Anti-Inflammatory and Antimicrobial Effects of Herbal Formulation Called Apo-Taat Using Extended-Spectrum ß-Lactamase-Producing Escherichia coli Isolates
Abstract
Pathogens contaminate drinking water in tropical countries causing diarrheal diseases. The conventional treatment for diarrhea is antibiotics. However, overuse and misuse of antibiotics has enabled pathogens to adapt, causing global antibiotic resistance and proliferation of extended-spectrum ß-lactamase-producing Escherichia coli (ESBL-E. coli), which causes diarrhea and high levels of inflammatory cytokines. Apo-taat, consisting of equal proportions of Phyllanthus emblica and Caesalpinia sappan, has been used to treat diarrhea and bloody diarrhea. Its antibacterial activity against E. coli ATCC 25922 has been reported, but its inhibitory effect against ESBL-E. coli has yet to be documented. This study investigated the antibacterial effect of Apo-taat extract against ESBL-E. coli and its anti-inflammatory activity. Antibacterial activity was determined by the microtiter plate–based method. HPLC was used to determine the brazilin and gallic acid contents in Apo-taat extract. Effects of herbal extracts on nitric oxide, IL-6, and TNF-α were investigated in RAW 264.7 cells. Results were that Apo-taat extract showed MIC values against ESBL-E. coli in the range of 0.625 to 2.5 mg/mL. Its 50% inhibitory concentration against nitric oxide and IL-6 production was 83.96 ± 10.60 and 83.06 ± 2.07 μg/mL, respectively, and it had slight inhibition against TNF-α. These findings suggest that Apo-taat may have an antibacterial impact on ESBL-E. coli and anti-inflammatory activity. Furthermore, safety and clinical trials should be conducted in the future.
1. Introduction
Infectious pathogens cause diarrhea leading to morbidity and mortality in children and adults, especially in the tropics [1]. Symptoms include frequent watery or loose bowel movements for 24 h. When the immune system detects these pathogens, it triggers inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and nitric oxide, which induce colitis [2–5]. The pathogen most frequently isolated in newborns and travelers to tropical regions is Escherichia coli [6]. The prevalence of E. coli infection is increasing because improper consumption of antibiotics has led to microbial resistance [7, 8]. Extended-spectrum beta-lactamase (ESBL) is an enzyme produced by the Enterobacteriaceae family that can deactivate a wide range of antibiotics, including broad-spectrum oxyimino-cephalosporin, penicillin, and monobactam, but not ß-lactamase inhibitors such as sulbactam, tazobactam, and clavulanic acid. The ESBL genes, which are plasmid-mediated, can be classified into families, including blaTEM, blaSHV, blaCTX-M, and blaOXA. The TEM type contains specific amino acid residues Gly238, Glu240, Arg164, and Glu104, which can provide resistance to ampicillin, penicillin, and first-generation cephalosporins, but not to oxyimino-cephalosporins. The SHV-type and TEM-type amino acid residues are similar; specifically, Ser238 and Glu240 are altered in this type. The blaSHV family confers resistance to penicillin, tigecycline, and piperacillin, but not to oxyimino-cephalosporin. The CTX-M type, which is the predominant ESBL family, is categorized into five groups according to sequence homologies: CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, and CTX-M-25. They prefer hydrolyzing cefotaxime rather than ceftazidime, and their activity is hindered by tazobactam. The OXA type is a diverse group that exhibits variation in amino acid sequences and can potentially break down cephalosporins and monobactams [9, 10]. Among the Gram-negative bacterial species, E. coli is the primary host for ESBL, and ESBL on E. coli is increasingly prevalent in Asia, making antibiotic treatment ineffective [11]. While microbial resistance has been increasing, a few new antibiotics have been discovered [12]. Therefore, alternative antibiotic substances are being developed to combat microbial resistance. Plants containing antimicrobial agents, such as flavonoids and tannins, are natural sources of potentially potent antibiotics [13, 14]. Apo-taat is a traditional Thai remedy for bloody diarrhea. It was recorded in King Narai’s pharmacopeia during the Ayutthaya dynasty. This herbal mixture was prepared by boiling Phyllanthus emblica bark and Caesalpinia sappan wood in equal proportions [15]. The chemical constituents of P. emblica bark are gallic acid, phyllanthol, glycoside compounds, 16-dehydropregnenolone, betulin, periplogenin, and methyl gallate [16]. The chemical constituents of C. sappan are tannins, saponin, and various phenolic constituents such as protosappanin and brazilin [17, 18]. The phytochemical compounds of the 21st Apo-taat remedy are tannins, phenolic acids, and flavonoids, which have antimicrobial and anti-inflammation activity [19–21]. A previous study found that an aqueous extract of the 21st Apo-taat remedy inhibited Gram-positive and Gram-negative bacteria with a minimum concentration of 0.156 to 1.25 mg/mL. Its effective compounds were brazilin and gallic acid [22]. However, the activity of Apo-taat against antibiotic-resistant bacterial species and against pathogen-induced inflammation has not been reported. This study assessed aqueous 21st Apo-taat remedy for the inhibition of ESBL-E. coli and anti-inflammatory activity.
