Untargeted Metabolomics and Antibacterial Properties of Streptomyces Species Sourced From Thai Mangrove
Abstract
Novel antimicrobial agents are urgently needed to combat the global threat of antimicrobial resistance. Actinobacteria are remarkable producers of bioactive compounds, which are crucial for the discovery of novel drugs to combat antimicrobial resistance. In the current study, bioactivity, phylogenetic analysis, and a dereplication approach were employed to quickly analyze the metabolomic profiles of selected strains and aid in selection strain prioritization. The aim of this study was to screen Thai actinobacteria isolated from the mangrove ecosystem for antibacterial activities. Taxonomic identification confirmed the classification of these isolates within the genus Streptomyces. Eleven strains identified as Streptomyces yogyakartensis, S. globisporus, S. albiaxialis, S. misionensis, S. iranensis, S. sanyensis, and S. diastaticus. The crude extracts of the selected actinobacteria strains exhibited antibacterial activities, with minimum inhibitory concentrations ranging from 9.9 to 1250 μg/mL against methicillin-resistant Staphylococcus aureus, Acinetobacter baumannii, and Klebsiella pneumoniae. Mass-guided molecular networking analysis of the crude extract revealed the metabolomic complexity of the isolates. Structurally unique compounds such as desferrioxamine E, elaiophylin, kanchanamycin C, bisacuberin, dehydroxynordicadamine, desmethylenylnocardamine, chymostatinol A, chymostatin B, desferrioxamine G, ferrioxamine E, and legonoxamine A were detected in the crude extracts. Notably, we report the potential of bioactive compounds isolated from Thai mangrove actinobacteria, demonstrating diverse biological activities. This study demonstrated the use of metabolomics to annotate putative bioactive compounds, prioritize strains, and discover novel bioactive metabolites.
1. Introduction
Antimicrobial resistance (AMR) is a growing global health threat with serious implications [1, 2]. Antibiotic resistance has been a persistent issue since the introduction of the first class of antibiotics and has continued to evolve dynamically [3]. Key factors contributing to the rise of antibiotic resistance include overpopulation, increased global movement, misuse of antibiotics in healthcare and agriculture, selection pressure, inadequate hygiene, and poor waste disposal systems [1, 4]. In developing countries, the lack of adequate AMR surveillance, poor quality control, and clinical misappropriation of antibiotics are major contributors to drug resistance. In contrast, poor hospital regulations and the overuse of antibiotics in food production are significant drivers of AMR in developed countries [5, 6]. If precautionary measures are not implemented, AMR-related deaths are projected to reach 10 million annually by 2050 [7]. In 2019, the first global report on AMR estimated that bacterial AMR was linked to 4.5 million deaths, with 1.27 million directly attributed to bacterial infections [5]. ESKAPE pathogens—Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species—are now the leading cause of hospital-acquired infections worldwide, with some strains lacking effective treatments [8]. Additionally, the Centers for Disease Control and Prevention (CDC) has identified 16 bacterial and fungal species as urgent threats to human health [9]. To combat AMR, a global emphasis on the One Health approach is essential. While multiple protocols exist for AMR surveillance, the implementation of a unified One Health strategy across different countries is hindered by the lack of standardized guidelines for monitoring and evaluation [10]. Nature bestowed with unlimited resources in the form of bioactive compounds and intensive research on these resources is very important in drug discovery. These bioactive molecules, known as natural products, are produced by living organisms such as plants, animals, and microbes as by-products [11]. Microbes are remarkable producers of various distinctive natural compounds that are crucial for the development of novel drugs. Majority of antibiotics and anticancer medications that are clinically used today were isolated from microbes during the “golden era” [12]. Conventional bioactivity screening and taxonomy-based methods often lead to the reisolation of known compounds [13, 14]. Metabolomics, an advanced “omics” approach, integrates high-throughput analytical techniques with bioinformatics to comprehensively assess metabolites. Dereplication tools such as GNPs, METLIN, and NP-MS help prioritize novel bioactive compounds for isolation [15–17].
Actinobacteria are naturally occurring microbes that play a vital role in decomposing complex organic matter. They are renowned for producing the majority of clinically used drugs, including antibiotics, anticancer agents, and immunosuppressants [18]. In recent years, actinobacteria have garnered significant attention from researchers, research organizations, and pharmaceutical companies for their applications across various fields, including medicine, agriculture, biotechnology, food, and enzyme industries [19, 20]. The focus has shifted from screening terrestrial actinobacteria to exploring those from unique ecosystems such as marine environments, mangroves, caves, and Arctic regions [21]. The extreme conditions in these habitats drive microbial adaptations, leading to the production of bioactive metabolites essential for survival, many of which hold potential for drug discovery [22]. To date, research on mangrove-derived actinobacteria has led to the discovery of at least 88 new species, including eight novel genera. Additionally, over 80 bioactive compounds have been identified, among them promising molecules such as halichoblelide D, xiamycins, and indolocarbazoles [22, 23]. Four Streptomyces strains (B475, B486, B353, and B98) isolated from mangrove sediments exhibited strong antibacterial activity, particularly against Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Bacillus subtilis, and Micrococcus luteus. Metabolite profiling of strain B475 identified seven quinoxaline-type antibiotics, including quinomycin A, quinomycin monosulfoxide, and five novel analogs [24]. Eudesmane-5β,11-diol (1), a polycyclic sesquiterpene isolated from the endophytic Streptomyces JMRC: ST027706 associated with the mangrove plant Bruguiera gymnorhiza in Xiamen, China, exhibited potent antibacterial activity. It demonstrated broad-spectrum efficacy, particularly against drug-resistant pathogens such as MRSA, vancomycin-resistant Enterococcus faecalis, and Escherichia coli [25]. These findings highlight the potential of mangrove environments as a valuable source of new antibiotics.
