Intermittent Administration of Human Parathyroid Hormone(1–34) Prevents Immobilization-Related Bone Loss by Regulating Bone Marrow Capacity for Bone Cells in ddY Mice
Abstract
ddY mice, 6 weeks of age, were neurectomized (Nx) in the right hindlimbs and sham-operated (Sham) in the left limbs for evaluation of the effects of intermittent injections of human parathyroid hormone (hPTH) on trabecular bone turnover and bone marrow cell development in unloaded and loaded limbs. Mice were given subcutaneous injections of hPTH(1–34) five times a week at a dose of 0 (vehicle), 4 (low dose), or 40 (high dose) μg/kg of body weight for 2, 4, or 6 weeks. Histomorphometric analyses of the trabecular bone of the proximal tibiae revealed that high-dose hPTH injections preserved the trabecular bone volume of the Nx limbs, which was reduced after neurectomy, at the same level as that of the contralateral Sham limbs. The mineral apposition rate in the Nx limbs was elevated to values above even that of the Sham limbs by high-dose hPTH injections. The bone formation rate reduced by neurectomy was maintained at the Sham level by low- and high-dose hPTH injections. The neurectomy-induced increase in osteoclast number was suppressed by high-dose hPTH injections. In the bone marrow cells, the numbers of nonadherent and adherent cells per tibia obtained from the Nx and Sham limbs did not change. The hPTH injections decreased the numbers of nonadherent cells and increased those of adherent cells in both the Nx and the Sham limbs, but the effects were less marked in the Nx than in the Sham limbs even at high-dose injections. The formation of osteogenic nodules in the marrow cultures obtained from the Nx limbs was decreased after surgery and was maintained at the level of the Sham limbs by high-dose hPTH injections. The number of osteoclast-like multinucleated cells was reduced in the Sham limbs by high-dose hPTH injections. The value was increased at 2 weeks after neurectomy, but it was maintained at the Sham level by high-dose hPTH injections through the experimental period. The numbers of colony forming units-fibroblastic, which were reduced by neurectomy, and those of colony forming units for granulocytes and macrophages were not altered by hPTH injections. These results demonstrate that intermittent high-dose hPTH administration in the Nx limbs as well as in the contralateral Sham limbs has similar anabolic effects, stimulating osteoblast cell lineage and suppressing osteoclast cell lineage. The anabolic effects at 4 μg were reduced, but the effects at 40 μg seemed to be less affected by unloading due to sciatic neurectomy.
INTRODUCTION
Unloading of bone by immobilization such as that due to long-term bed rest, paralysis after spinal cord injury, and plaster cast fixation leads to systemic or local bone loss known clinically as disuse osteoporosis. Previous histomorphometric analyses of trabecular bone surfaces (BS) in immobilized tibiae revealed that the rates of mineral apposition and bone formation were reduced and the number of osteoclasts was increased and that an imbalance between bone formation and resorption led to rapid bone loss.1-3 The number of adherent stromal cells and osteogenic nodule formations were reduced in an immobilized bone marrow cell culture.3, 4 Microgravity reduced the gene expression related to osteoblastic differentiation.5 Thus, the loss of mechanical stimulation affects the bone turnover by regulating the developments of osteoblasts and osteoclasts in bone marrow.
The effect of parathyroid hormone (PTH) on bone seems to depend on loading.6 The loss of PTH action by parathyroidectomy or by the administration of PTH antagonist lessened the increase in osteoclast number (Oc.N) in immobilized tibia by neurectomy or tenotomy1, 7 and increased PTH secretion due to calcium deficiency augmented the increase in a rat model.8 The formation of osteoclast-like multinucleated cells obtained from bone marrow of neurectomized (Nx) tibiae in mice was transiently enhanced in the presence of PTH.3 No osteogenic response to mechanical stimulation was seen in the vertebral body of thyroparathyroidectomized rats, and the osteogenic response was restored by a single injection of PTH before stimulation.9 These results may suggest that the presence of PTH helps to adjust the bone mass to the loading by regulating the bone formation and resorption.
Intermittent administration of PTH increases bone mass in many rat models under normal and estrogen-deficient conditions.10-12 It stimulates bone formation and reduces resorption. The anabolic effects of intermittent PTH administration in these models appear to differ depending on the site of the skeleton. In ovariectomized rats, the increase in lumbar trabecular bone mass induced by PTH injections is markedly larger than that in the cortical bone of the femur.11 Thus, the regulation of bone formation and resorption by intermittent PTH administration may depend on the conditions of mechanical loading. Against this background, to clarify whether the effects of intermittent PTH administration on bone formation and resorption differ in unloaded and loaded bones, we performed experiments with sciatic neurectomy in mice. We compared the parameters of the trabecular bone turnover and the bone marrow cell developments for osteoblastic and osteoclastic cells in tibiae of Nx and contralateral sham-operated (Sham) sides.
