Stimulatory Effects of Basic Fibroblast Growth Factor and Bone Morphogenetic Protein-2 on Osteogenic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells
Abstract
Bone marrow stroma contains multipotential mesenchymal progenitor cells which can differentiate into osteoblastic cells; we refer to these cells as mesenchymal stem cells (MSCs). Basic fibroblast growth factor (bFGF) and bone morphogenetic protein-2 (BMP-2) have been implicated in the osteogenic regulatory process by virtue of their mitogenic and differentiation activities, respectively. This study examines and compares the effects of bFGF and BMP-2 on dexamethasone (Dex)-dependent in vitro osteogenic differentiation of rat marrow–derived MSCs. A 6-day exposure to bFGF markedly stimulated cell growth and induced osteoblastic differentiation as shown by osteocalcin mRNA expression (day 14), bone nodule formation (day 18), and calcium deposition (day 18). These results indicate that bFGF enhances both mitogenic activity and osteogenic development of Dex-treated marrow MSCs. In contrast, BMP-2 did not induce osteogenesis as strongly as bFGF. Thus, exposure to BMP-2 slightly increased bone nodule number and calcium content compared with the control. Exposure of MSCs to both BMP-2 and bFGF induced expression of osteocalcin mRNA and mineralizing bone-like nodules as early as day 11 and resulted in enhancement of bone formation more markedly than either factor alone. Consistent with these results, porous calcium phosphate ceramic cubes implanted in vivo, which were loaded with MSCs pre-exposed to both bFGF and BMP-2, showed higher histologic score for bone formation than those with MSCs pre-exposed to either bFGF or BMP-2 alone. These data indicate that combined treatment with bFGF and BMP-2 synergistically enhances the osteogenic potency of bFGF in rat marrow MSC culture.
INTRODUCTION
BONE MARROW IS a complex tissue composed of hematopoietic and mesenchymal elements. The stroma of bone marrow is composed of a highly organized network of mesenchymal cells and extracellular matrix that prov
ides structural and functional support for hematopoiesis. Within the marrow stromal cell population, mesenchymal progenitor cells exist which are capable of differentiating into several different mesenchymal tissues including bone and cartilage1; we refer to these cells as mesenchymal stem cells (MSCs).2,3 Production of a bone-like mineralized tissue from bone marrow-derived mesenchymal cells has been demonstrated in vivo in diffusion chambers and porous calcium phosphate ceramics loaded with whole bone marrow or culture-adherent marrow cells and has also been observed in vitro, where bone-like tissue is synthesized by bone marrow-derived mesenchymal cells cultured in medium containing ascorbic acid, β-glycerophosphate, and the synthetic glucocorticoid dexamethasone (Dex).4-10 These results support the concept that marrow contains osteoprogenitor cells that are involved in bone remodeling and repair in adults.
Potential regulators of osteogenic differentiation from marrow mesenchymal progenitor cells include both soluble and bone matrix-derived factors. Numerous factors are known which exert modulatory effects on cells with the osteoblastic phenotype. Among them, basic fibroblast growth factor (bFGF) is a strong mitogen for bone-derived cells,11-13 and bone morphogenetic protein-2 (BMP-2) has been shown to be an active inducer of osteoblastic differentiation of both immature osteoblasts and less committed cells.14,15 Recent studies indicate that bFGF also induces bone formation by stimulating proliferation and differentiation of mesenchymal osteoprogenitor cells when administrated systemically or locally to fracture sites.16,17 However, the effects of this factor on the osteogenic differentiation of bone marrow-derived MSCs are still controversial. Pitaru et al.18,19 reported that bFGF stimulates osteogenic differentiation of Dex-treated bone marrow MSCs as evidenced by enhanced alkaline phosphatase (ALP) activity, osteocalcin production, and bone nodule formation. In another study, bFGF induced proliferation but reversibly inhibited the differentiation of bone marrow-derived osteoblastic progenitors.20 With regard to BMP-2, a number of studies indicate that this factor can up-regulate markers for the mature osteoblastic phenotype, such as ALP activity, collagen synthesis, and osteocalcin expression.21-23 In addition, studies have shown that BMP-2 increases expression of osteoblast indicators from pluripotent stem cell cultures, which suggests that BMP-2 regulates the entrance of uncommitted cells into specific differentiation pathways.14,23
Our laboratory has developed an in vitro culture technique for isolation, expansion, and maintenance of the bone marrow-derived MSCs from various animal species.2,3,6-8,24 The aim of this study was to examine the effects of bFGF and BMP-2 on the proliferation and osteogenic differentiation of rat bone marrow-derived MSCs in this culture system. Furthermore, we investigated the combined effects of these factors on MSC differentiation to determine if these growth factors can work cooperatively. Combined treatment with the mitogenic factor bFGF and differentiation factor BMP-2 could be expected to stimulate osteogenesis more than exposure to each factor alone. The results may provide a rational basis for their clinical application.
