Yield of prolonged wireless pH monitoring in achalasia patients successfully treated with pneumatic dilation
Abstract
Background
Gastro-oesophageal reflux disease (GORD) is a long-term complication of achalasia treatments. The aim of our study was to evaluate the yield of prolonged wireless pH monitoring in patients with successfully treated achalasia and its influence on proton pump inhibitor (PPI) use.
Methods
Twenty-five patients with achalasia who underwent prolonged wireless pH monitoring after a successful treatment with pneumatic dilation were enrolled. pH variables were analysed in the first 24 hours of monitoring to determine if tracings were indicative of GORD; the same variables were analysed in the following 24-hour period in order to obtain a worst-day diagnosis of GORD. PPI therapy before and after the test was recorded.
Results
Five out of 25 patients had GORD diagnosis during the first day of monitoring and four of them had oesophagitis at endoscopy. During the following days of monitoring four more patients had a diagnosis of GORD. Out of the 25 patients, PPIs were started after the test in six asymptomatic GORD-positive ones, whereas prescription of PPIs was stopped without detrimental effect on symptoms in three GORD-negative patients.
Conclusions
Prolonged wireless pH monitoring is a useful test to be added to endoscopy in order to evaluate GORD and to optimise antisecretory treatment in successfully treated achalasia patients.
Introduction
Oesophageal achalasia is a rare disease characterised by impaired relaxation of the lower oesophageal sphincter (LOS) and loss of normal peristalsis in the oesophageal body.1 All available treatments (i.e. pneumatic dilation (PD), Heller myotomy (HM) and more recently per-oral endoscopic myotomy (POEM)) aim to disrupt the LOS and reduce its pressure in order to allow oesophageal emptying.2 Gastro-oesophageal reflux disease (GORD) may occur as a complication. Studies with 24-hour pH monitoring have shown increased oesophageal acid exposure time (AET) in a variable percentage of patients treated with PD, HM with or without anti-reflux procedure and POEM, ranging from 4.7% to 53%.3-6 However 24-hour reflux tests have some intrinsic limitations such as physiological day-to-day variability of oesophageal AET due to different daily dietary habits and physical activity.7-9 These limitations could be particularly relevant in achalasia patients in whom reflux is characterised by a few long-lasting episodes.3, 10, 11 Thus, studies with 24-hour pH monitoring could have underestimated prevalence of pathological GORD in this particular population similarly to what has been shown in patients with GORD.12-14 Although it could be argued that presence of post-treatment GORD can be evaluated with symptoms (heartburn, chest pain, regurgitation) and need for proton pump inhibitors (PPIs),5, 15 a purely symptomatic approach has limitations because on one hand achalasia patients have alterations in sensory neural pathways16-18 and may have a higher threshold for perception, and on the other symptoms may not be related to GORD with consequent misuse of PPIs.4, 19, 20
The Bravo wireless technique has been developed in order to overcome limitations of traditional catheter-based pH testing. In non-erosive reflux disease patients (NERD) prolonged wireless pH monitoring has been shown to diagnose GORD in those with a normal AET during the first 24 hours of monitoring or with previously negative 24-hour catheter-based monitoring.13, 14 Moreover, one recent study regarding an achalasic cohort treated with POEM21 evaluated reflux after the endoscopic treatment with prolonged wireless pH monitoring and showed a higher incidence of reflux (58%) compared to a review of previous studies6 in which 24-hour pH monitoring was used. However, this study did not formally assess if the likelihood of GORD detection was higher when pH monitoring covers more days. Furthermore, to our knowledge no studies have evaluated the prevalence of GORD in achalasia patients treated with PD using prolonged wireless pH monitoring.
The aim of our study was to evaluate the yield of prolonged wireless pH monitoring in GORD diagnosis in a cohort of achalasia patients successfully treated with PD and its influence on PPI use.
Methods
Study population
Twenty-five consecutive achalasia patients (12 males; 47 years of age; 38–63) who underwent prolonged wireless pH monitoring after a successful treatment with single or multiple PD were reviewed from our cohort of patients and included in the study. Treatment was defined successful if stable clinical remission was achieved (Eckardt score ≤ 3 evaluated at three and 12 months after dilation).3 In our centre additional criteria for success are a negative intraoesophageal pressure at oesophageal high-resolution manometry (HRM) at three and 12 months and a decrease in the maximum diameter of the middle third of the thoracic oesophagus compared with pre-treatment at barium oesophagogram 12 months after dilation. Both additional criteria were present in all our 25 patients.