MATERIALS AND METHODS
Experimental animals
ddY male mice, 5 weeks of age, were purchased and acclimated for 1 week during which they were given a standard diet (CE-2; Japan Clea Co., Tokyo, Japan) containing 1.18% calcium, 1.06% phosphorus, and 200 IU/g of vitamin D3. The body weights of the mice at 6 weeks of age ranged from 30–40 g. All mice were allowed free access to food and water, and they were housed in metal mesh cages in an air-conditioned environment (temperature 24 ± 1°C, humidity 55 ± 5%) that was illuminated from 07:00 to 19:00.
Experimental design
Two hundred and fifty mice were assigned to one start control group (n = 25) and three body weight–matched groups (groups 1, 2, and 3; n = 75 each). All mice of groups 1, 2, and 3 were anesthetized with ether and subjected to surgery on day 0. Neurectomy was performed by resecting an ∼5-mm segment of sciatic nerve in the right hind limb. The sham surgery (Sham) was performed by only identifying the left sciatic nerve. Synthetic human PTH(1–34) (Asahi Chemical Industry Co., Tokyo, Japan) was dissolved in a vehicle of acidified saline containing 0.1% bovine serum albumin. PTH at the respective doses of 0 (only vehicle) for group 1, 4 μg/kg of body weight (bw) for group 2 and 40 μg/kg of bw for group 3 was administered five times a week by subcutaneous injection. Mice of groups 1, 2, and 3 were sacrificed with ether and exsanguination 24 h after the last injections 2, 4, and 6 weeks postoperatively.
To first determine the effects of PTH injections on the local trabecular bone turnover in the Nx limbs, we performed histomorphometric analyses on the proximal tibial metaphyses at 2, 4, and 6 weeks after the Nx. Then, after confirming the time course effects of PTH on trabecular bone formation and resorption, we performed in vitro experiments to evaluate the effects of PTH on tibial bone marrow capacity for bone cell development, also at 2, 4, and 6 weeks post-Nx. The numbers of total, nonadherent, and adherent bone marrow cells were counted. Alkaline phosphatase (ALP) activity and mineralized nodule formation were assessed as measures of the potential osteogenic activity of bone marrow cells. We then measured the development of osteoclast-like multinucleated cells from bone marrow cells in vitro under stimulation with PTH. Assays of colony forming units-fibroblastic (CFU-F) and colony forming units for granulocytes and macrophages (CFU-GM) were also performed.
Experiment for histomorphometry
One hundred mice were used for the experiment. Ten mice were used for the start control group, and three weight-matched groups (PTH at doses of 0, 4, and 40 μg/kg of bw) of 30 mice each were also studied. All mice of the weight-matched groups were anesthetized with ether and subjected to surgery on day 0. For all mice, bone labeling with a calcein injection (6 mg/kg of bw, intraperitoneally) was performed twice, at 5 and 2 days before the sacrifice. The 10 start control mice were sacrificed at day 0, and mice of each of the other three groups were sacrificed 2, 4, and 6 weeks postoperatively. The right (Nx side) and the left (Sham side) tibiae were harvested. The samples from five limbs in each group were fixed with 10% buffered formalin and then embedded in methyl methacrylate resin (MMA) after Villanueva's bone staining. Serial undecalcified 5-μm-thick frontal sections were obtained with a microtome (Model 2050 Supercut; Reichert-Jung, Heidelberg, Germany). Samples from five other limbs in each group were fixed with ice-cold 5% paraformaldehyde in 0.1 M phosphate buffer containing 2% sucrose, at pH 7.4 and 4°C. The samples were then embedded in a mixture of MMA, hydroxyglycol methacrylate, and 2-hydroxyethylacrylate.3 Polymerization was performed at 4°C. The specimens were sectioned in the center to yield 5-μm frontal undecalcified sections, which were then stained for tartrate-resistant acid phosphatase (TRAP).