Thailand harbors diverse bioresources that remain largely unexplored, particularly in mangrove environments. The majority of Thailand’s maritime ecosystems have been minimally investigated [26]. While terrestrial Streptomyces species have been extensively studied, only a small fraction of marine and mangrove-derived Streptomyces have been explored, leaving a significant gap in our understanding of their potential to produce novel antibiotics.
2. Materials and Methods
2.1. Sample Collection and Isolation of Actinobacteria
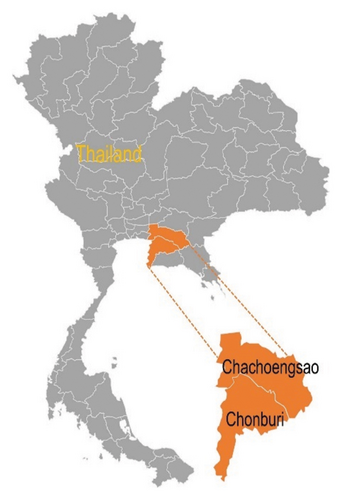
A total of 56 mangrove sediment samples were collected from the mangrove areas of Chachoengsao and Chonburi provinces (Figure 1). The samples were placed in sterile bags, stored at 4°C, and transported to the laboratory for further processing. They were then air-dried at room temperature for 1 week and ground into a fine powder. The dried sediment samples were used for the selective isolation of actinobacteria following the protocol described by Ruttanasutja and Pathom-aree [27].
2.2. Cultivation of Actinobacteria
Pure cultures of actinobacteria were routinely grown on ISP2 agar and incubated at 30°C for 7–14 days. For broth cultivation, a single colony was inoculated into a 5-mL tube of ISP2 broth and incubated on a rotary shaker at 150 RPM and 30°C for 4 days to establish a starter culture. This starter culture was then transferred to a 100-mL flask containing ISP2 broth and incubated under the same conditions for 7–14 days.
2.3. Extraction of Metabolites From Actinobacteria
Actinobacterial crude extracts were prepared in accordance with the growth medium conditions. Actinobacteria were cultured mostly in ISP2 medium either in agar or in a broth media; hence, the three crude extracts, agar media, supernatant (broth), and mycelia, were prepared for each strain. Firstly, the agar extracts were prepared from fully grown actinobacteria under the necessary conditions (14 days, 30°C) on ISP2 agar media, and then, the agar media along with actinobacteria were cut into small pieces inside the biosafety cabinet and macerated in 50 mL of 100% methanol (RCI LABSCAN, Thailand) with continuous shaking in an incubator for 24 h. Secondly, the pure colonies of actinobacteria were allowed to grow in broth media for 7–14 days, then on the last day of incubation, the broth culture were centrifuged at 5000 rpm/minute for 20 min to separate the supernatant and mycelia. The supernatant was extracted by the addition of activated XAD 16N resin (Sigma-Aldrich, France) and allowed to extract the necessary metabolites for 12 h on a rotary shaker at 90 rpm, followed by separation of the resin from the supernatant using a Buchner funnel and washing with sterile distilled water. The washed resin was then placed in a 125-mL flask with 50 mL methanol and extracted three times for 1 h on a rotary shaker at 90 rpm. Lastly, the mycelia were extracted once with 100 mL acetone on a rotary shaker at 90 rpm Thereafter, all the three extracts were pooled together and filtered through Whatman paper filter no. 1, and the resulting filtrate was concentrated using a rotary evaporator (Heidolph, Germany) to evaporate the organic solvents and then freeze-dried in a lyophilizer machine (Christ, Germany) to obtain the dry crude extract. All dried crude extracts were stored in airtight containers at −20 °C until further use for liquid chromatography–mass spectrometry (LC-MS) and biological screening.
2.4. Assessing the In Vitro Antibacterial Activities of the Crude Extracts
2.4.1. Determination of Minimum Inhibitory Concentration (MIC)
The antibacterial activity of the crude extracts from each actinobacterial strain was assessed using the microdilution technique to determine the MIC against MRSA, PW01, Acinetobacter baumannii (PW01), and Klebsiella pneumoniae (PW01), following the protocol outlined by Wiegand et al. 2008 [28] with minor modifications. Briefly, the crude extract (5 mg/mL) of each active actinobacteria extract was dissolved in 5% dimethyl sulfoxide (DMSO) and dispensed into the 96-microtiter plate. A positive control with a known antibiotic was used and 2.5% DMSO was used as the negative control. Fresh test pathogens, adjusted to a turbidity of OD 625 nm at 0.1 (1 × 108 CFU/mL), were prepared in Mueller–Hinton broth. These were diluted 20-fold and then inoculated into 96-well microtiter plates. A series of two-fold dilutions were performed across all columns, with each well containing a total volume of 100 μL. The 96-microtiter plate was incubated at 37°C for 16–18 h, and then 20 μL of resazurin was added and incubated again for 3–4 h. Colistin sulfate (GoldBio, USA, Lot#:0603.073120A) and vancomycin hydrochloride (GoldBio, USA, Lot#:0111.011921A) were used as the positive controls for Gram-negative and Gram-positive bacteria, respectively. The MIC of the crude extract was determined as the lowest concentration inhibiting visible bacterial growth. All experiments were conducted in triplicate (n = 3).