MATERIALS AND METHODS
Materials
A selected lot of fetal bovine serum (FBS),24 Dulbecco's modified Eagle's medium containing low glucose (DMEM-LG), trypsin-EDTA, antibiotic-antimycotic solution (penicillin, streptomycin, and fungizon), Superscript II reverse transcriptase, dNTP mix, dithiothreitol, 5× first strand buffer, oligo(dt)12–18, RNAse H, 10× polymerase chain reaction (PCR) buffer, MgCl2, Taq DNA polymerase, and HaeIII restriction fragments of φX174 DNA were purchased from Gibco BRL (Gaithersburg, MD, U.S.A.). Tyrode's salts, dexamethasone (Dex), calf thymus DNA, 3,5-diaminobenzoic acid dihydrochloride (DABA), and calcium assay kit were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Fibronectin was purchased from Collaborative Biomedical (Bedford, MA, U.S.A.). Calf serum was procured from Hyclone Laboratories (Logan, UT, U.S.A.). Total RNA isolation kit was purchased from Qiagen Inc. (Chatsworth, CA, U.S.A.). Recombinant bovine basic fibroblast growth factor (bFGF) was purchased from Boehringer-Mannheim (Indianapolis, IN, U.S.A.). Recombinant human bone morphgenetic protein-2 (BMP-2) was a generous gift from the Genetics Institute (Cambridge, MA, U.S.A.). Falcon plasticware, including 24- and 96-well culture plates, was procured from Becton-Dickinson Labware (Franklin Lakes, IN, U.S.A.). Porous calcium phosphate ceramic cubes were generously provided by Zimmer/Bristol Myers Squib (Warsaw, IN, U.S.A.). Fisher 344 rats were purchased from Charles River Laboratory (Wilmington, MA, U.S.A.), and 10% neutral buffered formalin was from Fisher Scientific (Orangetown, NY, U.S.A.).
Marrow MSC culture
MSC cultures were prepared from the bone marrow of femurs and tibias harvested from 2-month-old male F344 rats by a technique previously described.25 Briefly, the bones were cleaned of adherent soft tissue, the epiphyses removed with a rongeur, and the marrow harvested by inserting a syringe needle (18-gauge) into one end of the bone and flushing with complete medium (DMEM-LG supplemented with antibiotic-antimycotic solution and 10% FBS) into a 60-mm culture dish. A cell suspension was obtained by drawing the marrow into syringes sequentially three times through needles of decreasing size (gauge 18, 20, 22, respectively). The cells were then centrifuged, counted, seeded at a density of 5 × 107 in 7 ml of complete medium per 100-mm culture dish, and cultured at 37°C in 95% humidified air and 5% CO2. At 3 days after seeding, nonadherent cells were removed by changing the medium; thereafter, the medium was changed every 3–4 days.
After 12–14 days of primary culture, when large cell colonies developed on the dishes, the cells were liberated by exposure to 0.25% trypsin/1 mM EDTA for 5 minutes at 37°C, followed by the addition of 0.5 vol of calf serum to stop the reaction. The released cells were then centrifuged, resuspended in complete medium, and seeded at 5 × 103 cells/cm2 in 24-well plates for biochemical and PCR analyses or in 100-mm culture dishes to generate cells for in vivo ceramic cube assays. On day 1 following plating, the attached cells were exposed to bFGF and/or BMP-2.
Exposure of the marrow MSC cultures to bFGF and BMP-2
The MSC cultures were exposed continuously to 2.5 ng/ml recombinant bovine bFGF and/or 50 ng/ml recombinant human BMP-2 in 0.5 ml of complete medium in the presence of 10−7 M Dex for 6 days. An optimal dose of bFGF was determined by measuring DNA content, ALP activity, and calcium content at various time points; these values plateaued at a dose of 2.5 ng/ml. A maximal effect of BMP-2 was seen at a dose of 50 ng/ml, which was examined by measuring bone nodule number and calcium content of the cultures treated with 0–100 ng/ml BMP-2. Control cultures were maintained without added bFGF or BMP-2, but in the presence of 10−7 M Dex. The medium was changed once on day 4 and replaced with medium containing fresh growth factors. On day 7, the cultures were rinsed with Tyrode's balanced salt solution, and the medium was replaced with “osteogenic” medium (complete medium plus 10−7 M Dex, 50 μg/ml ascorbate, and 10 mM β-glycerophosphate) to induce bone formation.26 Freshly prepared ascorbate was added to the cultures every other day. Cultured cells were harvested for PCR and biochemical analyses on days 7, 11, 14, and 18. All biochemical assays were carried out with at least triplicate wells.