Ninety-six-hour wireless pH monitoring
After external calibration in buffer solutions pH 1.0 and 7.0, the Bravo pH capsule (Medtronic Inc, Shoreview, MN, USA) was attached 6 cm above the squamocolumnar junction during upper endoscopy under mild conscious sedation (1–5 mg midazolam intravenously (i.v.)) as previously described.22 pH monitoring was then activated and data were stored in the receiver for 48 hours. During the pH study patients were encouraged to engage in their usual activities including work and exercise with the only restriction that no sipping of acidic beverages (e.g. orange juice, cola) occur between meals so as not to alter readings. They were asked to press the event marker button on the receiver whenever they experienced their symptoms. Furthermore, patients kept diaries documenting timing of food intake, change of posture and occurrence of symptoms according to their watch, which was synchronised with the Bravo receiver. After 48 hours of pH recording, patients returned to the hospital and gave receivers and diaries back. Recorded data were downloaded to a computer using commercial software (Polygram Net, Medtronic, Denmark), and patients were invited to participate in a second 48-hour recording. Antisecretory drugs (PPIs) were stopped at least eight days before pH monitoring; patients were instructed not to use antacid formulations during the whole recordings.
Oesophageal manometry
HRM (Solar HRM, MMS, the Netherlands) was performed in all patients before prolonged wireless pH monitoring using a disposable 20-sensor catheter (MMS G-90500). The oesophageal manometry catheter was passed trans-nasally under topical anaesthesia (Lidocaine spray 10%) after an overnight fast, and positioned so that it straddled the LOS. The whole test was performed with the patients in a recumbent position on their right side. The information obtained with a standard manometric protocol included baseline tone of the LOS (recorded for at least 30 seconds) and intraoesophageal pressure.
Outcome evaluation
At our Gastrointestinal Unit patients with oesophageal disorders undergo a standardised medical interview during patient evaluation.22, 23 Patient outcome data were obtained by reviewing hospital files with regards to consultations which occurred before and after wireless pH monitoring. Regarding treatment for GORD, information was sought on PPI treatment before wireless pH monitoring and whether the treatment was stopped/continued after the test and during the follow-up period.
Data analysis
pH variables were analysed in the first 24-hour periods in order to determine if tracings were diagnostic of GORD: percentage of time at pH <4 more than 4.7%24 and/or positive symptom index (SI) or symptom association probability (SAP). Number of reflux episodes were considered increased if >57/24 hours.24 The same variables were analysed in the following 24-hour periods in order to obtain a worst-day diagnosis of GORD. Moreover, SI and SAP were measured during the whole period of monitoring. Furthermore, percentage time at pH < 4 in upright and supine position and number of long-lasting episodes of reflux (>5 min) were calculated in tracings of patients. In order to exclude intraoesophageal acidification due to fermentation, tracings were manually scanned in order to detect slow steady drops of pH not reaching values below 3 and exclude them from AET calculations.4, 25 Only days with at least 21-hour recordings were included.
In the outcome analysis, patients were considered GORD positive or negative both according to wireless pH monitoring and endoscopy.
Data were expressed as median (first and third quartile). Non-parametric statistics and chi-square test were used when appropriate.
Results
Wireless pH monitoring was performed after at least one year of clinical well-being from PD. Oesophagitis was found in seven patients (three grade A, three grade B and one grade C). No complications occurred during upper endoscopy and wireless capsule positioning.
All patients (N = 25) reported normal activities of daily living during wireless pH study and completed at least 48 hours of monitoring; 13 patients decided to stop registration at 48 hours because they were living at quite a distance from our hospital. Of the remaining 12 patients who agreed to undergo the second 48-hour recording, capsule detachment occurred on the third day in four and on the fourth day in one, thus in seven patients pH monitoring lasted 96 hours. No further dilation was needed during the follow-up after wireless pH monitoring.
Impact of prolonged wireless pH monitoring on GORD diagnosis
Characteristics of achalasia patients divided according to worst-day GORD diagnosis at prolonged wireless pH monitoring are described in Table 1. The two groups were similar with regards to clinical and manometric features. As depicted in Figure 1 five out of the nine GORD-positive patients had GORD diagnosis during the first day of monitoring; four of them had oesophagitis at endoscopy. Prolonging the monitoring over the first day, four more patients had a worst-day diagnosis of GORD: Two during the second day, one during the fourth day and one during both the second and the fourth day of monitoring; two out of these four patients had oesophagitis at endoscopy. All these patients received a GORD diagnosis based on percentage of time at pH < 4, whereas in the eight patient reporting symptoms during the monitoring (six had heartburn and two had chest pain), two with increased and six with normal AET, SI and SAP were negative both during each 24 hours and the whole period. Only one patient with negative prolonged wireless monitoring had oesophagitis. No pH drops indicative of fermentation were present in any patient.