2.4.2. Determination of Minimum Bactericidal Concentration (MBC)
A 10-μL sample from the MIC of each test pathogen was taken and inoculated onto a sterile, antibiotic-free Mueller–Hinton agar plate. A spreader was used to spread inoculated serial dilutions to determine the concentration of the crude extract that killed 99.99% of the bacteria. The inoculated plates were incubated at 37°C for 16–18 h, and the results were recorded. The concentration of the crude extract that killed 99.99% of the bacterial pathogens was determined as the MBC. All the tests were carried out in triplicates (n = 3).
2.5. Assessment of Actinobacteria Against Bacterial Pathogens
The agar overlay method was used to evaluate the ability of selected actinobacteria to inhibit the target pathogens MRSA (PW01), A. baumannii (PW01), and K. pneumoniae (PW01). Actinobacteria were grown on marine and ISP2 agar for 5 days at 30°C, with regular monitoring for colony growth. Semisolid Mueller–Hinton agar (0.7% w/v agar) was prepared under aseptic conditions and inoculated with test pathogens, adjusted to an optical density (OD) of 0.1 at 625 nm (1 × 108 CFU/mL). The standardized pathogen suspension was mixed with the semisolid Mueller–Hinton agar and overlaid onto the agar plates where the actinobacteria were already grown. The plates were then incubated at 37°C for 16–18 h. Active actinobacterial strains were identified by the presence of clear zones of inhibition around their colonies. All tests were performed in triplicate for each strain (n = 3).
2.6. LC-MS Analysis
Approximately 100 mg of each extract was dissolved in 75% methanol (v/v). The dissolved sample was then mixed and adjusted to a concentration of 50–300 mg/L. The mixture was then centrifuged at 120,00 × g at 25°C for 15 min. After that, the resulting supernatant was carefully transferred to a clean 1.5-mL microcentrifuge tube and filtered using a hydrophilized PTFE membrane (pore size: 0.22 μm, diameter: 13 mm). A volume of 5 μL was then injected into an ultra-high-performance liquid chromatography–high-resolution mass spectrometer coupled with an Orbitrap mass analyzer (UHPLC-HRMS/MS) for mass identification.
The injected sample extract was separated using a Hypersil GOLD™ Vanquish C18 column (2.1 × 100 mm, 1.9 μm, Thermo Scientific) with a guard column. The separation process was performed at 40°C and a flow rate of 0.4 mL/min. Mobile Phase A consisted of 0.1% formic acid (FA) in water, whereas mobile Phase B consisted of 0.1% FA in acetonitrile. The elution gradient started with 5% B for 4 min, and the percentage of Phase B was then increased to 90% over a period of 10 min. Then, the column was flushed with 90% Phase B for 4 min, followed by a reduction in the concentration of Phase B to 5% within 1 min, before returning to the initial conditions, with a total runtime of 25 min.
Data acquisition was performed using a Thermo Scientific Q-Exactive HF-X hybrid quadrupole-Orbitrap mass spectrometer equipped with a heated electrospray ionization (HESI) source. Full scan MS and data-dependent MS2 spectra were acquired in both positive and negative ion modes. Ionization parameters included a spray voltage of 3.5 kV (positive) or 2.5 kV (negative), sheath gas of 45 arbitrary units, auxiliary gas of 10 arbitrary units, and sweep gas of two arbitrary units. The capillary temperature was set to 320°C, and the auxiliary gas temperature was set at 400°C for stable ionization. During the acquisition process, full-scan MS1 and data-dependent MS2 (dd-MS2) modes (top n) were acquired with resolutions of 120,000 (with a maximum injection time of 100 ms) and 30,000 (with a maximum injection time of 50 ms), respectively, covering a scan range from 100 to 1500 m/z. The automatic gain control target was set to 3e6 for consistent signal intensities. Stepped N(CE) values of 20 eV, 30 eV, and 40 eV were set to ensure the desired collision energies.
2.7. Data Processing and Molecular Networking
The acquired data files from LC-MS were processed using Compound Discoverer software 3.3. LC-MS data were processed using a peak-picking algorithm with a minimum peak intensity threshold of 10,000 counts, ensuring only significant metabolites were included in the dereplication analysis. For molecular networking, similarity thresholds for MS/MS fragmentation patterns were carefully chosen to cluster structurally related compounds while avoiding false positives. The ore-cleaned raw data from the data-dependent acquisition mode were converted to mzXL format using an MS converter and uploaded to the global natural products social molecular networking (GNPS) [29] (https://gnps.ucsd.edu) to create an online molecular network. The MS2 fragment tolerance and parent mass of tolerance were both set at 0.02 Da. The network was created with a cosine score of 0.65. more than three matched peaks. Edges were created between two nodes within the network and kept within the network if the node appeared in the top 10 similar nodes. The molecular family was set to a maximum size of 100. Background signals were eliminated by incorporating media and solvent blank spectra into the spectral library. Cytoscape Version 3.9.1 from the U.S. National Institute of General Medical Sciences was used to visualize the output of molecular networking [30]. The dereplication method excluded known compounds efficiently, allowing us to prioritize novel bioactive strains. [31]. All MS data are publicly available in GNPS (https://gnps.ucsd.edu) under the molecular network search id = e8d5e43b6b104702a07a10099a9644923 and MolNetEnhancer id = bef431f45fd54f3da385a5a50410022 d.