2. Materials and Methods
2.1. Plant Materials
The heartwood of C. sappan was collected at Suphanburi Province, Thailand (100° 07′ 19.85″ E longitude and 14° 28′ 27.05″ N latitude). It was identified and authenticated by the herbarium of the Southern Centre of Thai Medicinal Plants, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Songkhla Province, Thailand. The specimen reference number is SKP098031901. Bark of P. emblica was collected at Pathum Thani Province, Thailand (100° 28′ 59.99″ E longitude and 14° 02′ 60.00″ N latitude). It was identified and authenticated by the Princess Sirindhorn Plant Herbarium Building, Department of Agriculture, Kasetsart University, Phaholyothin Road, Lat Yao, Khet Chatuchak, Bangkok, Thailand. The specimen reference number is BK No.085336. All materials were collected in January 2023 (winter season).
2.2. Chemical and Reagents
Nutrient agar and Mueller Hinton broth were obtained from Difco, USA. Ampicillin, lipopolysaccharide, and resazurin were obtained from Sigma-Aldrich, Germany. Levofloxacin, gallic acid, phosphoric acid, and MTT were purchased from TCI, Japan. Brazilin was purchased from ChemFaces, China. Acetonitrile and dimethyl sulfoxide were purchased from RCI Labscan, Thailand. Fetal bovine serum (FBS), penicillin/streptomycin, and Dulbecco’s modified Eagle’s medium were acquired from Gibco, USA. We bought ELISA kits for TNF-α and IL-6 from R&D Systems in the USA.
2.3. Preparation of Herbal Formulation and Its Ingredient Extracts
The Apo-taat preparation was extracted using the decoction method. The dried heartwood of C. sappan and bark of P. emblica were ground into powder. 250 g of each sample was combined to make Apo-taat powder. The 500 g of Apo-taat powder was boiled in 2000 mL of water for 15 min and then filtered. The extract was re-boiled and filtered twice more. After that, the three filtered extracts were combined, evaporated, and dried in a lyophilizer. P. emblica and C. sappan were extracted using a similar method. The three extracts, Apo-taat aqueous extract (APW), C. sappan aqueous extract (CSW), and P. emblica aqueous extract (PEW), were investigated for antibacterial and anti-inflammatory activities. All extracts were stored at −20°C until use.
2.4. Antibacterial Activity
Clinical strains of ESBL-E. coli were obtained from a previous study in which the blaESBL gene was detected using the polymerase chain reaction with blaESBL as the primer [23]. In that study, there were 21 strains including SK3, SK4, SK6, SK8, SK11, SK20, SK23, SK56, SK76, SK77, SK80, SK81, SK82, SK83, SK85, SK89, SK91, SK93, SK100, SK101, and SK114. Each clinical strain of E. coli had different resistance gene patterns, as shown in the previous report [23]. All 21 strains of ESBL-E. coli were cultured together in nutrient agar and incubated at 37°C for 18–24 h. Then, the single colony was cultured in Muller Hinton broth (MHB) and incubated for 2 h at 37°C. After that, the bacteria suspension was adjusted to 0.5 McFarland using a McFarland densitometer (Liofilchem, Italy), and then, it was used as the inoculum. The antibacterial activity was evaluated using resazurin as an indicator [24]. All aqueous extracts were prepared, adjusted to 20 mg/mL in water, and filtered through a 0.22-μm sterile syringe filter (Millipore, USA). The stock solution was diluted 2-fold in MHB. All samples and bacteria suspensions were placed in triplicate 96-well plates (Corning, USA) at 50 μL/well. A negative control consisting of 50 μL/well of MHB and 50 μL/well of suspended bacteria was prepared to detect bacteria growth. Another well plate containing only MHB at 100 μL/well was prepared to detect media contamination. Then, the plates were incubated for 18–24 h at 37°C in an orbital shaker incubator [25]. After incubation, 10 μL of resazurin at a concentration of 1 mg/mL was added to each well and incubated for 3 h at 37°C. The resulting color change was monitored visually. The resazurin changed from blue to pink where the bacteria were not suppressed. The minimum concentration of extract with no change in the color of the resazurin was the minimum inhibitory concentration (MIC). Suspensions with no change in the color of resazurin were pipetted and placed in nutrient agar. Then, the plates were incubated at 37°C for 18–24 h to detect minimal bactericidal concentration (MBC) of extract [25]. MBC was the minimal concentration with no growing bacteria. Ampicillin, levofloxacin, trimethoprim, amikacin, and cefotaxime were used as positive controls. Ampicillin (100 mg/mL) and trimethoprim (50 mg/mL) stock solutions were prepared in dimethyl sulfoxide. Levofloxacin and amikacin were prepared and adjusted to 10 mg/mL in water, whereas cefotaxime was dissolved and adjusted to 20 mg/mL in water. All stock solutions were filtrated using a 0.22-μm sterile syringe filter (Millipore, USA). The antibiotic stock solutions were diluted 2-fold in MHB and assessed for MIC values.
