Preliminary Investigation of GC–MS Profiling and Antibacterial Activities of Different Solvent Extracts From Litchi chinensis Sonn. Seed
Abstract
Traditionally different parts of Litchi chinensis Sonn. (Family: Sapindaceae) have been used medicinally to treat a variety of diseases, including stomach ulcers, flatulence, obesity, cough, diabetes, and hernia-like situations. This study investigates the gas chromatography–mass spectrometry (GC–MS) profiling to detect different types of phytochemicals and antibacterial activity of various solvent extracts derived from the seeds of Litchi chinensis Sonn. Eventually, comprehending the potential biological functions of the detected compounds is explored. GC–MS analysis revealed a diverse array of chemical compounds, with 34, 35, and 25 compounds identified in the n-hexane, n-hexane–chloroform (2:1), and methanol extracts, respectively. The major compounds identified were 2,4-bis (1,1-dimethylethyl) phenol (14.38%) in the n-hexane extract, hexadecenoic acid (13.35%) in the n-hexane–chloroform extract, and 2-(hydroxymethyl)-2-nitro-1,3-propanediol (39.16%) in the methanol extract. While most compounds exhibited biological activity, some were neutral or fatty acid derivatives. Notable bioactive compounds included bis (2-ethylhexyl) phthalate and pentadecane in the n-hexane extract, naphthalene in the n-hexane–chloroform extract, and 13-docosanamide (Z) and beta-longipinene in the methanol extract. Antibacterial activity was tested against Listeria monocytogenes and Salmonella choleraesuis, where methanolic extract showed the highest activity (ZOI-10 mm for both bacteria), followed by n-hexane extract and n-hexane–chloroform (2:1) extract, respectively. The study’s findings highlight the potential of L. chinensis seed extracts as sources of bioactive compounds, supporting their traditional medicinal uses and suggesting possible applications in antimicrobial therapy. Future studies should focus on isolating and characterizing the key bioactive compounds and broadening the scope to test against additional pathogens and assess other biological activities, such as anticancer and anti-inflammatory effects, could further validate the medicinal uses of L. chinensis Sonn.
Summary
- •
Sixty-three compounds were identified by GC–MS profiling from the litchi seed extracts.
- •
Biologically active compounds along with some neutral or fatty acid derivatives were found.
- •
All extracts exhibited antibacterial activity both gram-positive and gram-negative bacteria.
1. Introduction
Plants, due to their natural abundance, research on medicinal plants continues to thrive, ensuring their ongoing relevance as vital sources of medicine [1]. Infectious diseases pose a significant global health challenge [2], which account for 20% of all mortality and 25% of hospital deaths annually [3]. Based on recent advancements in clinical research, it has been discovered that bioactive phytoconstituents play expedient functions against these microbial attacks [3, 4]. Although there are many commercially available antimicrobial medications, rising resistance and related mortality are requiring researchers to consistently find new antibiotics [5]. Furthermore, to lower the death rate, researchers are being compelled by rising healthcare costs and mortality rates to develop novel antimicrobials with fewer adverse effects by employing natural product chemistry [4, 6]. The study of natural products has seen a minor decline in attention due to the recent advancements in combinatorial chemistry for drug discovery and development, but nature is still considered a priceless source of enormous chemical variety and fragments that help produce new lead compounds [7, 8]. Additionally, just 6% of plants have undergone biological screening globally [5, 9]. As a result, there is a growing need for thorough research into unknown medicinal plants with potential biological activity, which will hopefully lead to the identification of numerous interesting drug candidates [10, 11]. Since the ancient period, medicinal plants have played a crucial role in ensuring human welfare. It has been estimated that 66% of plant species in the world have therapeutic qualities [3, 12]. These therapeutic plants can be used in medications or formulations to treat a variety of human disorders since they have a variety of therapeutic components [4]. Due to their natural abundance, the possibility of doing studies with these medicinal plants will not disappear quickly [13]. As a consequence, they have been vital sources of medicine for at least 60 millennia, according to fossil records [12, 14]. These days, higher plants are used to manufacture 25% of natural items, whereas 50% of all appropriate medications come from natural products and their results [5].
Although various parts of Litchi chinensis Sonn. (Sapindaceae) have traditionally been used in medicine to treat a range of ailments, including stomach ulcers, flatulence, obesity, cough, diabetes, and hernia-like conditions [15], scientific research has increasingly focused on evaluating the pharmacological properties of Litchi chinensi Sonn. and its bioactive compounds [16]. Studies have demonstrated its potential antioxidant [17], anti-inflammatory [18], antimicrobial [19], anticancer [15], and antidiabetic [20] effects, which support its traditional uses. Additionally, modern research is exploring the molecular mechanisms behind its therapeutic actions [21], paving the way for more evidence-based applications in clinical settings [22].
In plant life cycle, seeds time span is short, but the active biochemical pathways are more similar with that of animal organism because as energy source they utilize similar kind of biomolecules such as carbohydrate, fat, and protein. That is why the smaller molecules available in seeds that regulate and rectify the metabolic pathway [23] can easily be exploited for design and development of drugs for animal organisms.
One of the previous finding has shown that seven flavonoid glycosides with one new compound (litchioside D) were isolated from the seeds of lychee using repeated column chromatography and high-performance liquid chromatography (HPLC) [22]. While studies on Litchi chinensis Sonn. seed extracts have demonstrated various bioactive compounds with potential antimicrobial, anticancer, and glucose-lowering properties, grown in various countries, limited research has been conducted in Bangladesh on Litchi chinensis Sonn seed extracts. A study was conducted to evaluate the antioxidant potential of two extracts (aqueous and ethanol) to explore the relationship between phytochemicals and antioxidant activities [24], but that study did not conduct gas chromatography–mass spectrometry (GC–MS) analysis and antibacterial screening.
The goal of the current investigation was to explore the bioactive phytochemicals present in different solvent extracts of L. chinensis seeds and to assess their antimicrobial properties. By correlating the phytochemical profiles with potential pharmacological actions, this research seeks to uncover new insights into the therapeutic potential of L. chinensis and contribute to the ongoing search for effective natural antimicrobial agents.
Additionally, most studies lack comprehensive in vivo and in vitro evaluations to confirm efficacy, toxicity, and pharmacokinetics. Furthermore, research on the broader spectrum of biological activities, the potential development of these compounds into clinical applications, remains underexplored. Addressing these gaps could unlock new insights into the medicinal value of L. chinensis and its applications in pharmaceutical development.