2.8. MicrobeMASST
A search tool within the GNPS platform, designed for taxonomically curating mass spectra in untargeted metabolomics of natural products, was also utilized in this study [32]. The USI or spectrum peaks, along with the precursor mass of the annotated compound, were copied and uploaded to the following link (https://masst.gnps2.org/microbemasst) to generate a taxonomic tree. The precursor and fragment ion tolerances were set to 0.05 Da, while the cosine score threshold was set to 0.7, requiring the merging of at least three peaks.
2.9. Molecular Identification by 16S rRNA Gene Sequencing
Selected actinobacteria were cultivated in ISP2 medium for 3 days. Total genomic DNA was then extracted using a modified protocol of the Gene Elute™ Bacterial Genomic DNA Kit (Sigma-Aldrich, USA), which included an extended incubation step. The purified genomic DNA served as a template for PCR amplification of the 16S rRNA gene using universal primers (27F: 5′-AGAGTTTGATCCTGGCTCAG-3′ and 1492R: 5′-TACGGCTACCTTGTTACGACTT-3′). The resulting amplicons were sequenced by the Sanger sequencing using the same primers at U2Bio Co., Ltd., Thailand.
2.10. Phylogenetic Analysis
Overlapping regions at the beginning and end of each 16S rRNA sequence from the actinobacterial strains were removed, and the resultant sequences were assembled into contigs using BioEdit Sequence Alignment Editor 7.2. The obtained nucleotide sequences were analyzed using 16S-based ID BLAST in the EzBiocloud database (https://www.ezbiocloud.net/) [33] to identify their closest phylogenetic neighbors. Molecular Evolutionary Genetics Analysis (MEGA) Version 11.0 was used for construction of phylogenetic using the neighbor-joining method with 1000 bootstrap replicates to assess the reliability of the branching patterns. Evolutionary distances were calculated using the maximum composite likelihood method, which was chosen due to its accuracy in estimating phylogenetic relationships in microbial populations [34].
3. Results and Discussion
3.1. Antibacterial Activities
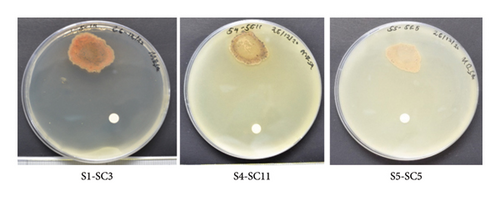
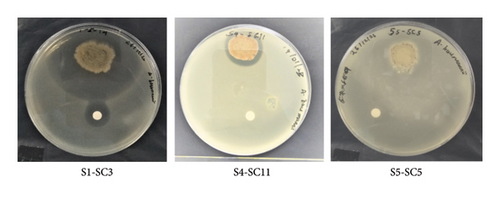
Preliminary screening of methanolic crude extracts of 165 actinobacteria showed that only 23 strains exhibited antimicrobial activities accounting for 14% of the total actinobacterial strains. The microdilution technique using the 96 microtiters well plate was used to determine the MIC of the selected active actinobacterial extracts in comparison with the positive control. Selected actinobacterial methanolic extracts exhibited antimicrobial activity against MRSA (PW01), K. pneumonia (PW01), and A. baumannii (PW01) with an MIC range of between (< 9.9 - > 1250 μg/mL; Table 1). The MIC and MBC values for strains S1-SC3, 1-3, 1-5-22, RH1-5-10, AV2-5-14, RH1-5-14, S2-SC16, S1-SC1, and RH1-5-22 ranged from 9.9 to 1250 μg/mL. These values are particularly noteworthy, as similar findings have been reported in only a few studies on mangrove-derived actinobacteria. This suggests a potentially unique antibacterial mechanism. Furthermore, agar overlay assay was used to confirm the antibacterial activity of the 23 strains and to narrow down to active specific strains. However, based on agar overlay assays only, 12 actinobacterial strains exhibited antimicrobial activity against MRSA (PW01), accounting for 48% of the active actinobacteria (Tables 1 and 2, Figure 1S), whereas 11 actinobacterial strains exhibited antimicrobial activity against A. baumannii, accounting for 44% of the active actinobacterial strains (Figure 2S, Tables 1 and 2). Additionally, two actinobacteria strains exhibited residual antimicrobial activity against K. pneumoniae (PW01), accounting for 8% of the total active actinobacteria (Figure 3S, Tables 1 and 2). Furthermore, agar overlay results highlight the unique potential of mangrove-derived actinobacteria as a valuable source of antibacterial compounds with 48% of active strains inhibiting MRSA, 44% targeting A. baumannii, and 8% showing residual activity against K. pneumoniae. It is also noteworthy that some of the actinobacterial strains exhibited stronger activity against Gram-positive bacteria than Gram-negative bacteria, likely due to differences in cell wall structure. Additionally, the unique bioactive metabolites produced by mangrove actinobacteria may have higher specificity for targets present in Gram-positive pathogens, further explaining the observed selectivity. Actinobacterial strains S1-SC3, S5-SC5, S6-SC2, S4-SC11, and S5-SC2 were selected for molecular networking in the GNPs platform based on their strong antibacterial activity and diverse metabolomic profiles. These strains exhibited potent inhibition against target pathogens, and their metabolic extracts contained unique or potentially novel bioactive compounds, making them ideal candidates for further dereplication and structural annotation (Figures 2 and 3, Tables 1 and 2).