In the tibial specimens, we measured the secondary spongiosa, which was the metaphyseal cancellous bone area located within 2.0 mm of the growth plate. At the growth plate–metaphyseal junction, the region within 0.15 mm of the growth plate was not measured, to exclude the primary spongiosa without contacting the bone marrow cavity. The region located within one cortical width of the endosteal surface was also excluded from the measurements. We measured the entire area described above of one frontal section per tibial sample, since we first cut three frontal sections per sample and there were no significant differences among the histomorphometric results of three series of these sections. The measurements were performed on a cathode ray tube monitor with a charge-coupled device camera (charge-coupled device High-Gain 1600 A color camera; Flovel, Tokyo, Japan) connected to a semiautomatic image analyzing system (Cosmozone 1S; Nikon, Tokyo, Japan). The abbreviations for histomorphometric parameters were derived from the recommendations of the American Society of Bone and Mineral Research Histomorphometry Nomenclature Committee.13
Parameters of trabecular bone volume and structure: MMA sections were measured at 100-fold magnification to determine the percentage of trabecular bone volume to tissue volume (BV/TV, %) and BS (mm). The parametric values of trabecular thickness (Tb.Th, μm) and trabecular number (Tb.N, /mm) were then calculated using the equation of the parallel plate model.14
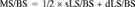
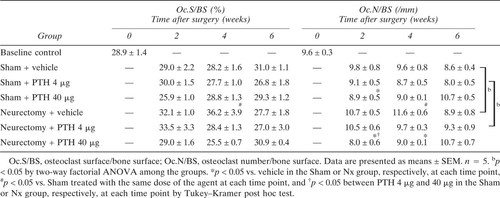
The mineral apposition rate (MAR, μm/day) was calculated as π/4 × Ir.L.Th/3 (μm/day). The ratio of the bone formation rate to the bone surface (BFR/BS, μm-%/day) was calculated by the formula MAR × MS/BS. The percentage of osteoclast surface to bone surface (Oc.S/BS, %) and the ratio of the trabecular osteoclast number to bone surface (Oc.N/BS, /mm) were obtained from the measurement on TRAP-stained sections at 200-fold magnification. TRAP-positive cells that formed resorption lacunae at the surface of the trabeculae and contained one or more nuclei were identified as osteoclasts.
Experiments to evaluate bone marrow cells
Marrow cell number, ALP activity and nodule formation: One hundred and fifty mice were divided into a start control group of 15 mice and three groups of 45 mice each and subjected to the same procedures described above for the histomorphometric experiment. The 15 start control mice were sacrificed at day 0, and 15 mice of the other three groups were sacrificed 2, 4, and 6 weeks after Nx/Sham surgery. Bone samples were taken from the bilateral tibiae and cleansed of all soft tissues. Five limbs in each group were used for the assays of the bone marrow cell number and the ALP activity, and five more limbs in each group were used for the measurement of nodule formation. Another five limbs in each group were used for the measurement of osteoclast-like cell formation and the CFU-F and CFU-GM assays.
Marrow cell number: The proximal epiphyseal end and the distal-most third of the cortex were cut away. Marrow cultures were initiated using the method of Maniatopoulos et al.15 Briefly, bone marrow was flushed out from the proximal cut end, a single-cell suspension was prepared by repeated aspiration, and the bone marrow cells were counted in a hematocytometer and cultured in alpha modified Eagle's medium (α-MEM) (Flow Laboratories Co., Irvine, U.K.) supplemented with 15% fetal calf serum (FCS) (GIBCO, New York, NY, U.S.A.), 2.0 g/l of NaHCO3, 100 μg/ml of streptomycin, and 100 U/ml of penicillin, 1.25 U/ml of Nystatin (Sigma Chemical Co., St. Louis, MO, U.S.A.), 50 μg/ml of ascorbic acid (Wako Pure Chemical Co., Osaka, Japan), 10 mM sodium-glycerophosphate (Sigma), and 10 nM dexamethasone (Wako). Cells were grown in 9.6 cm2 culture dishes. The medium was changed every other day to remove nonadherent cells. Nonadherent cells removed on the second culture day after the preparation were counted, and adherent cells were trypsinized and counted on the sixth culture day after preparation, following the methods described by Keila et al.4
ALP activity: Bone marrow cell cultures were obtained as described above, and extracted in 1 ml of 10 mM Tris-HCl (pH 7.6) containing 0.1% Triton X-100 and 10 mM MgCl2. The activity was measured by the colorimetric method using p-nitrophenylphosphate (Sigma) as the substrate at pH 10 and an optical density of 405 nm. The protein content of the cultures was determined by the Lowry method. The ALP activity is expressed as IU per milligram of protein.