2. Materials and Methods
2.1. Materials, Chemicals, and Reagents
Seeds of Litchi chinensis, methanol (analytical grade, purity 99.8%, Sigma-Aldrich, France), n-hexane (analytical grade, purity 97.0%, Sigma-Aldrich, France), chloroform (analytical grade, purity 98.0%, Merck, Germany), azithromycin (pharmaceutical secondary standard, purity 96.0%, Merck, Germany).
2.2. Plant Collection and Identification
From May 16 to 18, 2022, fresh seeds of Litchi chinensis Sonn. were collected from the Rajshahi city (24.3746°N and 88.6004°E) of Bangladesh. The plant specimens were identified by botanist Dr. A. N. Chowdhury, Principal Scientific Officer at Principal Scientific Officer, Bangladesh Council of Scientific and Industrial Research (BCSIR), Rajshahi Laboratories. The sample has been assigned the accession number FK-156.
2.3. Solvent Extraction of Litchi chinensis
The seeds were air-dried at room temperature for two days before being ground using a grinder (Mixture Grinder, India). Three portions (each of 300 g) of the powdered seeds were then subjected to cold extraction in three different solvent systems with increasing polarity: n-hexane < n-hexane: chloroform (2:1 ratio) < methanol. The mixtures were left to stand at room temperature for 72 h with regular manual shaking. Afterward, the extracting solvents were filtered through Whatman No. 1 filter paper, and the filtrates were concentrated using a rotary evaporator [25]. This process yielded three different extracts, which were stored at 4°C in refrigerator until further analysis.
2.4. Sample Preparation for GC–MS Examination
Three 50 mL Falcon tubes were labeled with serial numbers. In each tube, 350 mg of powdered Litchi chinensis seed was placed. The first tube received 15 mL of n-hexane, the second tube received 15 mL of a mixture of n-hexane and chloroform, and the third tube received 15 mL of methanol. The mixtures were vortexed in the tubes until they lost color. Subsequently, the tubes were heated in a water bath at 50°C for 2 h. After heating, the organic layer of each mixture was carefully extracted using a micropipette and prepared for GC–MS analysis.
2.5. GC–MS Analysis
The sample was analyzed using a GC–MS system (Shimadzu GCMS-QP-2020, Shimadzu, Japan) equipped with an autosampler (AOC-20s) and an autoinjector (AOC-20i) [26]. Helium gas was used as the mobile phase (carrier gas) with a flow rate of 1.72 mL/min, and the column flow rate was set at 0.75 mL/min. The column used was an SH Rxi 5MS Sill (30 m × 0.25 mm; 0.25 μm). The oven temperature was initially set to 80°C for 2 min and then increased at a rate of 5°C per min until reaching 150°C, where it was held for 5 min. The temperature was then further increased to a final temperature of 280°C, where it was maintained for 5 min.
The injector and ion source temperatures were set at 220°C and 280°C, respectively. A 5.0 μL sample was injected in splitless mode with a split ratio of 50:1. The mass spectrometer operated with an ionization potential of 70 eV, and mass spectra were recorded in the range of 45 m/z to 350 m/z over a 50-min run time. The solvent cut time was 3.00 min. Compounds were identified by comparing the mass spectra to the NIST08s, NIST08, and NIST14 libraries. The relative percent quantity of each component was determined by comparing the average peak area of each component to the total areas.
2.6. Identification of Chemical Constituents
The identification of bioactive compounds in various extracts of L. chinensis seeds was accomplished by analyzing the GC–MS retention time (RT) on the SH-Rxi 5Sill MS column and the molecular fragment m/z values. The obtained RT and m/z values from the spectra were compared using NIST08s, NIST08, and NIST14 software. The database contains approximately 60,000 recognized compound patterns. The spectra of Litchi chinensis seed extracts were compared to reference mass spectra of known components stored in the NIST08 and NIST14 libraries.
2.7. Plant Extracts Antibacterial Activity Test
The antibacterial activity of various solvent extracts of L. chinensis seed powder was evaluated using the disc diffusion method with slide modifications [27, 28] on nutrient agar (NA) plates. Two bacterial strains were tested in this study: Salmonella choleraesuis ATCC 10708, a gram-negative bacterium, and Listeria monocytogenes ATCC 13932, a gram-positive bacterium. The test organisms were first inoculated into nutrient broth and incubated overnight at 37°C. In this study, standard antibiotic azithromycin (15 μg/disc) used as the positive control, along with dimethyl sulfoxide (DMSO) serving as the negative control, was subsequently positioned on the NA media. The turbidity of the bacterial suspension was then adjusted to match a 0.5 McFarland standard, resulting in a final inoculum concentration of 1.5 × 108 CFU/mL. After allowing the extracts to diffuse at room temperature for 30 min, the plates were incubated at 37°C for 24 h. Post incubation, the plates were examined for the presence of clear zones around the wells, indicating antibacterial activity. The diameter of these zones of inhibition (ZOIs) was measured in millimeters (mm).
2.8. Statistical Analysis
The SPSS software (IBM, Version 20) was used to conduct the statistical analysis, which included one-way analysis of variance (ANOVA) test (Duncan as post hoc) and independent sample t test (p < 0.05). All the experimental findings were represented as mean ± standard deviation (SD).
3. Results
Across three different extracts, a total of 34 compounds were identified in the n-hexane extract, 35 in the n-hexane–chloroform (2:1) extract, and 25 in the methanol extract. The majority of these compounds were bioactive. The chromatograms are shown in Figures 1, 2, and 3, while the chemical constituents, along with their RT, mass-to-charge ratio (m/z), and biological activity, are presented in Tables 1, 2, and 3.