Measurement of DNA content
DNA content of the cultures was assayed with the technique described by Gillery et al.27 Briefly, the cells in each well of 24-well plates were rinsed with Tyrode's solution and then fixed with ethanol. Freshly prepared DABA solution (80 mg/ml, 0.2 ml) was added to each well. The standard curve was obtained by performing the DABA reaction in culture wells containing various concentrations of calf thymus DNA. The plates were then incubated for 45 minutes at 60°C. The reaction between DABA and DNA was stabilized by adding 1.5 ml of 1 M HCl to every well, and the intensity of fluorescence was measured at 420 nm excitation and 490 nm emission in a spectrophotofluorometer (American Instrument Co., Silver Spring, MA, U.S.A.). The DNA content was determined from a standard curve.
Total RNA extraction, cDNA synthesis, and reverse transcribed polymerase chain reaction analysis
Total RNA was extracted with a commercial kit following the manufacturer's instructions. The purity and amount of isolated RNA were assessed by spectrophotometric measurement at 260 and 280 nm. Total RNA (1.5 μg) was reverse transcribed to cDNA at 42°C for 50 minutes in a volume of 20 μl containing the following reagents: 0.5 mM dNTP mix, 10 mM dithiothreitol, 0.5 μg oligo(dT)12–18, 1× first strand buffer (5× = 250 mM Tris, pH 8.3, 375 mM KCl and 15 mM MgCl2), and 20 U of Superscript II (RNAse H-free reverse transcriptase). After terminating the reaction at 70°C for 15 minutes, 1 U of RNAse H was added to the reaction mixture, which was incubated at 37°C for 10 minutes to remove the RNA.
Aliquots of the total cDNA were diluted 1:50,000 and then amplified in 50 μl of a PCR reaction mixture which contained 20 pmol of primer sets for osteocalcin or actin, 1× PCR buffer (10× = 200 mM Tris, pH 8.4, and 500 mM KCl), 0.2 mM dNTP mix, 1.5 mM MgCl2, and 2.5 U of Taq DNA polymerase. Primers for osteocalcin were purchased from Operon Technologies Inc. (Alamedo, CA, U.S.A.) which synthesized them based on the sequences described by Araki et al.28 Primers for actin were kindly provided by Dr. E.M. Greenfield (Department of Orthopeadics, Case Western Reserve University, Cleveland, OH, U.S.A.). Amplifications were performed in a Robocycler Gradient 40 temperature cycler (Stratagene Cloning Systems, La Jolla, CA, U.S.A.). DNA amplification included an initial denaturation at 94°C for 2 minutes, followed by 35 cycles of denaturation at 94°C for 1 minute, annealing at 58°C (osteocalcin primers) or 60°C (actin primers) for 1 minute, and extension at 72°C for 1 minute. The final cycle included 5 minutes for extension.
Abundance of PCR products was analyzed by the electrophoresis of samples in 2% agarose gels stained with ethidium bromide; HaeIII restriction fragments of φX174 DNA were used as molecular weight markers.
Bone nodule assay
For quantification of bone nodule formation, the cultures were fixed with neutral buffered formalin and stained by the method of von Kossa.26 Freshly prepared 2% silver nitrate was added to the plates (0.5 ml/well), which were incubated in the dark for 10 minutes. The plates were rinsed with distilled water and then exposed to bright light for 15 minutes. The reaction was terminated by rinsing thoroughly with distilled water. The strongly stained nodules were counted under a dissecting microscope.
Determination of calcium content
After fixation with neutral buffered formalin, 0.5 ml of 0.6 N HCl was added to each well to decalcify the cultures.29 After 24 h, the HCl supernatant was recovered, appropriately diluted with 0.6 N HCl, and transferred into wells of a 96-well plate. Thereafter, reagents from a commercial calcium assay kit were added to the samples and the absorbance read at 575 nm with a model 2550 EIA reader (Bio-Rad Laboratories, Hercules, CA, U.S.A.); the concentration of the reaction product was determined from a standard curve.
