Downregulation of mucosal mast cell activation and immune response in diarrhoea-irritable bowel syndrome by oral disodium cromoglycate: A pilot study
Abstract
Background and goal
Diarrhoea-predominant irritable bowel syndrome (IBS-D) exhibits intestinal innate immune and mucosal mast cell (MC) activation. MC stabilisers have been shown to improve IBS symptoms but the mechanism is unclear. Our primary aim was to investigate the effect of oral disodium cromoglycate (DSCG) on jejunal MC activation and specific innate immune signalling pathways in IBS-D, and secondarily, its potential clinical benefit.
Study
Mucosal MC activation (by ultrastructural changes, tryptase release and gene expression) and innate immune signalling (by protein and gene expression) were quantified in jejunal biopsies from healthy (HS; n = 16) and IBS-D subjects after six months of either treatment with DSCG (600 mg/day, IBS-D-DSCG group; n = 18) or without treatment (IBS-D-NT group; n = 25). All IBS-D patients recorded abdominal pain and bowel habits at baseline and in the last 10 days prior to jejunal sampling.
Results
IBS-D-NT exhibited significant MC activation and over-expression of immune-related genes as compared to HS, whereas in IBS-D-DSCG MC activity and gene expression were similar to HS. Furthermore, DSCG significantly reduced abdominal pain and improved stool consistency.
Conclusion
Oral DSCG modulates mucosal immune activity and improves gut symptoms in IBS-D patients. Future placebo-controlled clinical trials are needed for confirmation of clinical benefit of DSCG for IBS-D.
Introduction
Increased immune activity, particularly mast cell (MC) activation, has been described in the gastrointestinal tract of a subgroup of diarrhoea-predominant irritable bowel syndrome (IBS-D) patients,1 and is related to its pathophysiology.2-5 Moreover, association between MC activation and IBS manifestations, such as visceral hypersensitivity and altered bowel habits,5-9 supports targeting MCs in the management of IBS.10 In fact, several experimental11 and clinical studies have already shown the potential clinical benefit of MC stabilisation, including disodium cromoglycate (DSCG) and ketotifen for chronic diarrhoea, food allergy and IBS.12-15 However, the mechanisms by which MC stabilisation leads to clinical improvement have not been defined.
MCs are multifunctional cells with a prominent role in both innate and adaptive immunity.16, 17 Upon activation MCs release biological mediators that promote inflammation, maintain tissue homeostasis and play a significant role in neuro-immune interaction in the gut.5 Indeed, MCs express a wide variety of surface receptors including immunoglobulin receptors that facilitate antibody-mediated responses, cytokines, chemokines receptors16 and Toll-like receptors (TLRs),18 which recognise microbial antigens and modulate inflammatory cascades through the nuclear factor (NF)-kB canonical pathway. The capacity of MCs to secrete a wide spectrum of preformed and newly synthesised mediators makes them highly versatile cells that can amplify or suppress innate or acquired immune responses.17 In IBS-D, MC activation is linked to altered intestinal permeability through mechanisms which involve changes in expression of intestinal tight junctional proteins and increased mucosal humoral activity, which have been associated with symptom severity.2, 19 Therefore, the aim of this study was to determine the ability of oral DSCG to downregulate mucosal MC activation and the mucosal innate immune system in the intestinal mucosa of IBS-D patients, and to explore its potential clinical benefit.
Methods
Participants
IBS-D-naïve patients, recently diagnosed and fulfilling Rome II criteria,20 were recruited from the outpatient gastroenterology clinic between 2007 and 2009. Healthy subjects (HS) were recruited by public advertising. All participants were ≥18 and ≤65 years of age. A complete medical history, physical examination, and skin prick tests were carried out in all participants. Skin prick tests included 22 common food antigens and 12 pneumo-allergens (Laboratorios Leti SA, Barcelona, Spain), using histamine and saline as positive and negative controls, respectively.21 Reasonable exclusion of past episodes of infectious gastroenteritis and gastrointestinal comorbidities in all patients was achieved by means of biochemical and serological analysis, including anti-transglutaminase antibodies and thyroid hormones. Other exclusion criteria for all individuals were pregnancy, major psychiatric and organic diseases and current or recent (last month) use of steroids, immunosuppressive drugs, anti-histaminics and MC stabilisers.2 Candidates with any positivity to food antigens or clinical history consistent with food allergy (digestive and/or extra-digestive symptoms associated with exposure to certain food components) were also excluded. Participants completed a gastrointestinal symptoms questionnaire based on the Rome criteria (to characterise digestive symptoms in patients and to verify the lack of symptoms in HS).20 At entry, all patients recorded their daily ratings of abdominal pain (0 to 10, by a visual analogue scale), the number of bowel movements and the stool consistency (Bristol Stool Form Scale-BSFS)22 during 10 consecutive days, before initiating treatment and prior to the biopsy. Background stress and depression levels were assessed in all participants using the validated Spanish versions of the Modified Social Readjustment Scale of Holmes-Rahe,23 Cohen’s Perceived Stress Scale,24 and Beck’s Depression Inventory.25 All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration. The study protocol was approved by the Ethical Committee of the Hospital Vall d’Hebron (PR(AG) 76/2006) and all participants gave written informed consent.