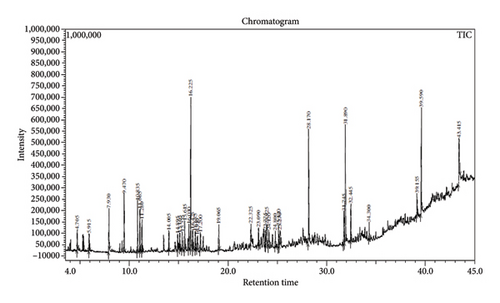
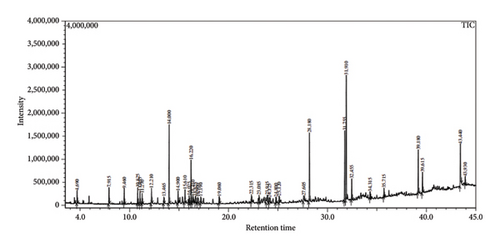
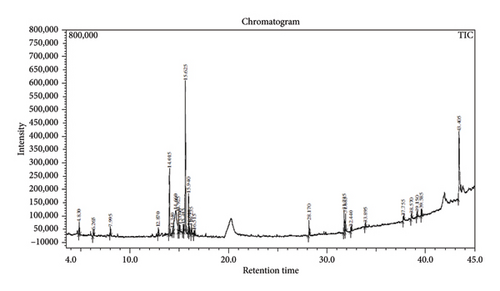
| No. | Name of compound | RT | m/z | Biological activity |
|---|---|---|---|---|
| 1 | Benzene acetaldehyde | 4.71 | 91.00 | Antibacterial activity |
| 2 | Nonanal | 5.92 | 57.00 | Antimicrobial activity |
| 3 | Naphthalene | 7.93 | 128.00 | Antimicrobial, antiseptic, carcinogenic |
| 4 | Benzene 1,3-bis (1,1-dimethylethyl) | 9.48 | 175.00 | Lipid oxidation |
| 5 | 1-Decanol, 2-hexyl- | 10.84 | 69.00 | Commercially used |
| 6 | Isotridecanol | 11.07 | 69.00 | Antimicrobial |
| 7 | 2-Isopropyl-5-methyl-1-heptanol | 11.29 | 69.00 | Antimicrobial |
| 8 | Caryophyllene | 14.01 | 91.00 | Cardioprotective, hepatoprotective, and immune-modulatory activity |
| 9 | Dodecane, 2,6,11-trimethyl | 14.90 | 71.00 | Antifungal, antibacterial activities |
| 10 | Hexadecane | 15.11 | 57.00 | Antifungal, antibacterial, antioxidant |
| 11 | Dodecane 4,6-dimethyl | 15.34 | 71.00 | — |
| 12 | Aromadendrene | 15.62 | 93.00 | Antimicrobial, antifungal |
| 13 | Pentadecane | 16.04 | 57.00 | Suger-phosphatase inhibitor, chymosin inhibitor, antibacteria |
| 14 | Phenol, 2,4, bis (1,1-dimethylethyl) | 16.23 | 191.00 | Antifungal, antimicrobial, antioxidant, antimalarial activities |
| 15 | 1-Dodecanol,3,7,11-trimethyl | 16.42 | 69.00 | — |
| 16 | 11-Methyl dodecanol | 16.69 | 69.00 | — |
| 17 | 1-Decanol, 2-octyl | 16.92 | 57.00 | — |
| 18 | 2-Hexyl-1-octanol | 17.20 | 69.00 | Antihelminthic |
| 19 | Eicosane | 22.33 | 57.00 | Antifungal, antitumor, larvicidal |
| 20 | Tetratriacontyl heptafluorobutyrate | 23.10 | 69.00 | Antimicrobial property |
| 21 | Carbonic acid, decyl undecyl ester | 23.78 | 57.00 | — |
| 22 | Eicosyl octyl ether | 23.93 | 57.00 | — |
| 23 | 1-Dodecanol, 2-octyl | 24.13 | 69.00 | Emollients, perfuming agents, cosmetics |
| 24 | Octatriacontyl trifluoroacetate | 24.80 | 69.00 | Insecticidal |
| 25 | Heneicosane | 25.12 | 57.00 | Antineoplastic, oviposition-attractant |
| 26 | Hexadecane, 2,6,10,14 tetramethyl | 25.28 | 57.00 | Biomarkers in petroleum studies |
| 27 | Hexadecanoic acid, methyl ester | 28.17 | 74.00 | Nematicide, pesticide, hemolytic, 5-alpha reductase inhibitor activities |
| 28 | 9,12-Octadecadienoic acid | 31.75 | 67.00 | Antibacterial, antifungal and antioxidative, hypolipidemic |
| 29 | 11-Octadecanoic acid | 31.90 | 55.00 | Antioxidants |
| 30 | Methyl stearate | 32.45 | 74.00 | GABA aminotransferase inhibitor, gastrin inhibitor, antinociceptive |
| 31 | 1-Decanol 2-hexyl | 34.30 | 69.00 | Commercially used |
| 32 | 13-Docosenoic acid, methyl ester, (Z)- | 39.16 | 55.00 | Anticancer |
| 33 | Bis (2-ethylhexyl) phthalate | 39.60 | 149.00 | Antimicrobial and cytotoxic activity |
| 34 | Cis-11-eicosenamide | 43.42 | 59.00 | Antioxidant and anti-inflammatory effects |
| No. | Name of compound | RT | m/z | Biological activity |
|---|---|---|---|---|
| 1 | Benzene acetaldehyde | 4.70 | 91.00 | Antibacterial activity |
| 2 | Naphthalene | 7.92 | 128.00 | Antimicrobial, antiseptic, carcinogenic |
| 3 | 1,3-Bis (1,1-dimethylethyl) benzene | 9.46 | 175.00 | Lipid oxidation |
| 4 | 2-Isopropyl-5-methyl-1-heptanol | 10.83 | 57.00 | Antimicrobial |
| 5 | Isotridecanol | 11.05 | 69.00 | Antimicrobial |
| 6 | 2,4-Diethyl, 1-heptanol | 11.27 | 69.00 | — |
| 7 | 2-Methoxy-4-(2-propenyl)-phenol, acetate | 12.21 | 164.00 | Antiinfective-agents, antioxidants |
| 8 | Tetradecane | 13.46 | 57.00 | Antimicrobial |
| 9 | Caryophyllene | 14.00 | 93.00 | Cardioprotective, hepatoprotective, and immune-modulatory activity |
| 10 | Alpha-caryophyllene | 14.91 | 93.00 | Anti-inflammatory |
| 11 | Beta-longipinene | 15.61 | 91.00 | Antibiofilm activity |
| 12 | Pentadecane | 16.02 | 57.00 | Sugar-phosphatase inhibitor, chymosin inhibitor, antibacterial |
| 13 | 2,4, Bis (1,1-dimethylethyl), phenol | 16.22 | 191.00 | Antifungal, antimicrobial, antioxidant, antimalarial activities |
| 14 | 11-Methyldodecanol | 16.41 | 69.00 | Antimicrobial |
| 15 | n-Tridecane-1-ol | 16.68 | 69.00 | Antibacterial activity |
| 16 | 1-Octadecane sulfonyl chloride | 16.90 | 57.00 | — |
| 17 | 2-Hexyl-1-octanol | 17.19 | 69.00 | Antihelminthic |
| 18 | Hexadecane | 19.06 | 57.00 | Antifungal, antibacterial, antioxidant |