In vivo ceramic cube assay
The ceramic composite assay was performed to test the in vivo osteogenic potential of cultured cells.6-8,26 First passage cells exposed to test agents for 6 days were rinsed with Tyrode's solution, harvested by trypsinization, resuspended at 5 × 106 cells/ml in serum-free DMEM-LG medium, and placed in a 5-ml tube containing 3-mm porous calcium phosphate ceramic cubes precoated with fibronectin.7 After producing a slight vacuum to release air pockets from the ceramic cubes, the tubes were placed in a CO2 incubator at 37°C for 2 h to allow the cells to attach to the ceramic surface. The cubes were then implanted subcutaneously into syngeneic F344 male rats. The ceramics were harvested 6 weeks postimplantation, fixed in 10% neutral buffered formalin, and processed for routine histology. The entire sample was serially sectioned and every 7th and 8th sections were stained with Mallory-Heidenhain. Each stained section was examined and scored for bone on a grading scale of zero to four as previously described.26 A score of zero means that bone was never observed within any pores of the ceramics. A score of four was given when more than 75% of the pores contained bone. The scores of all sections were combined and divided by the number of sections graded to determine the overall score of each ceramic cube.
Statistical analyses
The results obtained were expressed as the mean ± SD (standard deviation of the mean) of triplicate or quadruplicate cultures. Differences between experimental groups were determined with Student's t-test. For analyses of histologic score, Kruskall-Wallis one-way rank test was used. Differences at p < 0.05 were considered significant.
RESULTS
In pilot experiments, treatments with bFGF, BMP-2, and both factors did not induce bone nodule formation without Dex in the cultures. Therefore, the following experiments were performed with continuous exposure to Dex for the 17-day duration of the experiment.
Effects of bFGF, BMP-2, and their combined treatments on MSC growth
Marrow MSCs exposed to bFGF alone or in combination with 50 ng/ml BMP-2 showed an approximately 3-fold increase in DNA content compared with control cultures on day 7 (Fig. 1). There was no significant difference in DNA content between bFGF and bFGF + BMP-2–treated cultures. On the contrary, DNA content in the cultures treated with BMP-2 alone was the same as that of the control; similar results were obtained from the assays at later time points (data not shown). These results indicate that bFGF stimulates the proliferation of marrow-derived MSCs in culture in the presence of Dex, whereas BMP-2 does not stimulate a mitogenic response.
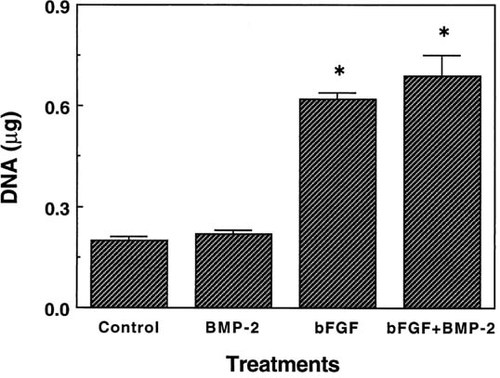
DNA content of MSC cultures on day 7. Rat marrow MSCs were plated at a density of 5 × 103 cells/cm2 in 24-well culture plates and treated for 6 days with no factors (control), 50 ng/ml BMP-2, 2.5 ng/ml bFGF, and a combination of bFGF and BMP-2 in complete medium containing 10% FBS and 10−7 M Dex. On day 7, cultures were washed and then assayed for DNA content. Each measurement is the mean of quadruplicate cultures. Standard deviation (SD) of the mean is shown by vertical bars. Mean ± SD of DNA content of untreated cultures on day 1 was 0.09 ± 0.01 μg. A significant difference (p < 0.05) in comparison with the control value is indicated by an asterisk.
Effects of bFGF, BMP-2, and their combined treatments on the osteogenic development of cultured marrow MSCs
For comparison of osteogenic activities of bFGF, BMP-2, and bFGF + BMP-2, osteocalcin mRNA expression, bone nodule formation, and calcium deposition were assessed as late markers of mature osteoblast functions.
Osteocalcin mRNA expression:
Reverse transcribed polymerase chain reaction (RT-PCR) analyses revealed that combined treatment with 2.5 ng/ml bFGF and 50 ng/ml BMP-2 induced early expression of osteocalcin mRNA on day 11, while treatment with bFGF alone produced a relatively weak expression (Fig. 2). On day 14, strong mRNA expression was detected in bFGF- and bFGF + BMP-2–treated cultures. In contrast, BMP-2–treated cultures had only low but detectable levels of osteocalcin mRNA even at this late time.