Experimental design and procedures
Intestinal fluid collection and jejunal biopsy
A single mucosal biopsy and fluid aspiration sample were obtained per participant, at inclusion in the HS group, and six months after inclusion in IBS-D patients. After an overnight fast, a Watson’s capsule (with an attached 3 mm diameter aspiration tube) was orally introduced under fluoroscopic control into the proximal jejunum, 5 cm distal to Treitz’s angle. Jejunal fluid (5 ml) was obtained by gentle aspiration, snap frozen and stored at −80℃ until analysed for tryptase content. A mucosal biopsy was obtained, immediately split into two similar pieces, and subjected to different experimental procedures (Figure 1 ). In all participants, one fragment was fixed in formalin and embedded in paraffin for further microscopic examination by an experienced pathologist and for immunostaining of specific cell types. Using a standard method,9 a subgroup of samples were selected for mucosal TLR2 and TLR4 analysis by immunofluorescence. The remaining fragment was allocated9 into two types of procedures and analysis: (a) tissue fixation and ultrastructural analysis of MC degranulation; or (b) RNA isolation and evaluation of gene expression. All samples were codified and analysed blindly.
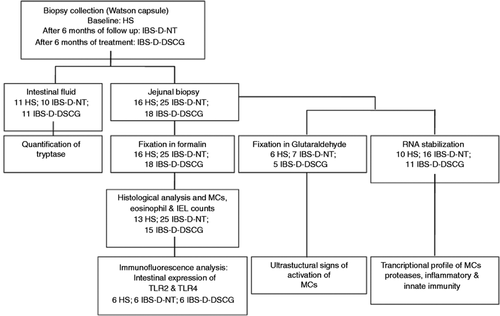
Flowchart for intestinal samples processing.
The chart indicates the number of samples subjected to the experimental procedures in each experimental group (healthy subjects, HS; IBS-D patients after six months’ follow-up with no treatment, IBS-D-NT; and after six months of treatment with oral disodium cromoglycate, IBS-D-DSCG). Intestinal fluid and a single jejunal biopsy per participant were obtained. Biopsy was immediately split into two similar pieces with a sterile scalpel. One fragment was fixed in formalin and embedded in paraffin for further microscopic examination; the remaining fragment was fixed in glutaraldehyde or in RNA stabilisation solution. Due to limited biopsy size, biopsy samples were randomised into three types of analysis (protein allocation and expression, gene expression and ultrastructure). IBS-D: diarrhoea-predominant irritable bowel syndrome; MC: mast cell; IEL: intraepithelial lymphocyte; TLR: Toll-like receptor.
Sample processing and analyses
Luminal jejunal tryptase content
Luminal tryptase was quantified by means of a specific fluoroenzyme-immunoassay (FEIA-UniCAP, Pharmacia Diagnostics, Uppsala, Sweden), as previously described.4 Briefly, frozen fluid samples were lyophilised to increase concentration by a factor of 10, reconstituted in phosphate-buffered saline (PBS) with bovine serum albumin 1%, and a multiprotease inhibitor cocktail (1:100 dilution; Sigma-Aldrich, Madrid, Spain) was added, and centrifuged. Tryptase concentration was assayed in supernatants, and expressed as µg/l.