| 19 | Heptadecane | 22.32 | 57.00 | Antioxidant |
| 20 | 2-Hexyl, 1-decanol | 23.08 | 69.00 | Commercially used |
| 21 | 2-Hexyl, 1-dodecanol | 23.91 | 69.00 | — |
| 22 | 2-Octyl, 1-decanol | 24.13 | 57.00 | — |
| 23 | 2-Octyl, 1-dodecanol | 24.80 | 69.00 | Emollients, perfuming agents, cosmetics |
| 24 | Heneicosane | 25.12 | 57.00 | Antineoplastic, oviposition-attractant pheromone |
| 25 | 2,6,10,15-Tetramethyl, heptadecane | 27.60 | 57.00 | Sex hormone in algae |
| 26 | Hexadecenoic acid | 28.18 | 74.00 | Nematicide, pesticide, hemolytic, 5-alpha reductase inhibitor activities |
| 27 | 9,12-Octadecadienoic acid | 31.75 | 67.00 | Antibacterial, antifungal, and antioxidative, hypolipidemic |
| 28 | 9-Octadecanoic acid | 31.91 | 55.00 | Antihypertensive increases HDL and decrease LDL |
| 29 | Methyl stearate | 32.46 | 74.00 | GABA aminotransferase inhibitor, gastrin inhibitor, anthelmintic antinociceptive |
| 30 | Octatriacontyl trifluoroacetate | 34.32 | 69.00 | Insecticidal |
| 31 | Methyl 9,10- methylene- octadecanoate | 35.72 | 55.00 | — |
| 32 | 13-docosenoic acid | 39.18 | 55.00 | Anticancer |
| 33 | Bis (2-ethylhexyl) phthalate | 39.62 | 149.00 | Antimicrobial and cytotoxic activity |
| 34 | Cis-11-eicosenamide | 43.44 | 59.00 | Antioxidant and anti-inflammatory effects |
| 35 | Squalene | 43.93 | 69.00 | Antibacterial, antioxidant, antitumor, cancer preventive, immunostimulant, pesticide |
| No. | Name of compound | RT | m/z | Biological activity |
|---|---|---|---|---|
| 1 | Benzene acetaldehyde | 4.83 | 91.00 | Antibacterial activity |
| 2 | Phenylethyl alcohol | 6.27 | 91.00 | Preservatives, antimicrobial |
| 3 | Naphthalene | 7.99 | 128.00 | Antimicrobial antiseptic |
| 4 | Copaene | 12.87 | 105.00 | Carcinogenic antioxidant and antiproliferative |
| 5 | Caryophyllene | 14.01 | 93.00 | Cardioprotective, hepatoprotective |
| 6 | Trans-alpha-bergamotene | 14.33 | 93.00 | Immune-modulatory activity antimicrobial |
| 7 | 2-(Hydroxymethyl)-2 nitro, 1,3-propanediol | 14.66 | 57.00 | Antimicrobial |
| 8 | Alpha-caryophyllene | 14.93 | 93.00 | Anti-inflammatory |
| 9 | Cis-alpha-bisabolene | 15.04 | 93.00 | Antibacterial effects |
| 10 | 1,8-Dimethyl-4-(1-methyl), spiro [4,5] dec-7-ene | 15.42 | 119.00 | Antifungal activity |
| 11 | Beta-longipinene | 15.62 | 93.00 | Antibiofilm activity |
| 12 | Gamma-elemene | 15.94 | 121.00 | Antitumor activity |
| 13 | 1,2,4a,5,6,8a-Hydroxy-4,7-dimethylethyl, naphthalene | 16.01 | 105.00 | — |
| 14 | 4,11,11-Trimethyl bicyclo [7.2.0] undec-4-ene | 16.24 | 93.00 | — |
| 15 | 1,2,3,5,6,8a-Hexahydro-4,7-dimethylethyl-naphthalene | 16.52 | 119.00 | — |
| 16 | Hexadecanoic acid | 28.17 | 74.00 | 5-Alpha reductase inhibitor |
| 17 | 9,12-Octadecadienoic acid | 31.74 | 67.00 | Antibacterial, antifungal, and antioxidative, hypolipidemic |
| 18 | 11-Octadecanoic acid | 31.88 | 55.00 | Antioxidants |
| 19 | Methyl stearate | 32.44 | 74.00 | GABA aminotransferase inhibitor |
| 20 | 4,8-Dimethyl, 3,7-nonadien-2-ol | 33.90 | 69.00 | |
| 21 | 2,3-Dihydro, 9,12-octadecadienoic acid, ZZ | 37.76 | 67.00 | — |
| 22 | 9,12-Octadecadienoyl chloride, ZZ | 38.57 | 67.00 | Anticancer, thyroid inhibitor |
| 23 | Methyl 9,10-methylene octadecanoate | 39.15 | 55.00 | — |
| 24 | 1,2-Benzene dicarboxylic acid, mono (2-ethylhexyl) ester | 39.58 | 149.00 | Antimicrobial activity, antifungal |
| 25 | 13-Docosanamide, Z | 43.41 | 59.00 | Antimicrobial, antinociceptive |
However, employing three solvent systems, a total of 63 compounds were found in the powdered L. chinensis seed (Table 4). The main compounds found by GC–MS analysis using methanol extract were 13-docosanamide, Z (10.94%), 2-(hydroxymethyl)-2-nitro 1, 3-propanediol (39.16%), and beta-longipinene (10.02%). However, phenol, 2,4-bis (1,1-dimethylethyl) (14.38%), bis (2-ethylhexyl) phthalate (10.69%), and hexadecanoic acid methyl ester (9.75%) were the primary compounds in the n-hexane extract, where hexadecenoic acid methyl ester (13.35%), phenol, 2,4, bis(1,1-dimethylethyl) (9.61%), and 9-octadecenoic acid (8.49%) were present in n-hexane–chloroform (2:1). Six common compounds were found in all three extracts from Table 4: caryophyllene, naphthalene, benzene acetaldehyde, 9,12-octadecadienoic acid methyl ester, hexadecanoic acid methyl ester, and methyl stearate.