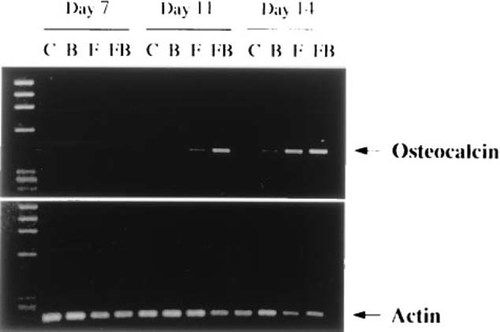
Treatment with bFGF and BMP-2 stimulates expression of osteocalcin mRNA in rat marrow MSC cultures. Rat marrow MSCs were plated at a density of 5 × 103 cells/cm2 in 24-well culture plates and treated for 6 days with no factors (control, C), 50 ng/ml BMP-2 (B), 2.5 ng/ml bFGF (F), and a combination of bFGF and BMP-2 (FB) in complete medium containing 10% FBS and 10−7 M Dex. Thereafter, the medium was replaced with “osteogenic” medium (complete medium plus 10−7 M Dex, 50 μg/ml ascorbate and 10 mM β-glycerophosphate). RNA was extracted on days 7, 11, and 14. Separate PCRs were done for actin and osteocalcin. Reaction products specific for actin (227 bp) and osteocalcin (414 bp) were visualized on ethidium bromide-stained agarose gels. The first lane on the left represents molecular weight markers of HaeIII restriction fragments of φX174 DNA.
Bone nodule formation:
Consistent with early expression of osteocalcin mRNA, mineralizing bone-like nodules appeared in bFGF + BMP-2–treated cultures on day 11 (Fig. 3). In bFGF-treated cultures, uncalcified cell groupings were observed. On day 18, cultures treated with bFGF alone or in combination with 50 ng/ml BMP-2 developed a substantial number of calcified bone nodules (Fig. 4). In contrast, the number of bone nodules in BMP-2–treated cultures was much smaller than that in bFGF-treated cultures; the size of the nodules in BMP-2–treated cultures was similar to that in bFGF-treated cultures.
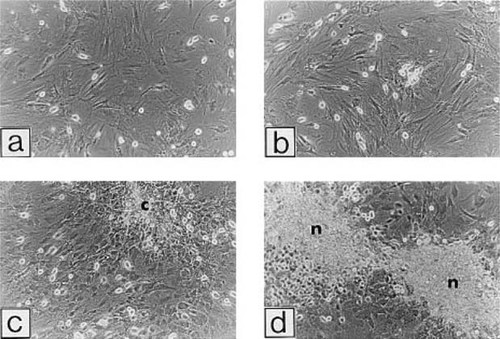
Phase contrast micrographs of MSC cultures on day 11 (×26); control (a), 50 ng/ml BMP-2 (b), 2.5 ng/ml bFGF (c), and combined treatment with bFGF and BMP-2 (d). The cultures were prepared as described in the legend for Fig. 2. Note the mineralizing early bone nodules (n) present in the cultures treated with bFGF and BMP-2. An uncalcified small cell colony (c) is also observed in the bFGF-treated culture.
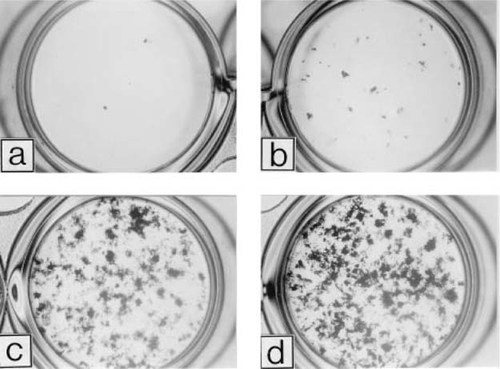
Photographs of von Kossa-stained MSC cultures on day 18 (×1.4); control (a), 50 ng/ml BMP-2 (b), 2.5 ng/ml bFGF (c), and combined treatment with bFGF and BMP-2 (d). The cultures were prepared as described in the legend for Fig. 2.
Calcium deposition:
To quantify the amount of bone-like tissue developed in the cultures, calcium content was measured (Fig. 5). A 6-day pretreatment with bFGF significantly increased calcium content on day 14 and induced a marked elevation on day 18. Continuous 17-day exposure to bFGF did not further enhance the calcium accumulation (data not shown). Treatment with 50 ng/ml BMP-2 also induced a significant elevation of this parameter on day 18. However, the calcium content of BMP-2–treated cultures was about half that of bFGF-treated cultures. Furthermore, 100 ng/ml BMP-2, continuous 17 day-exposure to 50 ng/ml BMP-2, or increasing MSC number at plating did not augment the calcium deposition and bone nodule formation (data not shown). Combined treatment with bFGF and BMP-2 induced an early increase in calcium deposition on day 14 and resulted in the highest levels of its accumulation on day 18. The maximum synergistic effect was observed in cultures exposed to 2.5 ng/ml bFGF in combination with 25 ng/ml BMP-2, which alone did not elevate the level of calcium deposition over control cultures. Calcium content of 14-day cultures treated with both bFGF and 25 ng/ml BMP-2 was 3-fold greater than that of bFGF-treated cultures. In comparison with cultures treated with 25 ng/ml BMP-2, exposure to both bFGF and BMP-2 showed a 20-fold increase. Thus, these results demonstrate that treatment with bFGF or BMP-2 alone stimulates Dex-dependent osteogenic differentiation of rat marrow MSCs, but the potency of BMP-2 is not as high as that of bFGF in this MSC culture system. Data further indicate that combined treatment with bFGF and BMP-2 enhanced in vitro osteogenesis more markedly than either factor alone.