2.5. Anti-Inflammatory Activity
2.5.1. Cell Culture
The macrophage RAW 264.7 cell line (reference number ATCC TIB-71) was obtained from the American Type Culture Collection. The cells were cultured in complete DMEM (DMEM containing 10% FBS, 50 μg/mL streptomycin, and 50 IU/mL penicillin). The cultured cell was grown at 37°C, 5% CO2, and subcultured by 0.125% trypsin every 4 days.
2.5.2. Cell Viability
2.5.3. Determination of LPS-Induced Nitric Oxide and Inflammatory Cytokine Production From RAW 264.7 Cells
Using the following modified method described in Tewtrakul and Itharat, the inhibitory effect of nitric oxide production from RAW 264.7 cells was assessed [27]. The RAW 264.7 cells were seeded in a 96-well plate with 1 × 105 cells/well and incubated at 37°C, 5% CO2 for 24 h. After that, cells were replaced with 100 μL/well of lipopolysaccharide (20 ng/mL) and different concentrations of extracts (100 μL/well). The plate was incubated at 37°C, 5% CO2 for 24 h. Following incubation, the supernatant was gathered to measure the production of IL-6, TNF-α, and nitric oxide. The nitric oxide production detection was performed using the Griess reagent assay by adding supernatant at 100 μL/well and Griess reagent at 100 μL/well. The plate was then measured at 570 nm, and the NaNO2 was calculated according to the standard curve of NaNO2 [27]. The production of TNF-α and IL-6 was quantified utilizing an ELISA kit. The supernatant was added to an ELISA plate coated with primary antibody and incubated for 2 h. Subsequently, the plate was washed, and a secondary antibody was applied. Subsequently, the 3,3′, 5,5″-tetramethylbenzidine substrate was added and measured with a microplate reader (Thermo Fisher Scientific, USA) at a wavelength of 450 nm, according to the manufacturer’s unmodified protocol [28]. The standard curve equation was utilized to determine the amounts of IL-6 and TNF-α.
2.6. HPLC Analysis
Gallic acid and brazilin were analyzed using high-performance liquid chromatography or HPLC (Shimadzu, Japan). The HPLC setup utilized a Phenomenex Luna 5 μ C18 [2] 100A analytical column (250 × 4.60 mm, 5 micron) with a C18 guard column (Phenomenex, USA). The injection volume was set to 10 μL. The mobile phase consisted of acetonitrile (A) and 0.1% phosphoric acid (B). For gradient elution, the initial conditions were set to 95% of eluent B, decreasing linearly to 85% over 0–12 min, and further to 75% at 32 min. The gradient then reached 0% of eluent B at 37 min, where it was held for 5 min. Before injecting the next sample, the mobile phase returned to its initial composition at 42.1 min and was left to stabilize for an additional 5 min. The flow rate was maintained at 0.65 mL/min, with detection wavelengths of 286 nm for brazilin and 272 nm for gallic acid [22].
2.6.1. Method Validation
The analytical method was validated following the International Conference on Harmonization (ICH) recommendations concerning linearity, accuracy, precision, limit of detection (LOD), and limit of quantitation (LOQ) [29].
2.6.1.1. Linearity
Gallic acid and brazilin were weighed and diluted in methanol to prepare standard solutions at 12.5, 25, 50, 100, 200, and 400 μg/mL. Each concentration was analyzed in triplicate. Calibration curves were plotted of peak area vs concentrations of gallic acid and brazilin. The ability of an analytical method to produce results that reflect the quantity of analyte in the sample is its linearity.
2.6.1.2. Accuracy
The relative standard deviation (%RSD) was calculated, and accuracy was expressed as the percent recovery of the known amount of analytes added to the sample, relative to its existing concentration. The APW sample was spiked with standard brazilin and gallic acid mixtures at concentrations of 200, 100, and 50 μg/mL. Three tests were conducted using triplicate preparations of spiked samples. The percent recovery was calculated using the formula as follows:
The recovery percentage = (detected amount/spiked amount) × 100.
2.6.1.3. Precision
Calculating the RSD can show the precision of a procedure by evaluating the consistency between individual test results. Standard solutions of gallic acid and brazilin in concentrations of 100, 200, and 400 μg/mL were analyzed in triplicate to assess intraday precision. Interday precision over 3 days was assessed using the same methodology.