Nodule formation: Cultures were obtained as described above and grown in 9.6 cm2 culture dishes. On the 21st culture day after preparation, they were fixed with a 1:1:1.5 solution of 10% formalin, methanol, and water for 24 h. The cultures were stained for 15 minutes with a saturated solution of Alizarin Red S at pH 4.0 (Sigma), washed with water, and dried in air. The number of nodules covered with dark red stain on the culture dish surface, representing mineralized nodules, was counted.16, 17
Osteoclast-like cell number and colony formation of CFU-F and CFU-GM
Thirty-five mice were subjected to the same procedures described above. The five start control mice were sacrificed at day 0, and five mice of the other three groups were sacrificed 2, 4, and 6 weeks after surgery. Bone samples were taken from the bilateral tibiae, cleansed of all soft tissues, and used for the assays of the osteoclast-like cell number, CFU-F, and CFU-GM.
Osteoclast-like TRAP-positive multinucleated cell development: We performed the cell culture experiments as described.18 Bone marrow cells obtained from the bilateral tibiae were disseminated into a 24-well plate at a concentration of 7.5 × 106 cells/ml. The culture medium was α-MEM containing 10% FCS, 2.0 g/l of NaHCO3, 100 μg/ml of streptomycin, and 10−8 M/ml of 1,25-dihydroxyvitamin D3 (Chugai Pharmaceutical Co., Tokyo, Japan). These cells were cultured in a 24-well plate (Corning, New York, NY, U.S.A.) for 8 days in 5% CO2 and 95% air in a humidified atmosphere. Half of the volume of the culture medium was replaced every other day. The culture medium was then removed, fixed in 10% formalin for 10 minutes, and refixed in ethanol-acetone for 1 minute. After the culture plate had dried, the samples were treated with 5 mg of Naphthol AS-MX phosphate (Sigma) dissolved in 0.5 ml of N,N-dimethylformamide (Wako) containing 50 mM sodium tartrate (Wako) and 30 mg of fast red violet LB salt (Sigma) in 50 ml of 0.1 M sodium acetate buffer (pH 5.0) for 15 minutes at room temperature. Each sample was washed with water, and the number of TRAP-positive multinucleated cells was counted under a light microscope.
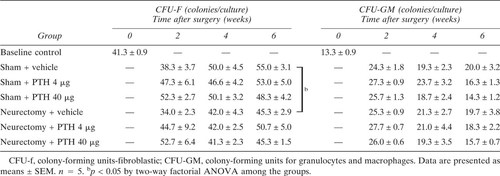
CFU-F and CFU-GM assays: Bone marrow cells of the bilateral tibiae were disseminated into a 6-well plate (Corning) at a concentration of 5 × 105 cells/ml in 2 ml of culture medium for both the CFU-F and CFU-GM assays and were cultured for 10 days. We then counted the CFU-F colonies stained with crystal violet and the CFU-GM colonies in agar with the culture dishes backlighted at 5-fold magnification. Colonies consisting of more than 50 cells were defined as CFU-F or CFU-GM. The medium components for the CFU-F culture were 8% bone marrow suspension cells (the final concentration was 4 × 104 cell/ml), 62% McCoy's 5A medium (Life Technologies Inc., Grand Island, NY, U.S.A.), 10% heat-inactivated horse serum (Bio Whittaker, Walkersville, MD, U.S.A.), 10% heat-inactivated FCS, and 10% L-cell conditioned medium, which was the supernatant filtrated through a 0.45-μm cellulose acetate filter (Sartorius, Göttingen, Germany) after a 7-day culture of 6 × 105 cells/ml of L929 B cells. The CFU-GM culture components were 10% bone marrow suspension cells (the final concentration was 5 × 104 cells/ml), a 20% double concentration of McCoy's 5A medium, 20% 1.5% Agar (Difco Laboratories, Detroit, MI, U.S.A.), 40% heat-inactivated horse serum, and 10% L-cell conditioned medium. We used the L-cell conditioned medium because it has colony stimulating activity for the formation of CFU-F and CFU-GM colonies, following the methods described by Worton et al.19
Statistical analysis
The results are expressed as the mean ± SEM. Statistical differences in the Sham and Nx groups were assessed by two-way factorial analysis of variance (ANOVA) for the time and the treatments. The Tukey–Kramer post hoc test was used to determine the difference at each time point. A p value of < 0.05 was considered to indicate a significant difference.