| No | Strain code | MRSA (PW01) | A. baumannii (PW01) | K. pneumonia (PW01) |
|---|---|---|---|---|
| MIC (μg/mL) | MIC (μg/mL) | MIC (μg/mL) | ||
| 1 | S1-SC3 | < 9.9 | < 9.9 | < 9.9 |
| 2 | 1-5-22 | < 9.9 | < 9.9 | < 9.9 |
| 3 | S2-SC16 | < 9.9 | < 9.9 | < 9.9 |
| 4 | S5-SC5 | 156.25 | 312.5 | 39.6 |
| 5 | 1–3 | < 9.9 | < 9.9 | < 9.9 |
| 6 | S4-SC11 | 625 | 312.5 | 625 |
| 7 | S2-SC19 | < 9.9 | < 9.9 | < 9.9 |
| 8 | S1-SC1 | < 9.9 | < 9.9 | < 9.9 |
| 9 | 2–27 | 78.125 | 156.25 | 39.6 |
| 10 | AV2-5-14 | 39.6 | 78.12 | 78.12 |
| 11 | RH1-5-14 | < 9.9 | < 9.9 | < 9.9 |
| 12 | S2-SC10 | 39.6 | 39.6 | 39.6 |
| P | Colistin | — | 4 | 2 |
| P | Vancomycin | 2 | — | — |
- Note: Vancomycin and colistin were used as positive control. The experiment was performed in triplicate (n = 3). P = positive control.
- Abbreviation: ND, not detected.
| No | Strain code | MRSA (PW01) | A. baumannii (PW01) | K. pneumonia (PW01) |
|---|---|---|---|---|
| MBC (μg/mL) | MBC (μg/mL) | MBC (μg/mL) | ||
| 1 | S1-SC3 | 1250 | 79.2 | 19.8 |
| 2 | 1-5-22 | 1250 | 39.6 | 312.5 |
| 3 | S2-SC16 | ND | 78.12 | 78.12 |
| 4 | S5-SC5 | 625 | > 1250 | ND |
| 5 | 1–3 | ND | 39.6 | > 1250 |
| 6 | S4-SC11 | ND | ND | ND |
| 7 | S2-SC19 | 1250 | 78.12 | ND |
| 8 | S1-SC1 | ND | 78.12 | 78.12 |
| 9 | 2–27 | ND | 1250 | ND |
| 10 | AV2-5-14 | ND | 625 | ND |
| 11 | RH1-5-14 | ND | 1250 | 312.5 |
| 12 | S2-SC10 | ND | 156.25 | 312.5 |
| P | Colistin | — | 8 | 4 |
| P | Vancomycin | 4 | — | — |
- Note: Vancomycin and colistin were used as positive control. The experiment was performed in triplicate (n = 3). P = positive control.
- Abbreviation: ND not detected.
3.2. Molecular Identification by 16S rRNA Gene Sequencing
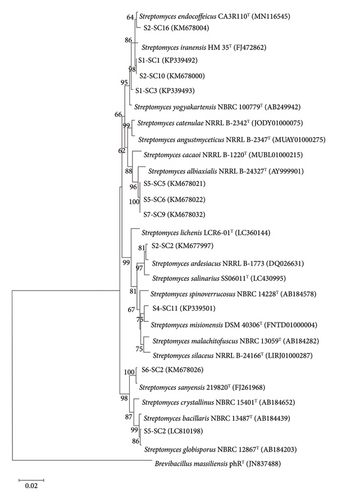
The BLAST analysis identified all selected actinobacteria as Streptomyces species with 16S rRNA gene sequence similarity values ranged between 99.21% and99.86% (Table 3). Three isolates were closely related to S. albiaxialis, and two isolates were closely related to S. iranensis. The remaining isolates were nearest to S. yogyakartensis, S. globisporus, S. misionensis, S. endocoffeicus, S. sanyensis, and S. ardesiacus. Phylogenetic tree further confirms the assignment of these selected actinobacteria as members of the genus Streptomyces (Figure 4).