2.6.1.4. LOD and LOQ
The lowest quantity of analyte that may be found in a sample is known as the LOD, and it was calculated as LOD = 3.3σ/S, where S is the calibration curve’s slope and σ is the standard deviation of the regression lines’ y-intercepts. The lowest quantity of analyte that can be quantitatively identified in a sample with appropriate precision and accuracy is known as the LOQ, and it was computed as 10σ/S.
2.6.2. Analysis of the Chemical Constituents of the Apo-Taat Extract and Its Ingredient
APW, CSW, and PEW were accurately weighed (10 mg) and adjusted to 10 mg/mL in methanol. The solution was subjected to ultrasonication for 5 min. The solution was filtered through a 0.45-μm nylon syringe filter and then analyzed by HPLC. The gallic acid and brazilin contents were calculated using the above calibration curve.
2.7. Statistical Analysis
Three independent experiments were performed in triplicate. The data were expressed as mean and standard deviation and analyzed using one-way ANOVA. A p value < 0.05 indicated statistical significance.
3. Results and Discussion
APW had the highest extraction yield (13.84%), followed by PEW (6.17%) and CSW (4.74%). All extracts were investigated for antibacterial and anti-inflammatory activities.
3.1. Inhibition Effect of Apo-Taat and Plant Ingredient Extracts Against ESBL-E. coli
The antibiotic sensitivity of each ESBL-E. coli strain was assessed, including their responses to various antibiotics, as shown in Table 1. All strains were resistant to ampicillin, and some strains were resistant to trimethoprim and cefotaxime. All strains were sensitive to levofloxacin and amikacin.
| Bacterial strain | Minimal inhibitory concentration (μg/mL) | ||||
|---|---|---|---|---|---|
| Ampicillin | Levofloxacin | Trimethoprim | Amikacin | Cefotaxime | |
| EC sk 3 | > 1000 | 0.24375 | > 500 | 6.25 | 1000 |
| EC sk 4 | > 1000 | 15.625 | 0.975 | 3.125 | 6.25 |
| EC sk 6 | > 1000 | 0.24375 | > 500 | 3.125 | 2000 |
| EC sk 8 | 1000 | 0.05 | 0.24375 | 3.125 | 6.25 |
| EC sk 11 | > 1000 | 0.4875 | > 500 | 6.25 | 250 |
| EC sk 20 | > 1000 | 0.24375 | 0.24375 | 3.125 | 1000 |
| EC sk 23 | > 1000 | 0.24375 | 0.12187 | 3.125 | 1000 |
| EC 190 sk 56 | > 1000 | 0.4875 | > 500 | 6.25 | 1000 |
| EC sk 76 | > 1000 | 0.025 | 0.24375 | 3.125 | 2000 |
| EC sk 77 | > 1000 | 0.05 | 0.24375 | 6.25 | 1000 |
| EC sk 80 | > 1000 | 7.8125 | 0.4875 | 6.25 | 2000 |
| EC sk 81 | > 1000 | 15.625 | 0.4875 | 12.5 | 5000 |
| EC sk 82 | > 1000 | 0.4875 | > 500 | 6.25 | 2500 |
| EC sk 83 | > 1000 | 7.8125 | > 500 | 6.25 | 10,000 |
| EC sk 85 | 1000 | 0.05 | 0.4875 | 12.5 | 0.03125 |
| EC sk 89 | > 1000 | 7.8125 | > 500 | 6.25 | 2500 |
| EC sk 91 | > 1000 | 15.625 | > 500 | 12.5 | 10,000 |
| EC 359 sk 93 | > 1000 | 7.8125 | > 500 | 6.25 | 1000 |
| EC 388 sk 100 | > 1000 | 15.625 | > 500 | 6.25 | 2500 |
| EC 410 sk 101 | 625 | 0.975 | 0.4875 | 12.5 | 0.0625 |
| EC 490 sk 114 | > 1000 | 7.8125 | > 500 | 3.125 | 200 |
Subsequent results showed that APW exhibited inhibitory effects against ESBL-E. coli, with MIC values ranging from 0.625 to 2.5 mg/mL (Table 2). The MIC values for PEW and CSW were 2.5 -> 5 and 1.25–2.5 mg/mL, respectively. APW and PEW exhibited high sensitivity to EC 190 sk 56, with MIC values of 0.625 and 2.5 mg/mL, respectively.