| No. | Compounds name | Structure | Formula | Relative concentration (%) | ||
|---|---|---|---|---|---|---|
| n-Hexane | n-Hexane–chloroform (2:1) | Methanol | ||||
| 1. | Benzene acetaldehyde | 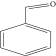 |
C8H10O | 3.36 ± 0.016b | 3.42 ± 0.005b | 4.75 ± 0.012a |
| 2. | Nonanal |  |
C9H18O | 0.936 ± 0.009 | ND | ND |
| 3. | Phenylethyl alcohol | 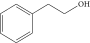 |
C8H10O | ND | ND | 2.76 ± 0.008 |
| 4. | Naphthalene | 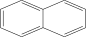 |
C10H8 | 7.24 ± 0.008a | 5.53 ± 0.008b | 4.20 ± 0.012c |
| 5. | Benzene 1,3-bis (1,1-dimethylethyl) | 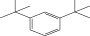 |
C14H22 | 4.866 ± 0.012a | 2.97 ± 0.012b | ND |
| 6. | 1-Decanol, 2-hexyl- | 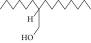 |
C16H34O | 2.29 ± 0.043 | ND | ND |
| 7. | Isotridecanol |  |
C13H28O | 2.34 ± 0.008a | 1.69 ± 0.012b | ND |
| 8. | 2,4-diethyl, 1-heptanol | 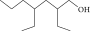 |
C11H24O | ND | 1.10 ± 0.017 | ND |
| 9. | 2-Isopropyl-5-methyl-1-heptanol | 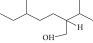 |
C11H24O | 1.536 ± 0.009a | 1.70 ± 0.016a | ND |
| 10. | 2-Methoxy-4-(2-propenyl)-phenol, acetate | 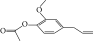 |
C12H14O | ND | 1.78 ± 0.008 | ND |
| 11. | Copaene | 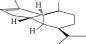 |
C15H24 | ND | ND | 1.15 ± 0.008 |
| 12. | Tetradecane | 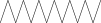 |
C14H30 | ND | 1.70 ± 0.012 | |
| 13. | Caryophyllene | 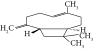 |
C15H24 | 0.503 ± 0.012c | 4.42 ± 0.012a | 4.59 ± 0.012a |
| 14. | Trans-alpha-bergamotene | 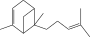 |
C15H24 | ND | ND | 0.17 ± 0.005 |
| 15. | 2-(Hydroxymethyl)-2 nitro, 1,3-propanediol | 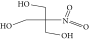 |
C4H9NO5 | ND | ND | 39.16 ± 0.012 |
| 16. | Propanediol |  |
C3H8O2 | 1.11 ± 0.008 | ND | ND |
| 17. | Alpha-caryophyllene |  |
C15H24 | ND | 1.71 ± 0.021b | 3.68 ± 0.008a |
| 18. | Cis-alpha-bisabolene |  |
C15H24 | ND | ND | 0.62 ± 0.012 |
| 19. | Heptadecane, 2,6,10,15-tetramethyl- |  |
C21H44 | 1.4 ± 0.008 | ND | ND |
| 20. | Dodecane 4,6-dimethyl |  |
C14H30 | 1.19 ± 0.016 | ND | ND |
| 21. | Spiro [4,5] dec-7-ene 1,8-dimethyl-4-(1-methylethyl) | 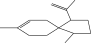 |
C15H24 | ND | ND | 0.72 ± 0.008 |
| 22. | Beta-longipinene | 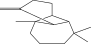 |
C15H24 | ND | 0.75 ± 0.012b | 10.02 ± 0.012a |
| 23. | Aromadendrene | 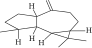 |
C15H24 | 0.75 ± 0.008 | ND | ND |
| 24. | Gamma-elemene | 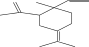 |
C15H24 | ND | ND | 4.03 ± 0.016 |
| 25. | 1,2,4a,5,6,8a-Hydroxy-4,7-dimethylethyl, naphthalene |  |
C15H24 | ND | ND | 0.75 ± 0.012 |
| 26. | Pentadecane |  |
C15H32 | 2.326 ± 0.024a | 1.62 ± 0.012b | ND |
| 27. | Phenol, 2,4, bis (1,1-dimethylethyl) |  |
C14H22O | 14.384 ± 0.014a | 9.61 ± 0.012b | ND |
| 28. | 4,11,11-Trimethyl bicyclo [7.2.0] undec-4-ene |  |
C15H24 | ND | ND | 0.77 ± 0.017 |
| 29. | 1-Dodecanol, 3,7,11-trimethyl |  |
C15H32O | 1.09 ± 0.008 | ND | ND |
| 30. | 1,2,3,5,6,8a-Hexahydro-4,7-dimethylethyl-naphthalene |  |
C15H24 | ND | ND | 0.52 ± 0.008 |
| 31. | n-Tridecane-1-ol |  |
C13H28 | ND | 1.38 ± 0.012 | ND |
| 32. | 11-Methyl dodecanol |  |
C13H28O | 1.28 ± 0.008a | 0.97 ± 0.008a | ND |
| 33. | 1-Octadecane sulfonyl chloride | 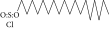 |
C18H37ClO2S | ND | 1.06 ± 0.005 | ND |
| 34. | 2-Octyl, 1-decanol | 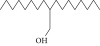 |
C18H38O | 1.3 ± 0.024a | 1.22 ± 0.017a | ND |
| 35. | 2-Hexyl-1-octanol | 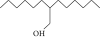 |
C14H30O | 1.246 ± 0.012a | 1.120 ± 0.008a | ND |
| 36. | Hexadecane | 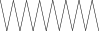 |
C16H34 | 3.48 ± 0.016a | 2.180 ± 0.012b | ND |
| 37. | Heptadecane | 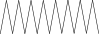 |
C17H36 | ND | 1.93 ± 0.012 | ND |
| 38. | Eicosane | 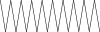 |
C20H42 | 2.24 ± 0.008 | ND | ND |
| 39. | Tetratriacontyl heptafluorobutyrate | 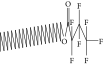 |
C38H69F7O2 | 1.23 ± 0.008 | ND | ND |
| 40. | 2-Hexyl-1-dodecanol | 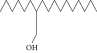 |
C18H38O | ND | 2.03 ± 0.017 | ND |
| 41. | Carbonic acid, decyl undecyl ester | 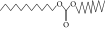 |
C22H44O3 | 0.763 ± 0.004 | ND | ND |
| 42. | Eicosyl octyl ether | 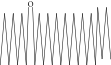 |
C28H58O | 2.34 ± 0.008 | ND | ND |
| 43. | 2-Octyl, 1-dodecanol | 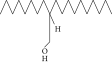 |
C20H42O | 1.686 ± 0.009a | 0.80 ± 0.012b | ND |
| 44. | Octatriacontyl trifluoroacetate | 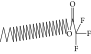 |
C40H77F3O2 | 0.963 ± 0.004a | 0.93 ± 0.012a | ND |