| No | Strain | Grown in ISP2 | Identification | % | Accession no | Location |
|---|---|---|---|---|---|---|
| 1 | S1-SC3 |  |
Streptomyces yogyakartensis NBRC 100777(T) | 99.58 | KP339493 | Chachoengsao, (13° 30′ 23″ N 101° 0′ 9″ E), Rhizophora mucronata Poir. |
| 2 | S5-SC2 |  |
Streptomyces globisporus NBRC 12867 (T) | 99.65 | LC810198 | Chonburi, (13° 20′ 40″ N 100° 56′ 30″ E), Avicennia alba |
| 3 | S5-SC5 |  |
Streptomyces albiaxialis NRRL B-24327(T) | 99.22 | KM678021 | Chonburi, (13° 20′ 40″ N 100° 56′ 30″ E), Avicennia alba |
| 4 | S5-SC6 |  |
Streptomyces albiaxialis NRRL B-24327(T) | 99.21 | KM678022 | Chonburi, (13° 20′ 40″ N 100° 56′ 30″ E), Avicennia alba |
| 5 | S7-SC9 |  |
Streptomyces albiaxialis NRRL B-24327(T) | 99.22 | KM678032 | Chonburi, (13° 20′ 35″ N 100° 56′ 35″ E), mangrove sediment (no vegetation) |
| 6 | S4-SC11 |  |
Streptomyces misionensis NBRC 13063(T) | 99.86 | KP339501 | Chachoengsao, (13° 30′ 11″ N 101° 0′ 5″ E), Avicennia marina (Forssk) Vierh. |
| 7 | 1-5-22 |  |
Streptomyces iranensis HM 35(T) | 99.55 | KM678004 | Chachoengsao, (13° 30′ 23″ N 101° 0′ 11″ E), Avicennia alba |
| 8 | S2-SC10 |  |
Streptomyces iranensis HM 35(T) | 99.58 | KM678000 | Chachoengsao, (13° 30′ 23″ N 101° 0′ 11″ E), Avicennia alba |
| 9 | S1-SC1 |  |
Streptomyces iranensis HM35(T) | 99.23 | KP339492 | Chachoengsao, (13° 30′ 23″ N 101° 0′ 9″ E), Rhizophora mucronata Poir. |
| 10 | S6-SC2 |  |
Streptomyces sanyensis | 99.85 | KM678026 | Chonburi, (13° 20′ 38″ N 100° 56′ 32″ E), Rhizophora mucronata Poir. |
| 11 | S2-SC2 |  |
Streptomyces diastaticus subsp. Ardesiacus NRRL B-1773(T) | 99.64 | KM677997 | Chonburi, (13° 20′ 40″ N 100° 56′ 30″ E), Avicennia alba |
3.3. Molecular Networking
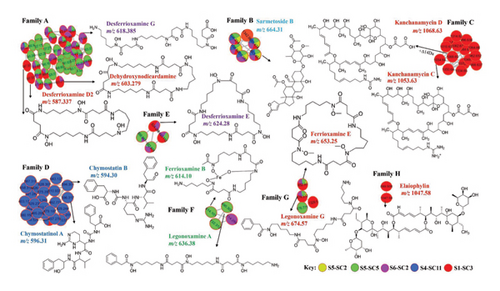
The adopted molecular networking approach via the GNPS molecular network search and MolNetEnhancer resulted in the annotation of various classes of compounds that were grouped into different molecular families, namely, Families A, B, C, D, E, F, G, and H. (Figure 5, Table 4). The dereplication method excluded known compounds efficiently, allowing us to prioritize novel bioactive strains. Molecular networking analysis revealed the presences of hydroxamate-type siderophores in Family A, E, F, and G (Figure 5). Family A consisted of mainly hydroxamate-type siderophores dehydroxynorcardamine (m/z 603.27, [M + 2H]2+, Figure 4S), and it was detected in crude extracts of strain S1-SC3 and S6-SC2, desferioxamine D2 (m/z 587.33 [M + H]+) was detected in crude extract of S1-SC3 only, desferioxamine G (m/z 618.38 [M + H]+, Figure 5S) was detected in crude extracts of S1-SC3 and S6-SC2 (Figure 5, Table 4). Desferrioxamine D2 (m/z 587.22 [M + H]+) was detected in the crude extracts of strains S1-SC3 (Figure 5). Family E consisted of legonoxamine A (m/z 690.30 [M + H]+), which was detected in crude extracts of S5-SC5, and Ferrioxamine B (m/z 614.10 [M + H]+) (Figure 9S), which was also detected in strain S5-SC5 (Figure 5). Family G consisted of ferrioxamine E (m/z 652.93 [M + H]+), (Figure 11S) which was detected in the crude extracts of S1-SC3, S6-SC2, S4-SC11, and S5-SC5 (Figure 5). Legonoxamine G (m/z 674.57, [M + H]+) (Figure 5) was detected in crude extracts of strains S1-SC3, S6-SC2, S5-SC5 and S5-SC2. Desferrioxamine E (m/z 601.35 [M + K]+, Figure 12S) was detected in crude extracts of S1-SC3, S6-SC2, and S5-SC5 and annotated in Family E (Figure 5, Table 4).