| Bacterial strain | APW | CSW | PEW | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | |
| EC sk 3 | 1.25 | 5 | 4 | 1.25 | 2.5 | 2 | 5 | > 5 | ND |
| EC sk 4 | 1.25 | 2.5 | 1 | 2.5 | 2.5 | 1 | 5 | 5 | 1 |
| EC sk 6 | 1.25 | 5 | 1 | 2.5 | > 5 | ND | 5 | > 5 | ND |
| EC sk 8 | 2.5 | 2.5 | 1 | 2.5 | > 5 | ND | 5 | > 5 | ND |
| EC sk 11 | 1.25 | 1.25 | 1 | 2.5 | > 5 | ND | 5 | > 5 | ND |
| EC sk 20 | 2.5 | 2.5 | 1 | 2.5 | 2.5 | 1 | 5 | > 5 | ND |
| EC sk 23 | 2.5 | 5 | 1 | 2.5 | 2.5 | 1 | 5 | > 5 | ND |
| EC 190 sk 56 | 0.625 | 0.625 | 1 | 1.25 | 2.5 | 2 | 2.5 | > 5 | ND |
| EC sk 76 | 1.25 | 1.25 | 1 | 2.5 | 2.5 | 1 | 5 | > 5 | ND |
| EC sk 77 | 1.25 | 1.25 | 1 | 2.5 | 2.5 | 1 | 5 | > 5 | ND |
| EC sk 80 | 2.5 | 5 | 1 | 2.5 | 2.5 | 1 | 5 | > 5 | ND |
| EC sk 81 | 2.5 | 2.5 | 1 | 2.5 | 2.5 | 1 | 5 | > 5 | ND |
| EC sk 82 | 1.25 | 5 | 4 | 2.5 | 2.5 | 1 | 5 | > 5 | ND |
| EC sk 83 | 1.25 | 1.25 | 1 | 2.5 | 2.5 | 1 | 5 | 5 | 1 |
| EC sk 85 | 1.25 | 1.25 | 1 | 2.5 | 5 | 2 | 5 | 5 | 1 |
| EC sk 89 | 2.5 | 2.5 | 1 | 2.5 | 2.5 | 1 | > 5 | > 5 | ND |
| EC sk 91 | 1.25 | 2.5 | 2 | 2.5 | 2.5 | 1 | > 5 | > 5 | ND |
| EC 359 sk 93 | 2.5 | 2.5 | 1 | 2.5 | 2.5 | 1 | > 5 | > 5 | ND |
| EC 388 sk 100 | 2.5 | 2.5 | 1 | 1.25 | 2.5 | 2 | 5 | 5 | 1 |
| EC 410 sk 101 | 2.5 | 2.5 | 1 | 2.5 | 2.5 | 1 | 5 | 5 | 1 |
| EC 490 sk 114 | 1.25 | 1.25 | 1 | 1.25 | 1.25 | 1 | 5 | > 5 | ND |
- Note: MIC refers to the minimal inhibitory concentration of extract that inhibited bacteria growth, and MBC refers to the minimal bactericidal concentration of extract that killed bacteria. An MBC/MIC value ≤ 4 defined a bactericidal drug, while a value > 4 defined a bacteriostatic drug [30].
- Abbreviation: ND, not detectable.
APW exhibited increased antibacterial activity when combined with the two herbal components. The enhanced effect may result from a synergistic interaction between the active compounds, supporting earlier research indicating that a combination of Origanum vulgare and Hypericum perforatum had better inhibitory activities against Staphylococcus aureus than individual extracts [31]. Another study demonstrated significant synergistic activity between Eucalyptus staigeriana and Alpinia galanga against E. coli [32]. Many phytomedicines have antibacterial properties that may function by activating enzymes to boost metabolism, interfering with nuclear-level enzymatic processes, rupturing cell walls, or interfering with secondary metabolism [33]. Synergistic action combines compounds to affect one or more of these mechanisms, producing an additive or synergistic antibacterial effect [34].
Our results expressed potent in vitro antibacterial activity of APW against different resistant strains of E. coli. APW had the most potent inhibitory effect on EC 190 sk 56 with MIC values of 0.625 mg/mL, followed by EC sk 3, EC sk 4, EC sk 6, EC sk 11, EC sk 76, EC sk 77, EC sk 82, EC sk 83, EC sk 85, EC sk 91, and EC 490 sk 114 with MIC values of 1.25 mg/mL. A previous study showed that the ESBL-E. coli used in that study had different patterns of blaESBL genes. For example, EC 190 sk 56 carried blaTEM, blaCTX-M, and blaOXA-2, while EC sk 3 and EC sk 91 carried blaSHV, blaTEM, and blaOXA-2 genes [23]. Notably, different gene patterns exhibit different types of antibiotic resistance [35]. The transformation of the blaOXA-2 gene could potentially cause hydrolysis of cephamycin, while the expression of blaTEM and blaCTX-M affect carbapenem resistance. The blaTEM transformation confers resistance to ampicillin, a first-generation cephalosporin, tazobactam, cefotaxime, and piperacillin/tazobactam [36, 37].