Histology and immunohistochemistry
All tissue specimens were deparaffinised and rehydrated following general procedures and processed for routine haematoxylin and eosin (H&E) staining to assess epithelial morphology and eosinophilic infiltration. For immunostaining, sections of 4 µm were blocked with Blocking Solution (Dako) for 10 minutes and incubated with anti-human CD117 or CD3 (mouse monoclonal; Dako, Madrid, Spain) for MC and intraepithelial T lymphocyte identification, respectively, followed by incubation with a secondary antibody, and developed with the Vectastain ABC kit (Vector Laboratories, Peterborough, UK). Positive cells in the mucosa were counted at high magnification (400×) in 10 contiguous non-overlapping fields, and results are expressed as the number of positive cells per high-power field (hpf) or per 100 epithelial cells, as previously described.4
Immunofluorescence
Tissue sections were deparaffinised, hydrated and blocked with blocking solution (Dako), followed by incubation with the primary antibodies anti-human tryptase and anti-human TLR4 or TLR2 (rabbit polyclonal antibody, Abcam, Cambridge, UK). Secondary antibodies were Alexa Fluor 594 goat anti-rabbit IgG and 488 goat anti-mouse IgG (Molecular Probes, Madrid, Spain). Nuclei were counterstained with 4',6-diamidino-2-phenylindole (DAPI) before mounting in Prolong antifade reagent (Molecular Probes, Madrid, Spain). For each staining, a negative control was set by exposing serial sections under similar conditions, without the primary antibody. Images were acquired using FV-10-ASW Olympus software (Olympus, Barcelona, Spain).
Transmission electron microscopy
Immediately after biopsy collection, tissue was placed in fixative buffer 2.5% glutaraldehyde, 2% paraformaldehyde in 0.1 M phosphate buffer. Following post-fixation in 1% (w/v) osmium tetroxide containing 0.8% (w/v) of potassium hexacyanoferrate (III) (Sigma-Aldrich, Barcelona, Spain) and dehydratation, tissue fragments were infiltrated in Epon’s resin, polymerised and contrasted. Ultrathin sections (90 nm) were mounted in copper grids, contrasted with standard uranyle acetate and lead citrate double-staining, and observed through a Jeol JEM-1400 transmission electron microscope equipped with a Gatan Ultrascan ES1000 CCD camera (JEOL LTD, Tokyo, Japan). Examinations were performed independently by two experienced investigators (BL and MV) in a blinded manner on a minimum of 20 sections per biopsy sample. Mucosal MCs were identified based on morphological characteristics: cell size and granule morphology. A number of 10–15 images of MC per subject were taken at 10,000×–15,000× magnification. The number of cytoplasmic granules and the degranulation status of each individual MC were analysed according to established criteria.26 The degranulation rate was calculated as the percentage of granules empty or with low electrodensity vs the total number of granules in each MC.
RNA isolation and quantitative real-time polymerase chain reaction (Q-RT-PCR)
Mucosal tissue was placed in RNAse-free tubes containing 500 μl of RNA Later Solution (Ambion, Madrid, Spain), and stored at −80℃ until processed. RNA was isolated using the RNAeasy Mini Kit (Qiagen, Madrid, Spain) and synthesis of cDNA was performed using 1 µg of total RNA with the High Capacity Reverse Transcription Reagents Kit (Applied Biosystems, Madrid). Gene expression was performed on an ABI PRISM® 7500 FAST Sequence Detection System (Applied Biosystems) using validated TaqMan Gene Expression Assays (GEA), and the human 18 S subunit ribosomal RNA gene as the endogenous control (Applied Biosystems). Each sample, including distilled water as negative control, was run in triplicate and data were analysed by the 2–ΔΔCt method, as previously described.27 The expression of each gene was normalised to the endogenous control and the fold-change was calculated individually with respect to the average of the control group. A panel of genes related to MC and immune activity was designed to evaluate mucosal immunity (Table S1, in the supplementary material).
Follow-up and treatment groups
After inclusion, patients were randomly allocated to follow-up for six months, under no treatment (IBS-D-NT group) to assess the natural evolution; or under oral DSCG (IBS-D-DSCG group), 200 mg, three times daily (t.i.d.), 10 minutes before meals. DSCG (Sigma-Tau, Madrid, Spain) was prepared at the hospital pharmacy as a powder mix on a starch base containing no lactose, and delivered in opaque gelatine capsules containing 200 mg. Dosage and intervals of administration had been inferred from published information.12, 13, 15 Patients were allowed to use symptomatic treatment (loperamide, codeine, acetaminophen, spasmolytics) for a maximum of three days/month during the study, in case of severe diarrhoea or abdominal pain. Other drugs were not allowed.
Statistical analysis
No sample size calculation was performed for this pilot study. The Kolmogorov-Smirnov test was used to check the normality of data distribution. Parametric normally distributed data are expressed as mean (±standard error); otherwise, data were expressed as median (interquartile range, IQR). Analysis of data among the three study groups was performed using a one-way analysis of variance (ANOVA) and followed by the multiple comparison Bonferroni post-test; otherwise, data were compared by Kruskal-Wallis test and followed by Dunn’s multiple comparison test. Correlations between clinical and biological variables were analysed using the Spearman’s r s test. The χ2 test was used for comparison of frequencies of clinical symptoms between groups. Paired comparisons within groups were analysed using Wilcoxon signed-rank test. Values of p ≤ 0.05 were considered significant and were further adjusted for multiple comparisons using the Benjamini and Hochberg method.28 Only adjusted p values showing differences in significance are indicated. Statistical analysis was performed using GraphPad Software (La Jolla, CA, USA).