| Compound no. | RT (min) | Precursor mass∗ | Formula | Exact mass | Error PPM | Cosine score | Sirius score | Matched MS/MS spectrum | Dereplication results# | Strains distribution |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.94 | 587.3374 [M + Na]+ | C26H46N6O9 | 586.3326 | 1 | 0.87 and 0.94 | 90.625% | 141 | Desmethylenylnocardamine | S6-SC2 and S1-SC3 |
| 2 | 5.49 | 619.3638 [M + K]+ | C27H50N6O10 | 618.3588 | 1 | 0.90 and 0.91 | 88.031% and 94.055% | 58 | Desferrioxamine G | S6-SC2 and S1-SC3 |
| 3 | 9.08 | 1054.6368 | C54H91N3O17 | 1053.6348 | 1 | 0.71 | 58.000% | 236 | Kanchanamycin C | S1-SC3 |
| 4 | 6.62 | 401.2605 | C18H32N4O6 | 400.2321 | 1 | 0.78 | 93.46% | 87 | Bisucaberin | S1-SC3 |
| 5 | 5.69 | 585.3591 | C36H50O5 | 584.3533 | 1 | 0.71, 0.90 and 0.92 | 89.503%, 92.127% and 100% | 114 | Dehydroxynorcardamine | S5-SC2, S1-SC3, S4-SC11 and S6-SC2 |
| 6 | 4.53 | 614.2703 | C25H45FeN6O8 | 616.2882 | 2 | 0.75 | 90.9% | 37 | Ferrioxamine-B | S4-SC11 |
| 7 | 5.01 | 561.3596 | C25H48N6O8 | 560.3533 | 1 | 0.87 | 85.206% | 44 | Deferroxamine | S4-SC11 |
| 8 | 5.04 | 654.2768 | C27H45FeN6O9 | 653.2597 | 1 | 0.84 | 53.427% | 130 | Ferrioxamine-E | S6-SC2 |
| 9 | 6.17 | 601.3534 | C27H48N6O9 | 600.3482 | 1 | 0.91 and 0.87 | 93.553% | 80 | Desferrioxamine E | S1-SC3, S6-SC2 and S5-SC5 |
- ∗Precursor mass are all [M + H]+or unless otherwise specified #Annotation of the compounds are based on dereplication spectral libraries search features of global natural product social molecular networking (GNPS), CSI: fingerID score and Sirius score.
Molecular network analysis of Family B revealed the presence of sarmetosides and other analogs annotated in the GNPS network. Family B consisted of sarmetoside B (m/z 663.45 [M + H]+) (Figure 5) as the only compound annotated in this family, which was detected in the crude extracts of strains S1-SC3, S6-SC2, S5-SC5, S4-SC11, and S5-SC2 (Figure 5). Family C was composed of the macrolide antibiotic kanchanamycin C with a parent mass of (m/z 1054.64 [M + H]+) (Figure 5, Figure 6S) and other possible novel compounds with a parent mass of (m/z 1068.65 [M + H]+ Figure 5), and it was only detected in crude extracts of strain S1-SC3. The parent mass of 1068.65 could be assigned to kanchanamycin D, which contains a molecular mass of 1069 in the literature review and an increase of Δ +14 Da (underwent methylation) (Figure 5). Family D contained ureylene-containing oligopeptides. The molecular network contained chymostatin B (m/z 594.30 [M + H]+) and chymostatinol A (m/z 596.31 [M + H]+), identified by the dereplicator plus in the GNPS dashboard (Figure 5). Moreover, these compounds were detected only in strain S4-SC11 only. Family H of macrolide antibiotic elaiophylin with a parent mass of (m/z 1047.53 M + Na) (Figure 5, Figure 13S), and it was only detected in strain S1-SC3 (Figure 5). Desmethylenylnocardamine (m/z 609.32 [M + Na]+) (Figure 5, Figure 8S), Bisacuberin (401.24 [M + H]+) (Figure 5, Figure 7S), deferoxamine (561.36 [M + 2H]2+) (Figure 5, Figure 10S), and ikarugamycin epoxide (m/z 495.29 [M + H]+) (Figure 14S) were all identified in the library search but not featured in the molecular network. The detection of elaiophylin, kanchanamycin C, kanchanamycin D, and bisacuberin in strain S1-SC3 is particularly significant due to their documented activity against both Gram-positive and Gram-negative pathogens, including Staphylococcus aureus, MRSA, Escherichia coli, and vancomycin-resistant Enterococci. Their presence in a mangrove-derived strain underscores a potentially untapped source of potent bioactive compounds, offering promising opportunities for discovering novel antibiotics essential in the fight against AMR.
3.4. MicrobeMASST
GNPS library searches using MicrobeMASST exclusively identified compounds produced by actinobacteria, primarily Streptomyces. The generated phylogenetic tree clearly illustrates the hierarchical structure from which the annotated compounds originate. The searches were conducted using the USI MS2 spectra deposited in the GNPS reference library, including desmethylenylnocardamine (CCMSLIB00004698381) (Figure 15S), desferrioxamine E (CCMSLIB00004695117) (Figure 16S), ferrioxamine E (CCMSLIB00005723618) (Figure 19S), desferrioxamine G (CCMSLIB00009918935) (Figure 18S), ikarugamycin epoxide (CCMSLIB00011906150) (Figure 17S), and deferoxamine (CCMSLIB00005435927) (Figure 20S). Results from the MicrobeMASST search clearly indicated that the annotated compounds were produced by known monoculture strains. The number of MS2 spectra deposited in the GNPS database is visually represented in pie charts, where the blue section indicates a match with a monoculture, while the yellow section signifies that it was not merged.
4. Discussion
Antibiotic resistance is spreading faster than the development of new antibacterial drugs for medical use, making it a major global cause of illness and mortality [35]. Although the emergence of antibiotic resistance in bacteria is a natural phenomenon, several factors exacerbate the crisis. These include the overuse of antibiotics, inappropriate prescribing practices, widespread agricultural use, weak or absent regulations on antimicrobial usage, regulatory barriers, and the lack of breakthroughs in novel antibiotic discovery [36]. Antibiotic resistance caused by the ESKAPE group of pathogens is linked to high mortality and morbidity rates, as well as a significant economic burden on affected individuals. Patients in intensive care units and those with underlying conditions are at the highest risk [37]. Although a few antibiotic classes have been commercialized in recent years, the last truly novel antibiotic classes were discovered in the 1980s. Despite four decades of research, no new classes have emerged, highlighting the urgent need for innovative strategies in antibiotic development [36]. Notably, while more than 4000 immuno-oncology agents are currently in the pipeline, only about 30–40 new antimicrobial compounds are in clinical trials. Most of these target the World Health Organization (WHO) priority pathogens and are derivatives of existing antibiotic classes [38].