| GORD positive (9) | GORD negative (16) | p value | |
|---|---|---|---|
| Age | 43; 35–56 | 52; 39–64 | 0.36 |
| Patients with oesophagitisa | 6 (A = 2, B = 3, C = 1) | 1 (A) | 0.0007 |
| Number of pneumatic dilation before pH monitoring | 2; 1–2 | 2; 1–2 | 0.69 |
| Interval between treatment and pH monitoring (months) | 15; 13–31 | 14; 13–32 | 0.90 |
| Follow-up time after pH monitoring (months) | 22; 10–46 | 47; 35–56 | 0.07 |
| Basal LOS pressure (mmHg) | 6; 3–10 | 4; 3–7 | 0.55 |
| Basal intra-oesophageal pressure (mmHg) | −2; −7 to −2 | −4; −6 to −3 | 0.20 |
- a In brackets are number of patients divided according to grade of oesophagitis. Median: interquartile range (IQR); GORD: gastro-oesophageal reflux disease; LOS: lower oesophageal sphincter.
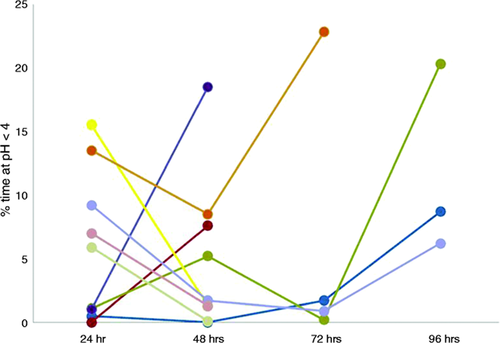
Daily AET variability in the nine GORD-positive patients according to worst-day wireless pH monitoring data. Each line represents a patient. Pathological AET was seen beyond the first day in four patients. AET: acid exposure time; GORD: gastro-oesophageal reflux disease.
Considering GORD-positive patients according to pH monitoring, six out of nine showed isolated pathological supine AET, whereas one patient had pathological AET both in upright and supine periods, and isolated pathological upright AET was seen in the remaining two patients. Of note, in five patients pathological supine values were seen during only one day of monitoring. In GORD-negative patients, on the contrary, only one had isolated pathological AET in the supine position with a percentage of pH < 4 over 24 hours of 4.5%.
Long-lasting episodes of reflux were seen in all GORD-positive patients whereas number of reflux episodes per day were normal in all patients (Table 2 ). Considering GORD-negative patients, number of reflux was normal in all patients and only six showed presence of long-lasting reflux but with a significantly lower duration compared to GORD-positive patients.
| GORD positive (9) | GORD negative (16) | p value | |
|---|---|---|---|
| Number of reflux episodes | 12; 7–20 | 3; 1–5 | 0.0008 |
| Number of long lasting reflux episodes | 5; 3–8 | 0; 0–1 | 0.0002 |
| Duration of long-lasting reflux episodes (min) | 60; 54–75 | 19; 8–33 | 0.01 |
- GORD: gastro-oesophageal reflux disease; median: interquartile range (IQR).
Outcome evaluation
Data are shown in Figure 2. Eight out of 25 patients were taking PPIs before the test: Six patients had GORD-like symptoms and two patients had dyspepsia. The remaining patients were asymptomatic for GORD like symptoms. Follow-up data are available in 24 patients because one was lost to follow-up after wireless pH monitoring. With regards to the GORD-positive group, in four symptomatic patients who were already taking PPIs before the prolonged pH monitoring (two twice daily (bid) and two at morning time), PPIs were continued. In four asymptomatic patients with both oesophagitis and pathological wireless pH monitoring, PPIs were started after the test and continued until the last follow-up (three at dinner time and one at morning time). In two additional asymptomatic patients with pathological wireless pH monitoring, PPIs were started at morning time after the test. With regards to the GORD-negative group, prescription of PPIs was stopped in three patients without detrimental effect on symptoms whereas it was maintained in the remaining one because he had dyspeptic symptoms.