| 45. | Heneicosane |  |
C21H42 | 2.093 ± 0.012a | 1.47 ± 0.008b | ND |
| 46. | Hexadecane, 2,6,10,14-tetramethyl | 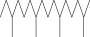 |
C20H42 | 3.033 ± 0.012 | ND | ND |
| 47. | 2,6,10,15-Tetramethyl, heptadecane | 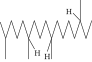 |
C21H44 | ND | 1.03 ± 0.012 | ND |
| 48. | Hexadecanoic acid, methyl ester | 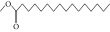 |
C17H34O2 | 9.753 ± 0.016b | 13.371 ± 0.012a | 3.16 ± 0.012c |
| 49. | 9,12-Octadecadienoic acid |  |
C18H32O2 | 1.196 ± 0.016c | 5.50 ± 0.021a | 1.90 ± 0.017b |
| 50. | 11-Octadecanoic acid | 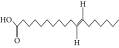 |
C18H34O2 | 3.673 ± 0.012a | ND | 1.57 ± 0.008b |
| 51. | 9-Octadecenoic acid | 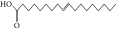 |
C18H34O2 | ND | 8.49 ± 0.0008 | ND |
| 52. | Methyl stearate | 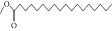 |
C19H38O2 | 2.79 ± 0.008b | 3.50 ± 0.012a | 0.56 ± 0.005c |
| 53. | 4,8-Dimethyl, 3,7-nonadien-2-ol | 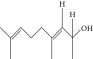 |
C11H20O | ND | ND | 0.68 ± 0.012 |
| 54. | 2-Hexyl 1-decanol | 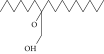 |
C16H34O | 0.796 ± 0.004b | 1.15 ± 0.012a | ND |
| 55. | Methyl 9,10-methylene-octadecanoate | 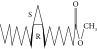 |
C20H38O | ND | 0.63 ± 0.012a | 0.46 ± 0.012a |
| 56. | 2,3-Dihydro, 9,12-octadecadienoic acid, ZZ | 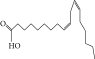 |
C18H32O | ND | ND | 0.48 ± 0.008 |
| 57. | 9,12-Octadecadienoyl chloride, ZZ | 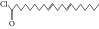 |
C18H31ClO | ND | ND | 0.53 ± 0.012 |
| 58. | 13-Docosenoic acid, methyl ester, (Z)- | 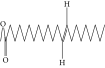 |
C23H44O2 | 0.79 ± 0.008b | 2.88 ± 0.008a | ND |
| 59. | 1,2-Benzene dicarboxylic acid, mono (2-ethylhexyl) ester |  |
C16H22O4 | ND | ND | 1.81 ± 0.008 |
| 60. | Bis (2-ethylhexyl) phthalate |  |
C24H38O4 | 10.69 ± 0.008a | 4.45 ± 0.012b | ND |
| 61. | Cis-11-eicosenamide |  |
C20H39NO | 3.333 ± 0.012b | 4.18 ± 0.008a | ND |
| 62. | 13-Docosanamide, Z |  |
C22H43NO | ND | ND | 10.94 ± 0.012 |
| 63. | Squalene | 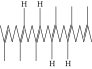 |
C30H50 | ND | 1.71 ± 0.008 | ND |
- Note: Relative concentration was calculated from the percentage (%) of peak area. Values (mean ± SD) are average of three samples (n = 3). Different superscript letters with in the same row indicate significant difference of means (p < 0.05). Here, independent sample t test was conducted for two variables, and one-way analysis of variance (ANOVA) (Duncan test as post hoc) was for three variables.
- Abbreviation: ND = not detected.
Other than the aforementioned six, following 16 compounds, for example, benzene 1,3-bis (1,1-dimethylethyl), 2-isopropyl-5-methyl-1-heptanol, isotridecanol, pentadecane, phenol, 2,4, bis(1,1-dimethylethyl), 2-hexyl-1-octanol, hexadecane, 1-decanol 2-hexyl-1-decanol, 2-octyl-1-dodecanol, 2-octyl-11-methyl dodecanol, heneicosane, octatriacontyl trifluoroacetate, 13-docosenoic acid, methyl ester, (Z), bis (2-ethylhexyl) phthalate, and cis-11-eicosenamide, were present both in the n-hexane and n-hexane–chloroform (2:1) extracts (Tables 1, 2); three compounds, alpha-caryophyllene, beta-longipinene, and methyl 9,10-methylene octadecanoate, were found in both n-hexane–chloroform (2:1) and methanol extract (Tables 2 and 3), and only 11-octadecanoic acid methyl ester was common compound detected in n-hexane and methanol extract. But the concentration of the compounds varied depending on the solvent system. Ten compounds, 2,6,11-trimethyl-dodecane, nonanal, 4,6-dimethyl dodecane, 3,7,11-trimethyl-1-dodecanol, aromandendrene, eicosane, tetratriacontyl heptafluorobutyrate, carbonic acid, decyl undecyl ester, eicosyl octyl ether, and 2,6,10,14-tetramethyl hexadecane, were available only in n-hexane extract, and another set of 10 compounds, 2,4-diethyl 1-heptanol, 2-methoxy-4-(2-propenyl)-phenol acetate, tetradecane, n-tridecane-1-ol, 1-octadecane sulfonyl chloride, 2-hexyl 1-dodecanol, 2,6,10,15-tetramethyl heptadecane, methyl ester 9-octadecanoic acid, and squalene, were detected only in n-hexane–chloroform (2:1) extract, and 15 compounds, phenylethyl alcohol, copaene, trans-alpha-bergamoetene, 1,3-propanediol, 2-(hydroxymethyl)-2 nitro, cis-alpha-bisabolene, spiro [4,5] dec-7-ene,1,8-dimethyl-4-(1-methyl), gamma-elemene, naphthalene 1,2,4a,5,6,8a-hydroxy-4,7-dimethylethyl, bicyclo [7.2.0]undec-4-ene, 4,11,11-trimethyl, naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethylethyl, 3,7-nonadien-2-ol,4,8-dimethyl, 9,12-octadecadienoic acid (Z,Z)-2,3-dihydro, 9,12-octadecadienoyl chloride, mono (2-ethylhexyl) ester, (Z,Z), 1,2-benzene dicarboxylic acid, and 13-docosenamide (Z), were present only in methanol extract.
