pQCT) analysis showed that the cross-sectional cortical area (CSA), the bone mineral content (BMC), and the bone mineral density (BMD) of the tibial diaphysis were lower in both legs of the immobilized animals than in age-matched controls, but the difference was mainly due to weight reduction. At the tibial metaphysis, CSA, BMC, and BMD were reduced in both legs of the immobilized animals, even after weight adjustment. At the femoral neck, CSA, BMC, and BMD were significantly lower in both legs of the immobilized animals, and the difference between the hind legs of the immobilized animals was also highly significant. The findings of the pQCT study were in good agreement with the changes in mechanical strength. The tibia was a more sensitive indicator of diaphyseal bone weakening than the femur when measuring the bending breaking force of the diaphysis. The femoral neck showed significantly decreased strength, and the difference between the immobilized leg and the contralateral leg was most clearly seen in lateral loading. We conclude that 3 weeks of hind limb immobilization weakened the tibia and femur significantly compared with their contralateral counterparts. The reduction was more significantly seen in the mechanical bending strength than in the pQCT evaluation, and the femoral neck was the most sensitive indicator of bone weakening.
INTRODUCTION
Experimental studies on osteoporosis using rat models have shown that, despite the anatomical and physiological differences between the rat and humans, the femoral neck is a relevant and sensitive site for studying the degree of osteopenia. This has been shown in ovariectomy,1-3 orchidectomy,2, 4 and immobilization2 rat models. Our previous studies5 have raised the question of whether such weakening also takes place in the mouse. Previously, the effect of bone loss on the mechanical strength of the femoral neck has been studied by loading the neck axially in a direction parallel to the femoral shaft axis.1-4 However, femoral neck strength tested in a lateral loading configuration gives additional information not explained by the strength in the standard axial loading direction.5 Typically, clinical fractures are associated with a fall to the lateral side, which justifies studies of the effect of the loading direction on the sensitivity of femoral neck strength in different experimental setups.
Mechanical stimuli are essential for the skeleton, and immobilization produces rapid bone loss in the weight-bearing bones, which is comparable in extent to the osteopenia induced by sex hormone deprivation.2 It is probable that a reduction of physical activity may also affect the involutional osteopenia seen in elderly people. Immobilization of a limb provides an experimental way to study mechanical influences on bone tissue without interfering with hormonal homeostasis. In experimental mouse studies, limb immobilization by neurectomy6 has been used to induce disuse osteopenia. Fixation by a plaster cast7, 8 or an elastic bandage9 has been used for unilateral hind limb immobilization in rat.
Peripheral quantitative computed tomography (pQCT) has proven to be an effective tool in evaluating the densitometric and geometric properties of bone in experimental studies.10-18 We have found pQCT fairly precise in evaluating the femoral and tibial shafts and the femoral neck in mouse.5, 19
The aim of this study was to evaluate the densitometric and mechanical changes in the tibia and femur during limb immobilization in mouse. The effect of loading direction on the sensitivity of femoral neck strength was also investigated.
MATERIALS AND METHODS
Animals
The study was performed on 30 outbred male Naval Medical Research Institute (NMRI) mice (age 10–12 weeks). The right legs of 20 animals were immobilized against the abdomen by an elastic bandage with the hip joint in flexion and the knee and ankle joints in extension (Fig. 1). The bandage was checked daily and replaced if necessary. Ten age-matched animals with no immobilization were used as controls. The animals were housed in individual cages. The control animals were submitted to the same treatment as immobilized animals, except that no bandages were applied. After 3 weeks of immobilization, the animals were killed by CO2 suffocation, and the tibiae and femora were dissected out. The bones were stored at –20°C with soft tissue and thawed at room temperature just before testing. The soft tissues were removed and the bones were stored moistened in closed plastic tubes until the experiment was finished.
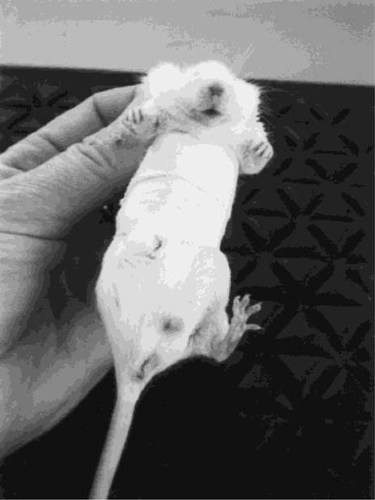
The right hind limb of a mouse immobilized against the abdomen by an elastic bandage.