A recent study demonstrated that some plants exhibited antibacterial activity against ESBL-producing E. coli strains expressing the TEM gene. The growth of ESBL-producing E. coli was reduced by various natural plants, such as Myrtus communis, Amaranthus retroflexus, Cyminum cuminum, Marrubium vulgare, and Peganum harmala. P. harmala exhibited significant efficacy against targeted ESBL-producing E. coli isolates, with a MIC range of 1.25–2.5 mg/mL. High quantities of polyphenols in plants may contribute to their strong antibacterial properties [35]. The presence of polyphenols, including brazilin and gallic acid, in APW is responsible for its strong antibacterial properties [22]. APW inhibited various strains of ESBL-E. coli. It has been reported to inhibit standard strains such as Pseudomonas aeruginosa, Salmonella Typhi, Shigella dysenteriae, S. aureus, and Bacillus subtilis [22].
Plant ingredients of APW, including CSW and PEW, showed antibacterial activity against ESBL-E. coli. CSW was the most potent inhibitor against EC sk 3, EC 190 sk 56, EC 388 sk 100, and EC 490 sk 114 with MIC of 1.25 mg/mL, while PEW showed low inhibition of E. coli. In a previous study, CSW and PEW inhibited Gram-positive and Gram-negative bacteria, such as E. coli, P. aeruginosa, S. Typhi, Enterobacter aerogenes, S. aureus, MRSA, and B. subtilis [22, 38].
Different plants possess different chemical components that exhibit different antibacterial properties. The major constituents of P. emblica bark are gallic acid and ellagic acid [39], while those of C. sappan are brazilin and protosappanin B [40, 41]. Gallic acid showed a potential antibacterial effect against Staphylococcus epidermidis, S. aureus, and Klebsiella pneumoniae [42]. Furthermore, gallic acid inhibited the TetK efflux pump for antibiotic resistance in the IS-58 strain of S. aureus and improved the inhibition effect of tetracyclin [43]. Ellagic acid inhibited the efflux pump of methicillin-resistant S. aureus [44]. Brazilin, which is the main constituent of C. sappan, also had antibacterial activity against E. coli O157:H7, enteroaggregative E. coli strain 042, and O104:H4 serotype and showed potential inhibition of swarming motility. In addition to swarming motility, it inhibits biofilm production in E. coli O157:H7 and E. coli strain 042 [40]. Antibacterial activity of brazilin decreased DNA and protein synthesis [45].
3.2. Effect of APW and Its Ingredient Extracts on RAW264.7 Cell Viability
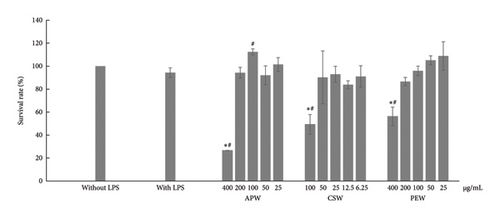
The results showed toxicity to RAW264.7 cells at incubation with 400 μg/mL of APW, 400 μg/mL of PEW, and 100 μg/mL of CSW, as shown in Figure 1. APW and PEW concentrations of up to 200 μg/mL had no toxicity to cells, while concentrations of CSW up to 50 μg/mL were also nontoxic on RAW264.7 cells. Therefore, APW and PEW were investigated for anti-inflammatory activities with concentrations ranging from 25 to 200 μg/mL, while CSW was investigated with concentrations of 6.25, 12.5, 25, and 50 μg/mL.
APW and CSW showed dose-dependent inhibition against nitric oxide, IL-6, and TNF-α production, while PEW markedly reduced nitric oxide and IL-6 in a dose-dependent manner. APW and CSW potently inhibited nitric oxide production with IC50 values of 83.96 ± 10.60 and 29.03 ± 0.38 μg/mL, respectively. APW and CSW inhibited IL-6 with IC50 values of 83.06 ± 2.07 and 41.88 ± 1.18 μg/mL, respectively. In contrast, the IC50 value of PEW was higher than the maximum concentration because the maximum inhibition was less than 50%. APW, PEW, and CSW concentrations at 100 μg/mL inhibited TNF-α production 8.15 ± 0.71, 6.42 ± 0.83, and 7.36 ± 0.33%, respectively, as shown in Figure 2.