Antibacterial activity of three different extracts of L. chinensis seed powder is presented in Table 5. At 10 mg/mL concentration, methanol extract showed the highest ZOI of 10 mm for test organisms L. monocytogenes and S. choleraesuis. At the same concentration, n-hexane extract showed 8 mm and 7 mm ZOI for L. monocytogenes and S. choleraesuis, respectively. In case of n-hexane–chloroform extract, ZOIs were 6 and 5 mm for L. monocytogenes and S. choleraesuis, respectively. On the other hand, the standard azithromycin (15 μg/disc) showed that the ZOIs for L. monocytogenes and S. choleraesuis are 40 and 33, respectively. The percentage differences of ZOI for all extracts from standard are shown in Table 5, indicated that all extracts offered relatively moderate antibacterial activity in comparisons with standard.
| Test organism | Zone of inhibition (ZOI) in mm | ||||||
|---|---|---|---|---|---|---|---|
| n-Hexane | n-Hexane–chloroform (2:1) | Methanol | Standard antibiotic (azithromycin 15 μg/disc) | ||||
| Extract ZOI | Percentage difference from standard | Extract ZOI | Percentage difference from standard | Extract ZOI | Percentage difference from standard | ||
| L. monocytogenes (gram +ve) | 8.0 ± 0.4c | 80% | 6.0 ± 1.1d | 85% | 10.0 ± 0.7b | 75% | 40.0 ± 1.5a |
| S. choleraesuis (gram −ve) | 7.0 ± 1.2c | 78.79% | 5.0 ± 0.6d | 84.85% | 10.0 ± 1.3b | 69.70% | 33.0 ± 0.9a |
- Note: For expressing zone of inhibition (ZOI), values (mean ± SD) are analyzed individually in triplicate for each type of microorganisms. Different superscript letters within the same row indicate significant (one-way ANOVA and Duncan test as post hoc, p < 0.05) difference of means.
Phenolic acids, tannins, flavonoids, triterpenes, anthocyanins, and sterols are among the phytochemicals of L. chinensis that may contribute to its many potential biological activities, including anti-inflammatory, antiviral, antimutagenic, anticancer, antioxidant, antimicrobial, antipyretic, antiplatelet, and antihyperlipidemic effects [15].
4. Discussion
The findings of this study align with previous research demonstrating the presence of bioactive compounds in Litchi chinensis extracts, which possess antimicrobial and other therapeutic properties. However, this study extends prior work by using GC–MS profiling to provide a more detailed identification of specific compounds in different solvent extracts, such as 2,4-bis(1,1-dimethylethyl) phenol, hexadecenoic acid, and 2-(hydroxymethyl)-2-nitro-1,3-propanediol, each found in higher concentrations within specific extracts (n-hexane, n-hexane–chloroform, and methanol, respectively). Three different types of esters of fatty acids were detected among which hexadecenoic acid, methyl ester, was the most prominent. The compound has been reported to have various biological properties such as antioxidant, nematicide, hypocholesterolemic, pesticide, antiandrogenic, 5-alpha reductase, and hemolytic inhibitor activities [29]. Two other fatty acid derivatives were the methyl ester of 9,12-octadecadienoic acid, which is the most prevalent fatty acid in human nutrition and is used to treat atherosclerosis and hyperlipoidemia [30] and the methyl stearate that possess different physiological functions such as GABA aminotransferase inhibition, gastrin inhibition, and antinociception [31].
The next outstanding compound was naphthalene which is hazardous for human health. A communication pheromone, benzene acetaldehyde, also called α-toluic aldehyde, phenylacetaldehyde, α-tolualdehyde, and hyacinthin, which is used for synthesizing fragrance and polymers, as well as caryophyllene, a ring-opened isomers β-caryophyllene [32] which is used as cardioprotective, hepatoprotective, and immune-modulatory in pharmaceuticals [33], were also identified. Caryophyllene elicits antimicrobial by altering membrane permeability and integrity of the target cell [34] and antitumor activity by condensing chromatin block and fragmenting DNA [35]. 1,1-Dimethylethyl-2,4-bis, the most prevalent of the 15 chemicals found in n-hexane and n-hexane–chloroform extracts, was phenol. The compound has antifungal, antimicrobial, antioxidant, and antimalarial activities [36]. It impedes quorum sensing of organism to reduce the growth rate of pathogenic fungi and bacteria [37] by inhibiting the biofilm formation [38]. The next prominent compound concurrently detected in these two extracts was bis (2-ethylhexyl) phthalates. The compound is highly antibacterial and cytotoxic [39]. Different hydrocarbon compounds such as hexadecane, benzene 1,3-bis (1,1-dimethylethyl), heneicosane, and pentadecane appeared in these two extracts are reported to have various types of medicinal properties, e.g., lipid oxidation [40], antifungal, antibacterial, antioxidant, sgar-phosphatase inhibitor, chymosin inhibitor, antibacterial, antineoplastic, and oviposition-attractant pheromone. Pentadecan’s sugar-phosphatase inhibitor activity [41] could be a clue to explain the cause of death of children that were reported in Bangladesh and India. Cis-11-eicosenamide, an anti-inflammatory and antioxidant [42], was available both in n-hexane (3.333%) and n-hexane–chloroform (4.18%) extracts. Although the six alcoholic hydrocarbon viz. isotridecanol, 1-decanol, 2-octyl 2-hexyl-1 octanol, 2-isopropyl-5-methyl-1-heptanol, 1-dodecanol, 2-octyl, 1-decanol 2-hexyl as well as octatriacontyl trifluoroacetate, 13-docosenoic acid, methyl ester (Z) in negligible amount, they are reported to antimicrobial and fragrance [43], anti-inflammatory, and anticancer properties. Alpha-caryophyllene, the major compound among the common three compounds detected in methanol and n-hexane–chloroform extracts, is an 11-member monocyclic sesquiterpene and is reported to possess potent anti-inflammatory properties [44]. 11-Octadecenoic acid methyl ester was detected both in n-hexane and methanol extract which is used as an antioxidant in oil-based formulation [45]. Hexadecane, 2,6,10,14-tetramethyl which is used as biomarker; eicosane that have antifungal, antitumor, larvicidal, and cytotoxic properties [41]; and eicosyl octyl ether that is available in different seed oils [46] were more than 2% among the 11 compounds detected only in n-hexane extract. 9-Octadecenoic acid methyl ester that has anticancer and antioxidant activities [47] was the most prominent compound available only in n-hexane–chloroform extract. 1,3-Propanediol, 2-(hydroxymethyl)-2 nitro, gamma-elemene, and 13-docosanamide (Z) were the most prominent compounds available only in methanol extract. The compound 1,3-propanediol, 2-(hydroxymethyl)-2 nitro has reportedly been shown to be microbicidal and is used as a bacterial growth inhibitor and disinfectants [48]. The second highest compound was 13-docosanamide Z (10.94%) in methanol extract. It exerts antinociceptive activities [49] with a possible mechanism of having its structural analogy with docosahexaenoic acid (DHA) which is a very important component of nerve of the brain [50]. An interesting future of all group of bioactive compounds detected is that they are not only diversified in biological activity but also some of them were opposing in their biological function. For example, naphthalene is a carcinogenic compound whereas eicosane is an antitumor one and octatriacontyl trifluoroacetate is an insecticidal, but heneicosane is oviposition-attractant pheromone chemical and bis (2-ethylhexyl) phthalate increases cellular proliferation [51]. Other anticancer compounds were 13-docosenoic acid, methyl ester (Z) [52]. Squalene, precursor of all plant and animal sterols, has also biological activities such as antibacterial, antioxidant, antitumor, cancer preventive, immunostimulant, and pesticide [31]. 9,12-Octadecadienoic acid, a ω-6 fatty acid, is not only antioxidative and hypolipidemic [30] but also an enhancer of antibacterial and antifungal activities. It exerts the later effect by increasing the permeability of the cell membrane. These results reveal the variation in bioactive content depending on the extraction solvent, a detail that previous studies may not have examined as thoroughly.