Results
Demographics and psychological features of the study population
Sixteen HS, 25 IBS-D-NT patients, and 18 IBS-D-DSCG patients were included in the study. At inclusion, no differences in clinical, demographic and psychological characteristics were found between the IBS-D-DSCG and IBS-D-NT groups. In both groups of IBS patients, the levels of perceived stress and depression were significantly higher than in HS at baseline (Table 1 ). At the end of the study, patients in the IBS-D-NT group remained classified as IBS-D, without detecting a shift to other IBS variants.
| HS (n = 16) | IBS-D-NT (n = 25) | IBS-D-DSCG (n = 18) | ANOVA p | |
|---|---|---|---|---|
| Age, years | 32.1 ± 2.3 | 37.4 ± 2.1 | 42.5 ± 3.8 | 0.137 |
| Gender, female:male (% female) | 7:9 (44%) | 17:8 (68%) | 11:7 (61%) | 0.225 |
| Holmes-Rahe scale | 100 (65.3–145.0) | 138.0 (57.0–237.0) | 116.5 (63.5–260.5) | 0.511 |
| Cohen scale | 14 (13–17) | 23 (20–32) | 19 (16–30) | <0.001 |
| Beck’s index | 1.8 ± 0.4 | 13.6 ± 2.9 | 8.3 ± 1.5 | <0.001 |
| Intensity of abdominal pain | – | 4.0 (4.0–5.8) | 4.4 (2.5–6.3) | NA |
| Stool Form Bristol Score | 3.6 (3.1–4.1) | 5.0 (5.0–5.4) | 5.0 (5.0–5.5) | <0.001 |
| Number of bowel movements per day | 1 (0.5–1.9) | 3 (2.2–3.9) | 3 (2.1–3.0) | <0.001 |
- ANOVA: analysis of variance; HS: healthy subjects; IQR: interquartile range; IBS-D: diarrhoea-predominant irritable bowel syndrome; IBS-D-NT: no treatment; IBS-D-DSCG: disodium cromoglycate treatment; SE: standard error; Holmes-Rahe scale: 0–150, low stress; 151–300, moderate stress; >300 severe stress. Beck scale: low depression (10–18), moderate (19–29) and severe (≥30); NA: not applicable. Stool consistency: 1 (hard) to 7 (entirely liquid). All values are expressed as median (IQR), except age and Beck’s index, which are expressed as mean ± SE.
Jejunal histology and cell counts
Epithelial architecture was normal in all specimens: No relevant lymphoplasmacytary infiltrate in lamina propria or excess intraepithelial lymphocytes (>40 per 100 enterocytes) were detected under routine histological evaluation. In addition, no parasites, microbial or viral inclusions were observed. Specific staining revealed no significant differences in mucosal MC and eosinophil counts between the three groups (Figure S1, supplementary material).
Mucosal MC activation
Luminal tryptase
The concentration of luminal tryptase was significantly different among the three experimental groups (p < 0.001): IBS-D-NT patients showed higher levels of tryptase (0.44 (0.38–0.61) µg/l) compared to both IBS-D-DSCG (0.09 (0.07–0.15) µg/l) and HS (0.20 (0.15–0.30) µg/l; p < 0.01), suggesting a downregulation of MC activation by oral DSCG.
Ultrastructural changes
In all specimens, we found a number of jejunal mucosal MCs displaying signs of piecemeal degranulation, including partial or total emptying of secretory granules, disassembled content and reduction of granular electron density (Figure 2(a) ). However, the percentage of activated MCs was different between groups (p = 0.034): MCs from IBS-D-NT patients exhibited significantly higher proportions of degranulation (68.5 (59.4–78.1)%) compared to HS (54.1 (48.6–61.6)%; p = 0.022); the difference between IBS-D-NT and IBS-D-DSCG patients did not reach statistical significance (62.5 (58.1–64.9)%; p = 0.268) (Figure 2(b) ).

Effect of oral disodium cromoglycate on MC activation.