The experimental manipulations were approved by the Committee on Animal Experimentation of the University of Oulu.
pQCT measurements
The bones were scanned with a pQCT system Stratec XCT 960 A (software version 5.20; Norland Stratec Medizintechnik GmbH, Birkenfeld, Germany) using a voxel size of 0.092 × 0.092 × 1.25 mm3. An attenuation threshold value of 0.930 cm−1 for cortical bone was chosen based on previous studies.5, 19 The tibial diaphysis was scanned at midshaft as previously reported.19 The scan line was adjusted using the scout view of the pQCT system. Another scan of the tibia was obtained at the proximal metaphysis, 3 mm from the proximal end of the bone. The femoral neck was scanned, with the femoral neck in an axial direction, and duplicate measurements were recorded for each sample as previously reported.5 The scan line was adjusted to midneck by using the scout view of the pQCT software.
The cross-sectional cortical area (CSA), the total and cortical bone mineral content (BMC and CtBMC), and the total and cortical mean volumetric bone mineral density (BMD and CtBMD) were used for analyses. The cross-sectional moment of inertia (CSMI) was calculated at the tibial diaphysis, but not in the femoral neck due to the low reproducibility and resolution at that site.5 An example of a cross-sectional image and segmentation of the cortical bone of a tibial diaphysis is shown in Fig. 2.
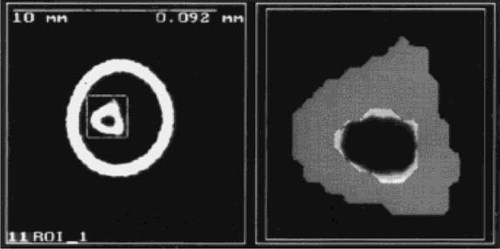
Cross-sectional pQCT image of a tibial diaphysis scanned inside a glass tube (outer circle). The CT scan on the left presents the whole cross-sectional scan and shows the region of interest (ROI) (scale bar = 10 mm, pixel resolution = 0.092 × 0.092 mm2). The segmentation of the cortical bone is shown in the image of the ROI on the right. Attenuation threshold = 0.93 cm−1.
The accuracy of the pQCT method was evaluated by scanning through one whole mouse tibia and one femur with 1.7 mm steps. After scanning, the bones were cut into corresponding slices using a 0.45-mm-thick circular diamond saw (Gillings-Hamco, Rochester, NY, U.S.A.), thus giving slices and slice thicknesses (1.25 mm) similar to those used in the pQCT scans. The slices were imaged with Sony 930-P3 CCD color camera (Sony, Tokyo, Japan) using a MicroNikkor 55 mm objective (Nikon, Tokyo, Japan) and extension tubes (36 + 20 mm; Vivitar, Tokyo, Japan), and analyzed by a digital image analysis system MCID M4 with software version 3.0, rev. 11 (Imaging Research Inc., St. Catherines, Canada). The pQCT-defined CSA was then compared with the morphometrically defined CSAs of these slices. The slices were then burned overnight at 600°C and the ash weight was used for comparison with the BMC. Using bivariate linear correlation analysis, this accuracy test of pQCT gave an intermediate correlation between the CSA and digitized morphometry (r = 0.85, p < 0.001) and a high correlation between the BMC and ash weight (r = 0.96, p < 0.001).
Mechanical testing
The tibial and femoral shafts and the femoral necks were subjected to mechanical testing after the pQCT measurements.
The three-point bending strength of the tibial and femoral shaft was measured with a span length of 6.5 mm.19 Briefly, the bone was positioned horizontally with the anterior surface upward centered on supports. The pressing force was directed vertically to the midshaft of the bone. Each bone was compressed with a constant speed of 0.155 mm/s until failure. The bending breaking force was defined as the maximal bending load at failure.
Two mechanical loading configurations were used to measure the strength of the femoral neck (Fig. 3). Both femora of 10 immobilized animals as well as the femora of 5 control animals were tested axially in a direction parallel to the femoral shaft axis.2, 4, 5 The femora of other 10 immobilized animals and 5 control animals were tested in the lateral loading configuration developed in our laboratory.5 The loading force in both configurations was applied to the femoral head, using a concave loading cup at a constant speed of 0.155 mm/s until failure. The strength of the femoral neck was determined as the maximal load at failure.