The antibacterial activity of methanolic extract showed high antibacterial activity (ZOI, 10 mm) against L. monocytogenes and S. choleraesuis followed by n-hexane extract and n-hexane–chloroform extract, respectively (Table 5). Methanolic extract showed high antibacterial activity (ZOI, 10 mm) against L. monocytogenes and S. choleraesuis followed by n-hexane extract and n-hexane–chloroform extract, respectively (Table 5). The result indicates that extractives of polar solvent system have notable, nonpolar solvent system has medium antimicrobial activity and mixed solvent system has lowest antimicrobial activity in comparison among them. The presence of highest concentration of 2-(hydroxymethyl)-2 nitro 1,3-propanediol (39.16%) in the methanolic extract elucidates its highest antibacterial activity against tested bacteria. The antibacterial activity of lychee probably attributed to a variety of mechanisms, as supported by the body of existing literature and the structural characteristics of the main compounds found within them. The primary mode of action involves disruption of the bacterial cell membrane, leading to increased permeability, leakage of intracellular contents, and eventual cell lysis. Additionally, certain bioactive constituents, such as terpenes and alcohols, are known to inhibit key enzymatic processes essential for bacterial survival and metabolism [53, 54].
In terms of antimicrobial activity, this study’s results align with previous findings that methanolic extract L. chinensis Sonn. seed exhibits effectiveness against certain pathogens: Klebsiella pneumoniae with a ZOI of 15 mm, Pseudomonas aeruginosa (ZOI-12 mm), and Staphylococcus aureus (ZOI-9 mm) [55]. Another study report showed by the disc diffusion method, L. chinensis of aqueous extract was found to have moderate antibacterial activity against gram-positive bacteria such as Staphylococcus aureus, Streptococcus pyogenes, and Bacillus subtillis, as well as Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa [56]. These findings are consistent with our research indicating that the methanol extract demonstrated notable activity against Listeria monocytogenes and Salmonella choleraesuis. This could be due to differences in extraction methods, solvent choice, or plant material, suggesting that extraction conditions significantly influence the antibacterial efficacy. Overall, while this study supports existing knowledge of L. chinensis’s medicinal properties, it offers new insights into solvent-dependent bioactivity and specific bioactive profiles, encouraging more targeted exploration of extraction techniques and compound-specific activities in future research.
5. Conclusions
In this study, GC–MS analysis of three different extracts n-hexane, n-hexane–chloroform (2:1), and methanol revealed the presence of 63 bioactive compounds. These compounds, which vary in structure and function, suggest their involvement in cellular differentiation, proliferation, and protection against oxidative damage and pathogenic attacks. Antimicrobial potentiality tested against certain pathogens; Listeria monocytogenes and Salmonella choleraesuis confirmed their antimicrobial activity. Furthermore, the detected compounds align with previously reported glucose-lowering effects, further validating the therapeutic potential of L. chinensis extracts. The diversity of these bioactive molecules supports the possible medicinal uses of the Litchi chinensis Sonn. seed. Overall, the findings highlight the therapeutic potential of L. chinensis extracts and justify further exploration of its bioactive compounds for their medicinal applications. Finally, we can conclude that methanolic extract shows the more prominent compounds from other two extract which help to express potential activity against bacteria, so this extract is relatively best from other two. Further studies are required to isolate compounds from the L. chinensis extracts, purification and structural elucidation bioactive compounds, along with in-depth pharmacological evaluations, to fully understand their mechanisms of action and potential applications in drug development. The results of this study offer significant understanding of the bioactive potential of L. chinensis extracts, paving the way for further research on compound isolation, purification, and their formulation for use in pharmaceuticals.
Nomenclature
-
- ANOVA
-
- Analysis of variance
-
- ATCC
-
- American Type Culture Collection
-
- CFU
-
- Colony forming unit
-
- GC–MS
-
- Gas chromatography–mass spectrometry
-
- NIST
-
- National Institute of Standards and Technology
-
- SPSS
-
- Statistical Product and Service Solutions
-
- ZOI
-
- Zone of inhibition
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Samia Sharmin: methodology and writing – original draft. Ali Ahsan Muzahid: methodology and writing – review and editing. Md. Mohibul Islam: formal analysis and resources. Mst. Sarmina Yeasmin: data curation and supervision. Amit Kumar Dey: visualization and methodology. Md. Jasim Uddin: investigation and formal analysis. G. M. Masud Rana: formal analysis and data curation. Jaytirmoy Barmon: validation and writing – review and editing. Safaet Alam: data curation and validation. Md. Nurul Huda Bhuiyan: resources and investigation. Nazim Uddin Ahmed: conceptualization and supervision. All authors read and approved the final manuscript.
Samia Sharmin and Ali Ahsan Muzahid contributed equally with all other contributors.
Funding
This study was conducted under Nurul Afsar Khan Post Graduate Fellowship grant (Ref: 39.307.054.00.01.110.2019/1078) from Bangladesh Council of Scientific and Industrial Research (BCSIR).
Acknowledgments
We would like to acknowledge the authority of the Bangladesh Council of Scientific and Industrial Research (BCSIR) for their financial and logistic support.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