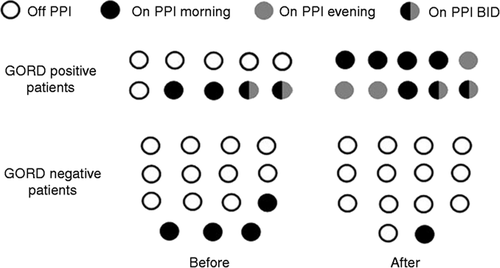
PPI treatment before and after evaluation in GORD-positive and -negative patients classified according to both wireless pH monitoring and endoscopy. PPI: proton pump inhibitor; GORD: gastro-oesophageal reflux disease.
Discussion
To our knowledge this is the first study evaluating the prevalence of GORD in patients treated with PD using prolonged wireless pH monitoring. Prolonged pH monitoring allowed us to diagnose GORD after the first 24 hours of monitoring in four patients and in particular in two of them who had no oesophagitis at endoscopy. It could be argued that during the day of capsule positioning patients may have eaten and moved less than during the following days because of the discomfort of the endoscopy and/or sedation. However, the literature looking at trends of AET during days of prolonged pH monitoring has generally reported similar AETs.22, 26-29
Furthermore, capsule pH-monitoring has proved useful for GORD management: In nine out of 25 patients (36%) PPI therapy was modified after the test: In three patients PPIs were stopped because symptoms were not related to reflux and AET was normal during the whole monitoring; in the other six patients, although asymptomatic, PPIs were started because oesophagitis was found at endoscopy or pathological AET was detected at the wireless pH-monitoring. In particular prolongation of pH monitoring allowed us to stop PPI therapy with stronger certainty when AET was normal. A negative single-day pH monitoring could be interpreted as a false-negative result due to AET day-to-day variability; indeed in a study comparing traditional 24-hour pH monitoring and 24-hour pH-multichannel intraluminal impedance (MII), physicians continued to prescribe PPIs in 45%–50% of their patients despite negative test results.23 Results on patients’ management should be cautiously considered due to the retrospective nature of our study; however, clinical and PPI status information were obtained during the consultations by standardised medical interview.22, 23 Another consideration is that there are no data regarding usefulness of lifelong PPIs in asymptomatic treated achalasia patients with increased reflux at pH monitoring, especially if they are young and endoscopy is negative for oesophagitis. It is our practice to discuss pros and cons of continuous PPI treatment with our patients, share the decision and check their upper gastrointestinal tract with an endoscopy every three to five years independently of being on long-term PPI or not.
In the present study AET variability was more pronounced than previously observed in a cohort of endoscopy-negative patients with typical GORD symptoms,14 probably because of a different pathophysiology of gastro-oesophageal reflux in achalasia, as detailed below.3, 10, 11 Total number of reflux episodes was normal in all patients and reflux episodes were long-lasting, suggesting that the main pathophysiological disturbance was delayed oesophageal clearance due to altered motility of the oesophageal body as described earlier by Benini et al.11 Some episodes lasted more than one hour also in asymptomatic patients. Furthermore, the majority of patients (seven of nine) with pathological pH monitoring had abnormal acid exposure in the supine position which was isolated in six of nine patients; the importance of prolongation of monitoring is underlined by the fact that five out of the seven patients with pathological supine values had this alteration during one day of monitoring only. These observations allowed us to start PPIs in three patients at dinner time for better acid inhibition during the night. Theoretically intra-oesophageal acidification in achalasia patients could be due to fermentation;4 however, this was not the case in any of the patients’ reflux; this observation is in line with the clinical characteristics of our patients who were all successfully treated with PD, had negative intra-oesophageal pressure at the oesophageal manometry and had no history of food regurgitation at the preliminary evaluation.
Achalasia patients with reflux after treatment are quite different from patients with classic GORD also in terms of both pathophysiology and genesis of GORD-like symptoms. They have alterations of sensory neural pathways,16-18 thus on one side reflux may not be perceived and on the other heartburn and chest pain could be related to the neuropathy and not to reflux. Furthermore, chest pain could be the result of dysmotility or oesophageal distension and increased intra-oesophageal pressure. In our study symptoms reported by achalasic patients during pH monitoring were not related to reflux, and increased AET remained asymptomatic in all patients. This observation is in line with previous studies in which no correlation between symptoms and acid reflux was observed.4, 21 For these reasons prolongation of pH monitoring is useful in order to increase the certainty of a negative/positive symptom-reflux association, by having a higher number of symptomatic episodes be evaluated.
In conclusion, prolonged wireless pH monitoring is a useful test to be added to endoscopy in order to evaluate GORD and to optimise antisecretory treatment in successfully treated achalasia patients. We suggest its use not only after pneumatic dilation, but also after per oral endoscopic or surgical myotomy.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.