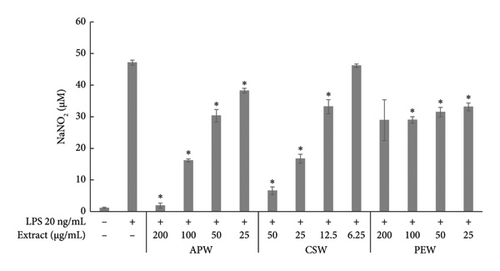
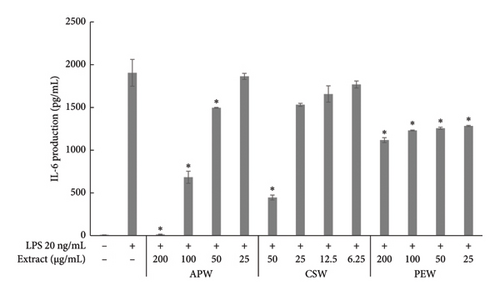
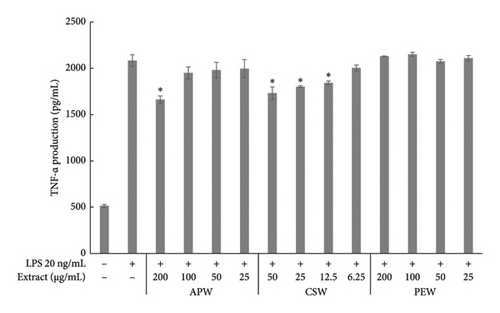
Pathogens induce the immune response and cause inflammatory diarrhea [46]. Phagocytosis by macrophages stimulates the inflammatory response and increases the synthesis of chemokines and cytokines, which are responsible for inflammation [47]. We demonstrated for the first time that APW has a dose-dependent inhibitory effect on nitric oxide, IL-6, and TNF-α production. A similar result was found with CSW. Our findings align with a previous study reporting that CSW had high inhibition of nitric oxide and IL-6 [48]. Moreover, brazilin and sappan chalcone, constituents of C. sappan, have expressed almost 100% inhibition of nitric oxide production and iNOS gene expression in LPS-induced J774.1 cells [49]. Among the several cytokines and chemokines associated with immunity and inflammation, macrophages produce typical inflammatory mediators such as nitric oxide, IL-6, and TNF-α. Excessive production of these mediators might have clinical implications [50]. Phytochemicals that show anti-inflammatory activity may improve clinical symptoms and control inflammation [51, 52]. Notably, in our study, PEW showed less inhibition effect on nitric oxide production, TNF-α and IL-6. In previous research, the ethanol and methanol extract of a branch of P. emblica inhibited COX-2, iNOS, TNF-α, IL-1ß, and IL-6 in LPS-induced RAW 264.7 [53]. Different extraction solvents can differentially impact the biological activity of different bioactive compounds [54].
3.3. Validation Parameters
Linearity regression curves for gallic acid and brazilin were plotted across six concentrations. The correlation coefficients (R2) for both standard gallic acid and brazilin were close to 1, indicating strong linear relationships and a high degree of precision in the method, as shown in Table 3. The LOD and LOQ were calculated from the calibration curves, providing clear detection limits for the method. The LOD values for gallic acid and brazilin were 11.47 and 12.12 μg/mL, respectively, while the LOQ values were 34.76 and 36.75 μg/mL. Additionally, the HPLC method demonstrated precision through both intraday and interday analyses, with RSD values below 2% (Table 4). The method’s accuracy was confirmed by spiking APW solutions with standards, resulting in recoveries of 100.64%–106.53% and 96.83%–109.19% for gallic acid and brazilin, respectively, as shown in Table 5.
| Parameter | Gallic acid | Brazilin |
|---|---|---|
| Range (μg/mL) | 12.5–400 | 12.5–400 |
| Regression equation (y = axe + b) | y = 50587x − 128834 | y = 18658x + 34889 |
| R2 | 0.9997 | 0.9997 |
| LOD (μg/mL) | 11.47 | 12.12 |
| LOQ (μg/mL) | 34.76 | 36.75 |
| Compound | Concentration (μg/mL) | Intradaya (n = 3) | Interdayb (n = 9) | ||
|---|---|---|---|---|---|
| Measured concentration (μg/mL) | %RSD | Measured concentration (μg/mL) | %RSD | ||
| Gallic acid | 100 | 109.33 ± 1.75 | 1.60 | 97.90 ± 1.80 | 1.84 |
| 200 | 210.48 ± 3.09 | 1.46 | 205.22 ± 3.91 | 1.90 | |
| 400 | 427.55 ± 7.47 | 1.74 | 418.79 ± 5.65 | 1.35 | |
| Brazilin | 100 | 99.01 ± 0.87 | 0.88 | 103.22 ± 1.92 | 1.86 |
| 200 | 205.00 ± 2.48 | 1.20 | 211.52 ± 3.94 | 1.86 | |
| 400 | 398.67 ± 4.86 | 1.21 | 400.67 ± 6.63 | 1.65 | |
- aAll data are expressed as mean ± SD; after doing triple analyses in a subsequent run.
- bAll data are mean ± SD, obtained by triplicate analyses per day over three different runs.
| Compounds | Spiked concentration (μg/mL) | Recovery (%) | Mean ± %RSD | ||
|---|---|---|---|---|---|
| N1 | N2 | N3 | |||
| Gallic acid | 50 | 103.64 | 102.68 | 104.15 | 103.49 ± 0.72 |
| 100 | 103.22 | 100.64 | 103.05 | 102.30 ± 1.40 | |
| 200 | 106.02 | 105.22 | 106.53 | 105.92 ± 0.62 | |
| Brazilin | 50 | 105.01 | 105.73 | 107.45 | 106.06 ± 1.18 |
| 100 | 99.67 | 96.83 | 99.81 | 98.77 ± 1.70 | |
| 200 | 109.19 | 105.78 | 107.72 | 107.56 ± 1.59 | |
- Note: All data are expressed as mean ± SD.