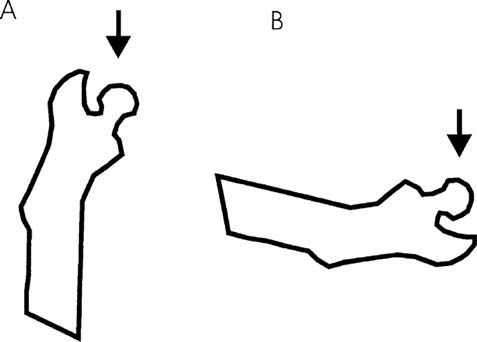
The mechanical loading configurations used to test femoral neck strength. (a) Axial loading. (b) Lateral loading.
Statistical analysis
The values are given as mean ± SD. Student's paired t-test was set to compare the parameters of the immobilized leg and the contralateral leg of the same animal. Independent t-test was used to compare the parameters between the immobilized animals and the control animals. Values of p < 0.05 were considered statistically significant.
RESULTS
The bandage immobilization model appeared to be easy to use and the bandage tape was well tolerated. Body weight decreased during immobilization (p < 0.001), being 39.1 ± 2.6 g at the beginning and 33.8 ± 2.9 g at the end of the study. Three femora broke during handling due to fragility, all of them from immobilized legs. This disabled the mechanical and pQCT analysis of two femoral necks and the mechanical testing of two femoral shafts.
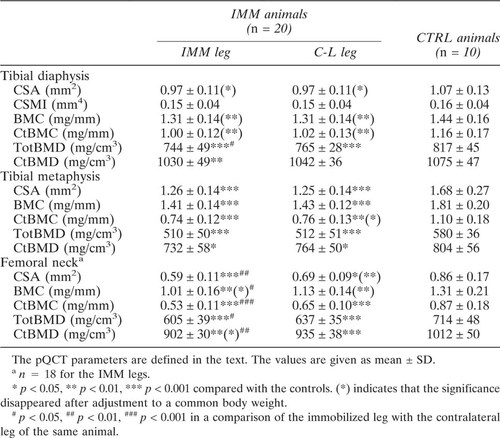
The results of the densitometric evaluation are shown in Table 1. CSA, BMC, and BMD were significantly reduced at all the three scan sites in both legs of the immobilized animals compared with the controls. However, with the exception of BMD at the tibial diaphysis, only the femoral neck showed a statistically significant difference between the legs of the immobilized animals. The most significant difference between the immobilized and contralateral leg was found in the CtBMC. The significance of the differences between immobilized and control animals disappeared at the diaphysis when adjusted to a common body weight but remained at the metaphysis and at the femoral neck.
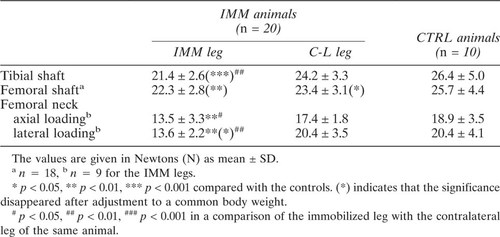
The results of mechanical evaluation are shown in Table 2. A significant reduction in bone strength was found in all the testing configurations when comparing the immobilized leg with the controls. The immobilized leg was weaker than the contralateral leg, the difference being statistically significant in the tibial shaft and the femoral neck. The femoral neck strength was more clearly reduced in lateral loading (33.3% lower than the controls, p < 0.001, and 33.3% lower than the contralateral femora, p < 0.01) than in axial loading (28.6%, p < 0.01, and 22.4%, p < 0.05, respectively). The bone strength in the leg contralateral to the immobilized leg was also decreased compared with the controls, but the reduction was only statistically significant in the bending strength of the femoral shaft. The significance of the differences between immobilized and control animals disappeared at the diaphysis when adjusted to a common body weight but remained at the femoral neck.
The bivariate linear relationships between the pQCT parameters and the mechanical strength were not statistically significant within any study group.
DISCUSSION
In mouse, ovariectomy20 and limb immobilization by neurectomy6 have been used to induce osteopenia. However, denervation is not easy to do in mice and it may also produce effects related to the denervation and not only to the disuse. We therefore tried to find a convenient, but precise way to induce immobilization osteopenia. In the rat model, plaster cast7, 8 and elastic bandage fixation9 has been used for unilateral hind limb immobilization. The bandage immobilization model used here appeared to be easy to use, and the bandage tape was well tolerated. Only a few bandages were renewed during the immobilization period. We consider the bandage the method of choice for immobilization in mice.