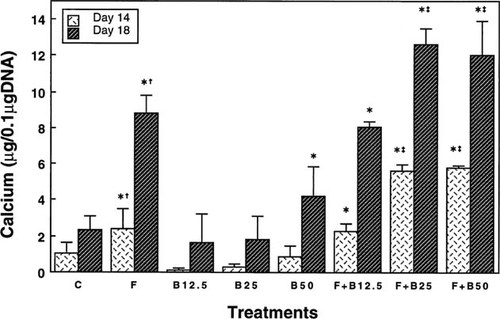
Calcium content of control cultures (C) and cultures treated with 2.5 ng/ml bFGF (F), 12.5, 25, and 50 ng/ml BMP-2 (B12.5, B25, and B50, respectively), and both 2.5 ng/ml bFGF and 12.5, 25, or 50 ng/ml BMP-2 (F + B12.5, F + B25, and F + B50, respectively) on days 14 and 18. The cultures were prepared as described in the legend for Fig. 2. In parallel with calcium assay, the DNA content of matching samples was also determined. All data are expressed per 0.1 μg of DNA. Each measurement is the mean of data obtained from three separate experiments. Standard deviation (SD) of the mean is shown by vertical bars. Significant differences are presented as *,†, and ‡ (p < 0.05) in comparison with the values for the control, 50 ng/ml BMP-2, and bFGF, respectively.
Early or late exposure to bFGF and BMP-2:
To define further the exposure timing for BMP-2 and bFGF in MSC differentiation, factors were added to cultures in the presence of Dex either “early” (days 1–4) or “late” (days 4–7), as illustrated in Fig. 6, and calcium content was measured on day 18. As for the experiment in Fig. 5, 6-day exposure to 25 ng/ml BMP-2 alone was ineffective at increasing calcium deposition. Early 3-day treatment with bFGF increased calcium content more effectively than late 3-day treatment, which indicates that bFGF acts at an early stage of MSC cultures. Treatment with bFGF at the early stage followed by BMP-2 at the late time resulted in a greater increase in calcium deposition than continuous treatment with bFGF alone. Early bFGF treatment followed by late BMP-2 treatment resulted in calcium accumulation equivalent to that in cultures treated for the entire 6 days with both bFGF and BMP-2. Treatments in the reverse order caused a small calcium accumulation. These data show that bFGF pretreatment followed by BMP-2 exposure was the most effective at stimulating in vitro Dex-dependent osteogenesis of marrow MSCs.
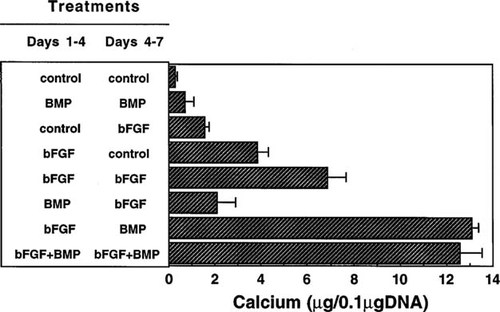
Effects of early (days 1–4) and/or late (days 4–7) addition of 2.5 ng/ml bFGF and 25 ng/ml BMP-2 on osteogenic differentiation of rat marrow MSCs. Factors were administered on day 1 to Dex-treated MSC cultures and removed on day 4, or added on day 4 and removed on day 7, or both. The cultures were prepared as described in the legend for Fig. 2. Calcium content was measured on day 18. In parallel with this assay, DNA content of matching samples was also determined. All data are expressed per 0.1 μg of DNA. Each measurement is the mean of triplicate cultures. Standard deviation (SD) of the mean is shown by transverse bars.