(a) Representative transmission electron photomicrographs of MCs in the jejunal mucosa. MCs from healthy subjects (HS), IBS-D patients after six months’ follow-up with no treatment (IBS-D-NT), and after six months of treatment with oral disodium cromoglycate (IBS-D-DSCG). Bars indicate 1 µm. Note MCs from IBS-D-NT patients displayed marked signs of piecemeal degranulation (white arrows) compared to IBS-D-DSCG patients and HS, in which a higher proportion of filled granules (black arrows) are observed. (b) Degranulation rate of individual MCs from each group was assessed according to ultrastructural characteristics of granules. Results are shown as box-and-whisker plot, horizontal bars indicate the medians, boxes indicate 25th to 75th percentiles. Groups were compared using Kruskal-Wallis test *p < 0.05, all after Dunn’s multiple comparison test *p < 0.05. Note significantly higher degranulation rates in NT patients compared to HS and DSCG. (c) mRNA expression of genes related with MC activation: tryptase (TPSAB1/B2) and chymase 1 (CMA1), assessed by Q-RT-PCR. The 18 S was used as house-keeping gene. Individual and mean values (horizontal lines) represent the fold-change against the average of the HS group in HS (n = 10), IBS-D-NT (n = 16), and IBS-D-DSCG (n = 11). Groups were compared using one-way ANOVA. *p < 0.05; after Bonferroni post-test for multiple comparison test. Note differences between IBS-D-NT patients vs IBS-D-DSCG patients and HS. MC: mast cell; mRNA: messenger RNA; Q-RT-PCR: quantitative real-time polymerase chain reaction; ANOVA: analysis of variance.
Transcriptional profile of MC proteases
In IBS-D, DSCG induced a significant reduction in tryptase gene expression compared to the untreated group, which was similar to that in healthy controls. Chymase-1 expression was not affected by DSCG (Figure 2(c) ).
Innate immunity and inflammatory and gene expression profile
To assess the role of MC in innate immunity, the expression of molecules involved in TLR4 signalling pathway was assessed (Figure 3 ). We identified higher expression of TLR4 and Ly96 IBS-D-NT patients compared to HS, which was restored to HS values by DSCG treatment. TLR2 transcripts showed the same transcriptional profile in all groups without significant differences among groups. Gene expression of the negative regulators of TLR signalling (Toll interacting protein (TOLLIP) and single immunoglobulin interleukin-1-related receptor (SIGIRR)) and the intracellular receptor nucleotide-binding oligomerisation domain-containing protein 1 (NOD1) were significantly upregulated in NT patients compared to HS, and also a trend to restoration to HS values for TOLLIP (p = 0.06) and NOD1 (p = 0.07) was observed in the IBS-D-DSCG group.
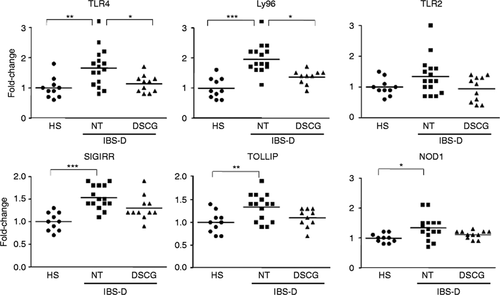
Mucosal expression of genes involved in innate immune activation.
TLRs signalling: mRNA expression of Toll-like receptor 4 (TLR4), lymphocyte antigen (Ly96), Toll-like receptor 2 (TLR2), nucleotide-binding oligomerisation domain-containing protein 1 (NOD1), single immunoglobulin interleukin-1-related receptor (SIGIRR), and Toll interacting protein (TOLLIP). mRNA expression was assessed by Q-RT-PCR. The 18 S was utilised as house-keeping gene. Individual and mean values (horizontal lines) represent the fold-change against the average of the HS group in HS (n = 10), IBS-D-NT (n = 16), and IBS-D-DSCG (n = 11). Groups were compared using one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.0001; all after Bonferroni post-test for multiple comparison test. Note differences between IBS-D-NT patients vs IBS-D-DSCG patients and HS. mRNA: messenger RNA; Q-RT-PCR: quantitative real-time polymerase chain reaction; ANOVA: analysis of variance.
The transcriptional profile of pro-inflammatory/anti-inflammatory-related genes involved in activation of innate immunity was also analysed (Figure S2, supplementary material). Both inflammatory (TLR4, NOD1, Ly96, HSP70, HSP27, ALOX5, TNFAIP3) and anti-inflammatory genes (PPARG, SIGIRR, TOLLIP, TGFB1) were significantly over-expressed in IBS-D-NT patients compared to HS. Interestingly, transcripts of TGFB1 and ALOX5 were restored to HS values with DSGC therapy. Moreover, significant correlations between MC activation, as measured by tryptase messenger RNA (mRNA) transcription, and TLR4, TLR2, Ly96, HSP27 were detected (Table S2, supplementary material).