We did not investigate different durations of immobilization but used a period of 3 weeks based on information given by Sakai et al.6 about trabecular bone loss after neurectomy in mice. This is also comparable to our own rat immobilization studies.8 We applied the model in adult (10–12 weeks) mice. Klein et al.21 found that the peak whole-body BMD defined by dual-energy X-ray absorptiometry was reached at approximately this age in female mice, which justifies our model.
The pQCT method provides high reproducibility but is limited in accuracy when applied to very small specimens. The axial resolution of pQCT is equal to the slice thickness (1.25 mm), which is thick in relation to the high cross-sectional resolution. When small bones are measured with high resolution, the partial volume effect is anticipated with decreased accuracy. This is particularly true with the femoral neck. Here, we studied the accuracy of the pQCT method by comparing the geometrical data with computerized histomorphometry and the mineral content with ash weight. The accuracy study showed that the evaluation of BMC with the pQCT is highly relevant, even with small specimens, but the accuracy in the geometrical analysis of cortical bone is not so good.
Beamer et al.10 found cortical bone parameters defined by pQCT sensitive in discriminating between mouse strains. We found that both the total bone and cortical parameters of the tibial diaphysis and metaphysis were able to differentiate between the study groups. However, cortex was a dominant discriminator in the femoral neck. This is in good agreement with the former findings that bone loss in aging bone of mouse does not occur solely in the trabeculae, but is accompanied by an enlargement of the marrow cavity of the femoral neck as well as by a decrease in cortical thickness.22 It has also been suggested that the cancellous bone of the femoral neck may be less important for strength in rats than in humans.23
When studying TGF-β1 knockout mice for postnatal bone development, Geiser et al.15 analyzed the BMC and BMD by pQCT in these mice. An analysis of the proximal tibial metaphysis showed a significant decrease in the BMC of TGF-β1(−/−) mice compared with TGF-β1(+/+) or TGF-β1(+/−) mice, but no significant difference was observed in BMD between the groups. This is in agreement with our findings, as we also failed to find a significant difference in BMD in the tibial metaphysis between the immobilized and contralateral legs. However, we found a significant difference in both BMC and BMD in the femoral neck, cortical BMC being the most significant. This indicates that the femoral neck is a sensitive site for the analysis of bone mineral loss in mice. We also calculated the differences after adjustment to a common body weight, which showed that the significance of the differences between the immobilized and control animals disappeared at the diaphysis, but not at the metaphysis and at the femoral neck. This provides further evidence for the sensitivity of the femoral neck as an indicator of bone loss.
We have previously reported that both CSA and CtBMD correlate significantly with the mechanical parameters.5, 19 Here, mechanical strength was found to indicate the difference between the immobilized and contralateral legs more specifically than the pQCT parameters, whereas the relationships between pQCT and mechanical testing were weak, with no statistical significance within any study group. This may be due to the relatively small group sizes (n = 9–20) and to the limitations in reproducibility and accuracy.
We have shown that one third of the change in the femoral neck strength in the two loading configurations used here was not explained by the strength in the other configuration.5 We here found that the sensitivity of indicating the disuse-induced bone loss was different in these two loading configurations. The new lateral loading configuration we designed was the most sensitive method to indicate bone weakening during immobilization, being more sensitive than the standard axial loading of the femoral neck and also more sensitive than the bending strength of either the tibial or the femoral shaft.
The purpose of this study was to evaluate the densitometric and mechanical changes in murine bones during immobilization. Therefore, an outbred strain was used, and the different genotypes were not considered. It has been shown that genes may modify the mechanical properties of bone.10, 24-29 In the future, experiments applying different murine genotypes may yield new information of factors contributing to disuse osteopenia.
In conclusion, hind limb immobilization by an elastic bandage is an effective immobilization model in mouse. The results indicate that immobilization weakened the tibia and the femur compared with the contralateral legs. The reduction was more significant in mechanical bending strength than in pQCT evaluation. Compared with the control animals, the nonimmobilized leg of the immobilized animal was also in relative disuse. The femoral neck showed more significant weakening than the diaphysis or the tibial metaphysis, and lateral loading was the most sensitive indicator of bone loss.
Acknowledgements
The authors thank Ms. Ulla Hirvonen and Ms. Tuula Inkala for taking care of the animals and Ms. Minna Vanhala for technical assistance.