In vivo osteogenic potential of marrow MSCs exposed to bFGF, BMP-2, or both factors in vitro
To examine their in vivo osteogenic potential, samples of MSCs pretreated with bFGF, BMP-2, or bFGF + BMP-2 were loaded into porous ceramic cubes and then implanted into syngeneic rats. Six weeks postimplantation, cubes were harvested and assessed for bone formation by histologic scoring. As presented in Table 1, bFGF and bFGF + BMP-2 treatments resulted in a high incidence of bone-positive ceramics and the histologic scores were significantly higher than those of the control. Cells pretreated with BMP-2 showed very low osteogenic potential. In good agreement with in vitro data, combined exposure to BMP-2 and bFGF increased the histologic score significantly in comparison with bFGF treatment. Figure 7 shows a representative section from the histologic analysis of a cube loaded with MSCs treated with bFGF + BMP-2. New woven bone, in which many osteocytes were embedded, was formed along the walls of individual pores within the cubes. The morphologic appearance of the bone which formed was histologically identical for all cubes seeded with the control cells or cells pretreated with bFGF, BMP-2, or both factors. The possibility that elevated in vivo osteogenesis of bFGF- or bFGF + BMP-2–treated MSCs could be ascribed to enhanced attachment of MSCs to the cubes has been excluded by assays on MSC attachment to porous ceramics. Thymidine-labeled MSCs were added to ceramic cubes, and no significant differences in cell number attached to the cubes was detected among control, bFGF-, BMP-2-, and bFGF + BMP-2–treated MSCs (data not shown). These results indicate that bFGF and bFGF + BMP-2 treatments enhance the in vivo osteogenic potential of marrow MSCs.

Histologic features of a section of a ceramic cube loaded with MSCs exposed to both bFGF and BMP-2 (×110). Cubes were harvested from host rats 6 weeks postimplantation. After fixation and decalcification, histologic sections were prepared and stained with Mallory-Heidenhain. The new bone (b) is formed along the walls of individual pores. Decalcified ceramic material (c) appears as acellular space stained lightly.
DISCUSSION
The studies described above demonstrate that bFGF is a mitogen for rat marrow MSCs and, in the presence of Dex, stimulates their osteogenic differentiation. The MSCs were cultured with Dex-supplemented medium, since it was reported to be essential for induction of in vitro osteogenesis in various culture systems.9,10 Treatment with bFGF in the absence of Dex results in no osteogenesis (unpublished observation). In the presence of Dex, an initial 3-day exposure to bFGF is more effective at inducing bone formation than if bFGF is added later (Fig. 6). This suggests that bFGF acts on early Dex-committed osteoprogenitor cells and/or uncommitted MSCs responsive to Dex at an earlier differentiation stage. Thus, the effects of bFGF are probably mediated predominantly by stimulation and proliferation of osteoprogenitor cells to form a larger pool of cells capable of responding to Dex. Thereafter, the Dex must drive these cells to terminal differentiation, which results in a significant enhancement of bone formation.
In a recent report by Boden et al.,30,31 it was shown that glucocorticoids up-regulate the expression of BMP-6 mRNA in subcultured rat calvaria cells and that blockage of BMP-6 expression by antisense oligonucleotides inhibits glucocorticoid-induced osteogenesis. Induction of BMP-6 expression is a potential mechanism of action for the bFGF potentiation of Dex-induced osteogenesis observed in this study. Preliminary results from semiquantitative PCR analysis of BMP-6 mRNA expression of MSCs treated with Dex + bFGF showed elevated BMP-6 mRNA in comparison with cultures treated with Dex only, during an initial 3-day exposure. However, our RT-PCR data indicate that BMP-6 was not enhanced by Dex treatment as compared with controls with no Dex; in fact, it may be diminished by Dex. When bFGF was added along with Dex, BMP-6 levels were elevated beyond the control level at an early stage of culture. Interestingly, we did see an increase in mRNA expression of BMP type IA receptor (ALK-3: the receptor for BMP-2 and −4), and we also observed an increase in BMP-2 mRNA expression in Dex + bFGF–treated cultures. An up-regulation of BMP receptor expression could be an alternative mechanism for regulating osteogenic progression in MSCs which may not be regulated in the same way as are calvaria cells used in the study of Boden et al.30,31 Clearly, it is difficult to compare directly our results with that of Boden et al.30,31 due of differences in the source of osteogenic cells, culture conditions, and the differences in glucocorticoid type and concentration used. The synthetic glucocorticoid dexamethasone was used at 10−7 M in this study, while Boden et al.30,31 used another glucocorticoid derivative, triamcinolone, at 10−9 M concentration.
Our current interpretation is that bFGF enhances osteogenesis by promoting the proliferation of an osteogenic subset of the MSCs. Moreover, preliminary experiments suggest that early expression of BMP-2 and −6 is involved in Dex + bFGF-induced osteogenic differentiation of rat marrow MSCs. Studies are under way in this laboratory to determine the mechanism of action of bFGF as related to BMP-superfamily expression and to resolve the differences between our results and those of Boden et al.