Mucosal expression of TLR2 and TLR4
To further assess local MC involvement in innate responses, the expression and co-localisation of TLRs and tryptase protein was evaluated. In all specimens TLR2 protein displayed cytoplasmic distribution within the jejunal epithelium. The density of TLR2-positive cells in the lamina propria was higher in IBS-D-NT (2.7 ± 0.6 cells/hpf) and IBS-D-DSGC (2.1 ± 0.3 cells/hpf) patients than in HS (0.8 ± 0.4 cells/hpf), but differences were not statistically significant (p = 0.055; p value adjusted = 0.110). TLR2 was not expressed by mucosal MCs in any specimen tested (Figure 4 ).
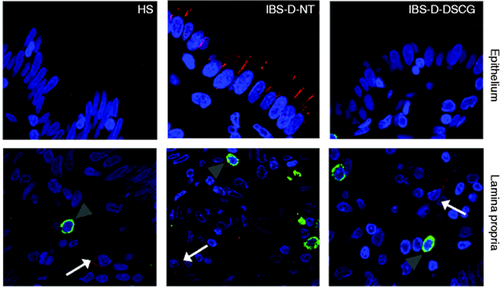
TLR4 and tryptase protein distribution in the jejunal mucosa.
Representative micrographs of double immunostaining of tryptase (green) and TLR4 (red) in the epithelium and in the lamina propria from HS, IBS-D-NT, and IBS-D-DSCG groups. Original magnification ×100. Note TLR4 (white arrows) independent of MCs (grey arrowheads). TLR4: Toll-like receptor 4; HS: healthy subjects; IBS-D-NT: diarrhoea-predominant irritable bowel syndrome patients after six months’ follow-up with no treatment; IBS-D-DSCG: diarrhoea-predominant irritable bowel syndrome patients after six months of treatment with oral disodium cromoglycate; MCs: mast cells.
Epithelial TLR4 protein, mainly located at the apical border, showed similar distribution in HS and IBS-D-DSCG groups; however, in biopsies from IBS-D-NT patients TLR4 was mainly distributed basolaterally in enterocytes. The density of TLR4-positive cells in the lamina propria was higher in IBS-D-NT patients (2.0 ± 0.9 cells/hpf) than in HS (0.8 ± 0.5 cells/hpf) and IBS-D-DSGC patients (1.6 ± 0.7 cells/hpf) but differences were not statistically significant. TLR4 was not expressed by mucosal MCs in any specimen tested (Figure 5 ).
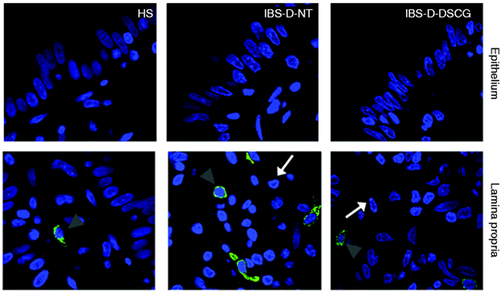
TLR2 and tryptase protein distribution in the jejunal mucosa.
Representative micrographs of double immunostaining of tryptase (green) and TLR2 (red) in the epithelium and in the lamina propria from HS, IBS-D-NT, and IBS-D-DSCG groups. Original magnification ×100. Note TLR2 expression (white arrows) independent of MCs (grey arrowheads). TLR2: Toll-like receptor 2; HS: healthy subjects; IBS-D-NT: diarrhoea-predominant irritable bowel syndrome patients after six months’ follow-up with no treatment; IBS-D-DSCG: diarrhoea-predominant irritable bowel syndrome patients after six months of treatment with oral disodium cromoglycate; MCs: mast cells.
Clinical evolution and clinical-biological correlations
Oral therapy with DSCG significantly reduced pain intensity, number of bowel movements per day and stool consistency, whereas in IBS-D-NT patients non-significant changes were observed (Figure S3 supplementary material). Notably, the proportion of patients reporting ≥50% improvement in the severity of abdominal pain by the end of the test period was significantly higher in the IBS-D-DSCG group than in the IBS-D-NT group (77% (14/18) vs 28% (7/25), respectively; p = 0.002)). Likewise, the proportion of patients reporting ≥50% reduction in the frequency of bowel movements, and normalisation of stool consistency, by the end of the treatment period, was greater in the IBS-D-DSCG group compared to the IBS-D-NT group (44.4% (8/18) vs 0% (0/25); 72% (13/18) vs 20% (5/25), respectively; p = 0.001).