3.4. The Gallic Acid and Brazilin Contents in the Apo-Taat Extract and Its Ingredients
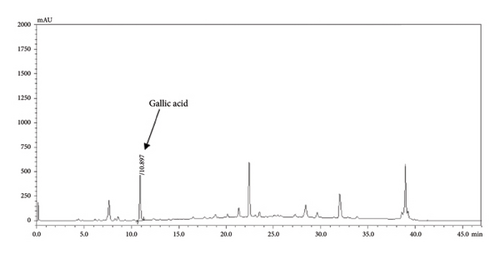
The HPLC profiles of gallic acid, brazilin, Apo-taat, and its ingredients are shown in Figures 3 and 4. The major constituents of Apo-taat are brazilin (1.49% ± 0.12%w/w) and gallic acid (0.32% ± 0.01%w/w), which were found in plant ingredients. P. emblica consisted of gallic acid, while C. sappan contained brazilin. The gallic acid content in P. emblica extract was 0.81% ± 0.03%w/w, and the brazilin in C. sappan extract was 4.66% ± 0.17%w/w, as shown in Table 6. Gallic acid and brazilin have been shown to have antibacterial activity against E. coli and to inhibit nitric oxide and proinflammatory cytokines [48, 55]. Furthermore, gallic acid expressed bactericidal activity against clinical isolates of multidrug-resistant E. coli [56]. Gallic acid and brazilin may be linked with enhanced antibacterial activity and anti-inflammatory properties of APW.
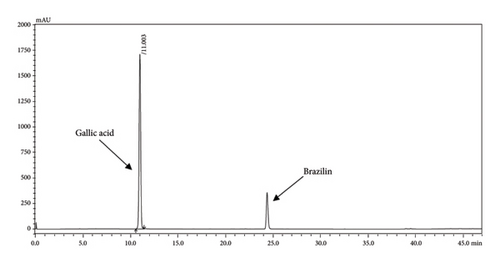
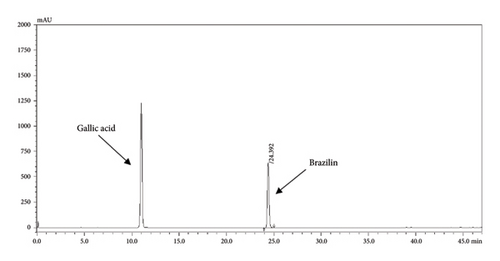
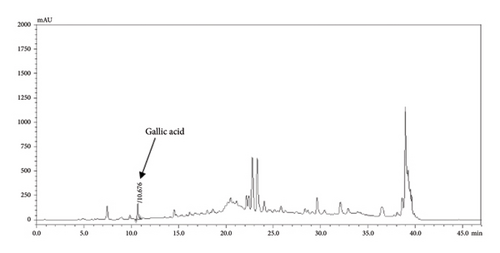

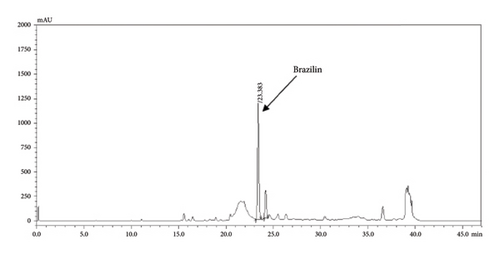
| Sample | Brazilin content (%w/w) | Gallic acid content (%w/w) |
|---|---|---|
| APW | 1.49 ± 0.12 | 0.32 ± 0.01 |
| PEW | Not detectable | 0.81 ± 0.03 |
| CSW | 4.66 ± 0.17 | Not detectable |
- Note: All data are expressed as mean ± SD.
4. Conclusions
Herbal medicine can be used as an alternate treatment for several illnesses. The Apo-taat formulation, comprising two botanical components, may serve as a possible antibacterial agent, particularly against antibiotic-resistant pathogens. Subsequent studies should evaluate the impact of the Apo-taat formulation on virulence characteristics, including toxin production, biofilm development, and its synergistic interactions with antibiotic agents. Safety and both acute and chronic toxicity of Apo-taat should also be investigated in vivo and in future clinical trial research.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This project was supported by the Thai Traditional Medical Knowledge Fund, Department of Thai Traditional and Alternative Medicine, Ministry of Public Health, Grant no. KPT10/2565.
Acknowledgments
The authors would like to thank the Research Group in Multidrug-Resistant Bacteria and the Antimicrobial Herbal Extracts, Faculty of Medicine, Thammasat University, for supporting ESBL-E. coli strains of our present study. The authors would like to acknowledge Michael Jan Everts, from the Clinical Research Center, Faculty of Medicine, Thammasat University, for English editorial assistance.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.