BMP-2 slightly induces bone nodule formation and calcium deposition compared with the control cultures. A weak osteogenic potential of BMP-2–treated MSCs was further confirmed with the in vivo ceramic cube assay. Recently, Rickard et al.21 demonstrated that BMP-2 (50 ng/ml) acted synergistically with Dex to increase ALP activity and vitamin D–induced mRNA expression for type I collagen and osteocalcin in rat primary stromal cell cultures. However, they did not assess actual in vitro bone formation represented by bone nodule formation and calcium deposition as presented here. Moreover, the methods of cell preparation were different from ours. They added BMP-2 to whole marrow primary cultures containing adherent and nonadherent cell populations for days 1–3 and then re-exposed the adherent cell fraction to BMP-2 after removing the nonadherent cells. In this study, only culture-expanded, first passage MSCs were used. In other culture systems with mouse marrow stromal cell lines and fetal rat calvarial cells, BMP-2 (25–100 ng/ml) greatly stimulated ALP activity, osteocalcin production, and bone nodule formation.32,33 Discrepancies between our results and those of others may be due to differences in the differentiation stage of the cells used, the age of the donor animals, and species differences.
BMP-2 was also reported to stimulate adipogenic and chondrogenic differentiation in cultures of a murine mesenchymal pluripotential line C3H10T1/2.23 However, after a 6-day treatment of MSCs with 50 and 100 ng/ml BMP-2, or during continuous exposure, there was no enhanced adipogenic differentiation by day 18 compared with the control cultures, and no chondrocytes appeared by histologic examination of the cultures (data not shown).
Surprisingly, combined exposure of MSC cultures to bFGF and BMP-2 markedly enhances bone formation in vitro and in vivo compared with exposure to bFGF alone. Moreover, the early appearance of mineralizing nodules and mRNA for osteocalcin (day 11) and an early increase in calcium deposition (day 14) indicate that osteogenic differentiation is accelerated in bFGF + BMP-2–treated cultures. These data demonstrate that Dex-dependent osteogenesis of marrow MSCs is enhanced by bFGF and BMP-2 cotreatment. As presented in Fig. 6, the successive exposure of MSCs to bFGF followed by BMP-2 results in an increase in calcium deposition equivalent to the combined treatment and was much more effective than when the factors were presented in the reverse order. This result suggests that early treatment with bFGF and Dex induced a large number of BMP-2–responsive osteoprogenitor cells, and thereafter these cells are driven to differentiate into fully mature osteoblasts by BMP-2 and Dex treatment. Preliminary experiments were conducted on mRNA expression of BMP type IA receptor (ALK-3) in MSC cultures treated with Dex or Dex + bFGF for days 0–7. MSCs showed detectable mRNA expression of the receptor on day 1 with slightly enhanced expression on day 4 by a Dex + bFGF treatment. Therefore, it is likely that a 3-day treatment with bFGF and Dex increases the number of osteoprogenitor cells that have already expressed BMP-2 receptors and induces the expression of receptors on them for exogenous as well as endogenous BMP-2. An enhancement of bone formation by co-treatment with bFGF and BMP-2 could be explained by this mechanism. A detailed analysis for the expression of BMP-2 receptors is now in progress.
It is widely accepted that the bone formation process is controlled sequentially and cooperatively by many growth factors.22 Therefore, it is unlikely that any single factor controls the entire sequence of differentiation from osteoprogenitor cell to mature osteoblast. The present study revealed that the mitogenic factor, bFGF, and differentiation factor, BMP-2, exhibit a strong synergism during relatively early stages of differentiation in rat marrow MSC cultures and result in enhancement of osteogenesis in vitro and in vivo. Each of these factors has been demonstrated to stimulate bone formation in normal and wounded animal models, such as those with a bone fracture and bone defect.16,17,34 Thus, it is reasonable to expect that combined treatment with bFGF and BMP-2 may provide more effective cell- and growth factor-based therapies for the repair of bone wounds or the reversal of bone loss in osteoporosis.
Acknowledgements
The authors are grateful for the expert technical assistance of Ms. Joanne L. Fiore, Mrs. Debra Fein-Krantz, and Mr. Amad Awadallah. We thank Dr. David A. Carrino and Dr. Scott Bruder for critical review of this manuscript and for thoughtful comments. We also thank Dr. Donald P. Lennon for useful discussions. This work was supported, in part, by grants from the National Institutes of Health.