To get further insight into mucosal immune activation as a potential mechanism linked to intestinal dysfunction, clinical-biological associations were analysed. Intraluminal tryptase protein positively correlated with abdominal pain severity and number of bowel movements, whereas tryptase gene expression correlated only with abdominal pain severity (p < 0.05; Figure S4, supplementary material). Ly96 and TLR2 expression positively correlated with number of bowel movements, and TLR4 expression positively correlated with abdominal pain severity (Figure S5, supplementary material).
None of the patients in the IBS-D-DSCG group required rescue medication (i.e. loperamide), whereas three patients in IBS-D-NT group needed to take loperamide once to control diarrhoea. Finally, DSCG was well tolerated throughout the study, and no major adverse effects were recorded. Mild transitory symptoms including dyspepsia, headache and sleepiness were reported by 3/18 (16.6%) of orally DSCG-treated patients.
Discussion
This pilot study shows that oral DSCG treatment modulates mucosal MC activity and decreases innate immune signalling in IBS-D patients. Compared to healthy controls, untreated patients exhibited features of mucosal MC activation, including increased expression and luminal release of mucosal tryptase, together with ultrastructural signs of piecemeal degranulation. The increase in tryptase protein and mRNA expression has previously been related to altered intestinal epithelial permeability and visceral pain.6, 9 Furthermore, in our study, MC activation was associated with IBS-D clinical manifestations, as indicated by the correlation of mucosal tryptase expression and release with abdominal pain severity and bowel habit. Overall, our findings show the effectiveness of oral DSCG to stabilise jejunal mucosal MCs, by decreasing ultrastructural signs of MC degranulation, downregulating tryptase expression and release to levels similar to healthy controls. Although Constipation-predominant irritable bowel syndrome (IBS-C) could have been considered as a more adequate control for pain in this study, MC hyperplasia and activation have been described only in the colon and terminal ileum in this group, and therefore we did not select these patients.
The innate immune system, involving TLRs, participates in intestinal tolerance and homeostasis, and experimental studies suggest that dysregulation of TLRs signalling contributes to intestinal inflammation.29 Increased permeability, a feature in IBS-D patients, could facilitate the activation of TLR-dependent immune responses. In fact, previous studies have demonstrated differential expression of TLRs in the colonic mucosa of IBS,30 and correlation of TLR2 and TLR4 mRNA expression with the duration of symptoms.31 We therefore designed a study to test whether TLR2 and TLR4 in the small intestine were also implicated in the pathophysiology of IBD-D. Of importance, our study extends previous findings on the contribution of innate immunity and MC involvement in the pathogenesis of IBS to the jejunum, as MC degranulation, TLR4 and innate immune-related molecules are increased in the mucosa of non-treated IBS patients and reduced after MC stabilisation. However, the small sample size limited the significance of the findings in this study, and we cannot conclude as to the involvement of TLR2 in the small intestine in IBS-D. Nevertheless, our data point at a role of TLR2 in the lamina propria while the TLR4 may be more implicated in the intestinal epithelium.
Heat-shock proteins (HSPs) are intracellular proteins constitutively expressed in all types of cells and inducible by various stimuli in response to environmental stress. HSPs could have a role in the maintenance of the epithelial barrier and in immune homeostasis, as in the gut HSP27 is involved in cytoskeleton dynamics and plays an essential role in maintaining the intestinal epithelium integrity.32 Our data show that increased HSP27 mRNA in the jejunal mucosa of non-treated patients correlates with tryptase expression, suggesting that HSP27 may have a role in counteracting the intestinal barrier impairment associated with MC activation. Moreover, IBS-D (non-treated) patients exhibited upregulation of two inhibitors of TLR signalling (TOLLIP and SIGIRR), suggesting that the homeostatic mechanisms that downregulate inflammatory responses are preserved in IBS.
As discussed above, oral DSCG reduced tryptase expression, as well as the expression of innate immune-related genes (TLR2, TLR4 and Ly96), and the potential interrelation of these two effects is not clear. TLR2 and TLR4 are expressed by epithelial and lamina propria cells, but not by mucosal MCs in our samples, and TLR expression was reduced by DSCG. Previous studies have also shown that DSCG could regulate the epithelial barrier itself by the inhibition of allergen absorption by Caco-2 cells in a dose-dependent manner.33 In addition, a recent study demonstrated that DSCG blocks the increase in small bowel permeability induced by experimental stress in humans.34 Besides a direct effect on the epithelium, modulation of permeability could be secondary to an effect on the underlying innate immune cells. DSCG can also modulate eosinophil responses, acting partly via the G-protein coupled receptor35 and neuronal activity.36 Notably, our study identifies modulation of TLR4 expression on epithelial cells; however, whether TLR4 contributes to DSCG-mediated changes in epithelial permeability remains to be elucidated. We cannot rule out that the clinical benefit also involves regulation of these and other cell types in the vicinity of the gut mucosa.