RESULTS
General conditions
Body weight in all groups increased time-dependently for 6 weeks after the Nx/Sham surgery (data not shown). There were no significant differences among the groups. The animals remained healthy and their behavior did not change.
Histomorphometry
Trabecular bone volume and structure: BV/TV was reduced after Nx (Table 1). The prevention of bone loss in the Nx limbs was partial with 4 μg of PTH injections, whereas the volume was maintained at the same level as that of the contralateral Sham limbs by 40 μg injections. Tb.Th was reduced after Nx. PTH (40 μg) injections significantly increased the Tb.Th values in the Sham limbs, preventing their decrease in the Nx limbs. The PTH injections significantly prevented the decrease in Tb.N after Nx, maintaining Tb.N at the same levels as in the contralateral Sham limbs.
Trabecular bone formation: The dLS/BS values were significantly decreased after Nx and increased by the PTH injections (Table 2). PTH (4 μg) injections increased dLS/BS in the Nx over the levels of that in the contralateral Sham limbs. The PTH injections increased the MAR and BFR/BS in both the Nx and the Sham limbs. MAR in the Nx limbs was elevated to values above even the Sham levels by 40 μg injections. BFR/BS reduced by Nx was maintained at the Sham level by 4 μg and 40 μg injections.
Osteoclast surface and number: The Oc.S/BS and Oc.N/BS were increased at 4 weeks after Nx compared with Sham + vehicle (Table 3). PTH (40 μg) injections suppressed these Nx-induced increases in Oc.N/BS to the same level as in the Sham limbs.
Bone marrow cells
The number of cells: The number of total bone marrow cells per tibia obtained from the Nx limbs or the PTH-treated limbs did not significantly differ among the groups (Table 4). The numbers of nonadherent or adherent bone marrow cells per tibia were not changed by Nx. The PTH injections decreased the number of nonadherent cells in both the Nx and the Sham limbs, but the reduction by PTH (40 μg) injections was less marked in the Nx than in the Sham limbs. PTH (40 μg) injections increased the number of adherent cells in both the Nx and the Sham limbs, but the number was still less in the Nx than in the Sham limbs.
ALP activity, osteogenic nodule formation, and osteoclast-like cells: ALP activity reduced by Nx was maintained at the Sham level upon PTH (40 μg) injections (Table 5). The number of osteogenic nodules formed from the Nx limbs showed a significant decrease postsurgery. The number of nodules was increased in the Nx limbs by the PTH injections and was maintained at the Sham level by 40 μg injections. The number of osteoclast-like cells formed in the Sham limbs was reduced by PTH (40 μg) injections. The value was increased at 2 weeks after Nx and was maintained at the Sham level by PTH (40 μg) injections.
CFU-F and CFU-GM: The numbers of CFU-F which were reduced by Nx, and those of CFU-GM were not altered by either 4 μg or 40 μg injections of PTH (Table 6).
DISCUSSION
The findings of our histomorphometric study clearly demonstrated that reduction of tibial trabecular bone in Nx was prevented in the PTH-treated animals by sustaining the bone forming surfaces and enhancing MARs to values above even those in the Sham limbs. We found anabolic effects of PTH on both the Nx limbs as well as the Sham limbs consisting in suppression of nonadherent bone marrow cells in favor of adherent bone marrow cells, and in favor of stimulating osteogenic nodule formation in culture. The PTH-treated Nx limbs showed maintenance of TRAP-positive osteoclasts on histologic sections and multinucleated cells in bone marrow cell culture. The anabolic effects of 4 μg PTH injections were reduced in the Nx limbs, but the effects of 40 μg were not affected by Nx. It seems that lower dose administration only partially counteracts the deleterious effects of skeletal unloading on bone formation and resorption. But the effects of the high-dose PTH injections are not substantially affected by unloading due to Nx.
The present PTH (40 μg) injections produced equal effects on both the Nx and the Sham limbs, consistent with previous observations20, 21 indicating the equal effects of PTH (30 and 80 μg) on the trabecular bone of both remobilized and continuously immobilized rats after 18 weeks of immobilization and the anabolic effects of PTH (200 μg) on immobilized rats compared with vehicle-treated aging controls. However, these results are inconsistent with Kostenuik's in vitro study6 that bone marrow osteoprogenitor cells in unloaded bone were resistant to the normally anabolic effects of in vivo PTH (80 μg) therapy for 7 days. This inconsistency would mainly be due to the type of treatment used, since female rats were subjected to both ovariectomy and tail suspension in Kostenuik's experiment.
The bone marrow cells of the PTH-injected tibiae seem to be characterized by a stimulated osteogenic potential. The number of adherent cells, the activity of ALP and the formation of mineralized nodules increased significantly. The number of nonadherent cells decreased in the PTH-injected mice. However, the number of total bone marrow cells did not change. Thus, PTH administration seems to increase the population of osteogenic cells in bone marrow. It is unclear why MAR in the Nx limbs was enhanced over the levels in the Sham animals by 40 μg of PTH injections. It may be that mechanical unloading stimulates individual osteoblastic function and possibly facilitates the PTH/PTH-related protein signaling pathway in mature osteoblasts. We analyzed the effects of PTH injections on osteogenesis at three developmental stages. In the first stage at the level of bone marrow cells, the reduction in nonadherent cells and the increase in adherent cells were down-regulated by unloading even with 40 μg PTH injections. In the second stage of osteoblastic development, such differences were not found between the Nx and the Sham limbs in the activity of ALP and the mineralized nodule formations. In the third stage at the tissue level, the bone forming surfaces, the MAR, and the BFR in the Nx limbs were enhanced at the same or over the levels in the Sham limbs. Thus, the mutual interdependence of PTH injections and loading may differ depending on the developmental stages of osteoblastic differentiation. PTH injections seem to stimulate functions of mature osteoblasts in unloading caused by Nx.
The effects of PTH injections on osteoclastic bone resorption remain controversial. Our present results are compatible with Lane's report12 that the values of trabecular Oc.S were dose dependently decreased by PTH injections in ovariectomized rats. Our study showed suppression in the numbers of trabecular osteoclasts and osteoclast-like multinucleated cells by PTH (40 μg) injections. However, the exposure to intermittent PTH in a human study showed that excretion of cross-linked N telopeptide increased by 20%.22 The reason for this apparent discrepancy in the results between our model and human studies is not clear. We suspect that the action of PTH on osteoclasts varies with experimental animals and conditions, including the exposure time of PTH,23 the period after the last injection of PTH,11 the types of cell lines used, and the differentiation stage. In addition, we should stress that the murine bone mass is highly strain specific and that our observations may apply only to ddY mice and have limitations of significance to the human situation.
The present results are compatible with our previous report3 that the transient increase in trabecular osteoclast number in the Nx limbs is related to the enhanced production of osteoclast-like cells in marrow cell culture. In this system, it is conjectured that PTH in the culture medium stimulates the final differentiation of cells committed to the osteoclast pathway.18 Mac-1 is expressed on macrophages, granulocytes, and natural killer cells. We observed no significant difference in the number of Mac-1–positive cells in bone marrow between the Nx and Sham limbs (data not shown). We thus concluded that Nx did not affect the differentiation and heterogeneity of macrophages. In our study, the number of CFU-GM was not altered in either the Nx or the PTH-treated limbs, while a transient increase in the TRAP-positive multinucleated cells was observed following Nx and was suppressed by 40 μg of PTH injections. While PTH physiologically stimulates the fusion of osteoclast-committed precursors,24 the PTH injections seem to paradoxically suppress the final steps in the differentiation of osteoclasts.
In this study, there were temporal gaps in the results between the in vivo and in vitro studies. Osteogenic nodules in the Nx limbs were decreased in number at 2 weeks and 4 weeks postsurgery in the in vitro study, but BFR/BS and BV/TV were still reduced at 6 weeks postsurgery in the in vivo study. The formation of osteoclast-like cells was transiently enhanced at 2 weeks after Nx in the in vitro study, but Oc.S/BS and Oc.N/BS showed transient increases 4 weeks after Nx in the in vivo study. We suspect that the histomorphometric findings reflect the in vitro changes in possible osteogenesis and osteoclastogenesis with a delay of 2 weeks.
In conclusion, intermittent 40 μg PTH administration in the Nx limbs as well as in the contralateral Sham limbs has anabolic effects stimulating osteoblast cell lineage and suppressing osteoclast cell lineage. These anabolic effects of 4 μg PTH injections were reduced by Nx, but the effects of 40 μg were not affected by Nx. The interactions of PTH injections and mechanical stimulation are not completely and mutually interdependent.
Acknowledgements
This work was supported by grants-in-aid from the Japan Ministry of Education, Science and Culture (No. 08671706), JOTF Grant (No. 0106) and UOEH Research Grant for Promotion of Occupational Health.