Although not our primary aim, our study suggests that long-term oral administration of DSGC reduces abdominal pain by 50% and improves bowel habits in most IBS-D patients. In addition, TLR4 mRNA expression correlates with the intensity of abdominal pain in IBS-D patients. This is consistent with previous studies showing the involvement of TLR4 in visceral hypersensitivity in a chronic psychosocial stress model.37 Our results should be interpreted with caution regarding their translational relevance to clinical practice. We are aware of the limitations of the experimental design: the lack of a baseline intestinal biopsy and of a placebo group, and the relatively small sample size. However, this pilot study has allowed us to identify MC involvement in the small intestine and potential DSCG clinical benefit, and sets the baseline for a future double-blind placebo-controlled study. We also acknowledge the relatively large amount of tests run in our study, which limit the interpretation of our results. Despite this, p adjusted values have been calculated and did not differ for most of the variables analysed in this study. Regardless, these preliminary clinical results need to be further substantiated. In conclusion, our study indicates that oral long-term DSGC modulates intestinal innate immune activity and MC activation in the jejunal mucosa of IBS-D patients with a promising clinical benefit.
Acknowledgements
We acknowledge all IBS-D patients participating in this study for providing their personal medical history and current clinical symptoms, and regularly responding to the questionnaires. M Gallart, M Casellas and C Alastrue contributed to sample analysis; A Aparici, MT Casaus and P Rodríguez contributed to assistance in the performance of jejunal biopsies; J Heredia and G Santaliestra contributed to secretarial assistance; and Dr A Sánchez-Chardi and F Cardoso from Servei de Microscòpia of Universitat Autònoma de Barcelona contributed to technical support.
BL and LR contributed to the experimental design, study coordination, acquisition and analysis of clinical data, and drafting of the manuscript. BL, LR, DG, CA-C and MP contributed to participant recruitment, performance of biopsies, and creation/maintenance of study database. MG performed the allergy tests and clinical assessment of participants. CM contributed to acquisition and analysis of Q-RT-PCR data. IT performed the histopathological analysis; AMG, ES-R, BKR-J, MF and CP-C conducted experimental analysis and data interpretation. FA contributed to clinical advice and critical revision of the manuscript. JS contributed to patient recruitment and collection of clinical data, project supervision and drafting of the manuscript. MV contributed to the experimental design, conducted electron microscopy analysis, project supervision and drafting of the manuscript. All authors discussed the results and discussed the manuscript at all stages. All named authors have approved the final version of the manuscript.
Declaration of Conflicting Interests
Beatriz Lobo, Laura Ramos, Cristina Martínez, Mar Guilarte, Ana M González-Castro, Carmen Alonso-Cotoner, Marc Pigrau, Ines de Torres, Bruno K Rodiño-Janeiro, Eloisa Salvo-Romero, Danila Guagnozzi, Marina Fortea, Cristina Pardo-Camacho and María Vicario have nothing to declare. Prof Dr Azpiroz has received fees for consulting in Allergan and Danone. Dr Santos has received fees for consulting for Allergan and Ipsen.
Funding
This study was funded in part by the Fondo Europeo de Desarrollo Regional (FEDER), Fondo de Investigación Sanitaria and CIBEREHD, Instituto de Salud Carlos III, Subdirección General de Investigación Sanitaria, Ministerio de Economía, Industria y Competitividad: B Lobo (CM08/00229), L Ramos (CM05/00055), M. Vicario (CP10/00502 and PI13/00935), C. Alonso (PI 15/00301 and PI12/00314), B.K. Rodiño-Janeiro (EII2011-0035 and CD15/00010), J. Santos (PI08/0940, EC07/90148, PI11/00716 and PI14/00994); Ministerio de Educación, Dirección General de Investigación: F Azpiroz (SAF 2009-07416); Agència de Gestió d’Ajuts Universitaris i de Recerca, de la Generalitat de Catalunya: F. Azpiroz (2009 SGR 219). The International Foundation for Functional Gastrointestinal Disorders (IFFGD Research Grant Award 2008), J Santos (IFFGD Research Grant Award 2009). Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas: F. Azpiroz (CB06/04/0021).