Microbes thrive in diverse ecosystems, including air, soil, oceans, plants, and animals, making them a rich source of bioactive metabolites for drug discovery. While extensive screening during the “golden era” led to the belief that novel microbial compounds are scarce, discoveries continue to emerge. With only a fraction of bioactive metabolites identified, the potential for new breakthroughs remains vast [39]. Microbial genomes contain a vast, largely unexplored reservoir of genes responsible for the biosynthesis of bioactive compounds [40]. However, a major challenge in drug discovery is the frequent reisolation of known compounds, often resulting in limited or no new findings. To overcome this, researchers now integrate multiple strategies—including bioactivity screening, phylogenetic analysis, and dereplication—to rapidly assess the metabolomic profiles of selected strains before conducting in-depth investigations of specific microbes [40].
GNPS is a publicly accessible web-based mass spectrometry platform that provides various tools for MS2 data analysis. Its molecular networking feature facilitates compound annotation through spectral library searches and clusters detected ions into compound families [41]. In this study, we utilized GNPS to enhance the visualization of nontargeted tandem mass spectrometry (MS2) data. This approach allowed us to highlight structural similarities within the sample extract and improve the annotation of detected bioactive secondary metabolites (Figure 4) [42]. The dereplication method excluded known compounds efficiently, allowing us to prioritize novel bioactive strains. The molecular networking approach, implemented using molecular networking ID = e8d5e43b6b104702a07a1099a9644923 and MolNetEnhancer ID = bef431f45fd54f3da385a5a50410022 d) on 29-01-2024, resulted in the annotation of various classes of compounds. These included macrolactams (ikarugamycin epoxide), polyol macrolides (kanchanamycin C), ureylene-containing oligopeptides (chymostatin B and chymostatinol A), macrolides (elaiophylin), polycyclic ether antibiotics of the nigericin group (Grisorixin), and hydroxamate-type siderophores (desferrioxamine E, G, and D2), along with bisucaberin, ferrioxamine E, and dehydroxynorcardamine (Figure 5). Previous studies employing untargeted metabolomics via the GNPS platform have identified novel antibacterial compounds from mangrove-derived actinobacteria, offering valuable potential in the fight against AMR [40, 43].
Ikarugamycin epoxide (m/z 495.29 [M + H]+), a polyketide macrolactam antibiotic isolated from Streptomyces phaechromogenes, possesses potent antiprotozoal, antiamoebic, anticancer, and antibacterial properties [44]. Previous studies have reported that ikarugamycin epoxide possesses antibacterial activity, including potent effects against MRSA, underscoring its potential as a therapeutic agent in combating AMR [45]. Kanchanamycin C, a polyol macrolide antibiotic first isolated from Streptomyces olivaceus, has a parent mass of m/z 1054.64 ([M + H]+). A closely related analogs, likely formed through methylation, exhibits a mass shift of +Δ14 Da (m/z 1068.65 [M + H]+) (Figure 4). Previous studies on Kanchanamycin C have highlighted its broad-spectrum antibacterial activities against MRSA, suggesting its potential role in combating AMR [46]. Elaiophylin (m/z 1047.53 [M + H]+) was detected exclusively in the crude extract of strain S1-SC3. Originally isolated from Streptomyces melanophores, this 16-membered macrolide exhibits diverse and potent biological activities. Notably, its antibacterial properties highlight its potential as a valuable candidate in the ongoing fight against AMR [47, 48]. While desferrioxamine, bisucaberin, kanchanamycin C, and elaiophylin are not new compounds, their discovery in mangrove-derived Streptomyces suggests a unique ecological source for bioactive metabolites that may produce previously uncharacterized analogs with enhanced activity.
Siderophores are low-molecular weight chelating agents produced by bacteria, fungi, and plants to facilitate iron uptake [49]. They also support plant growth and defense against phytopathogens [50]. Notably, the research has shown their activity against Mycobacterium tuberculosis [51]. Desferrioxamine D2, isolated from Streptomyces nicoyae, exhibits antimicrobial activity against Escherichia coli [52]. Emerging studies suggest that desferrioxamines can serve as adjuvants alongside antimicrobial agents, offering a promising strategy to combat mycobacterial infections and address AMR [53]. While dereplication prioritized some of the strains and effectively excluded known compounds, it may have overlooked minor metabolites with significant bioactivity due to their low abundance. This limitation highlights the need for complementary approaches, such as bioassay-guided fractionation, to ensure the detection and characterization of these potentially valuable compounds in future studies. Additionally, all selected strains were cultivated on both agar and in broth to maximize metabolite diversity. Extraction was carried out separately from agar, broth, and mycelia to capture both intracellular and extracellular bioactive metabolites. The resulting extracts were then pooled to ensure comprehensive metabolite recovery. Moreover, during dereplication, molecular networking helps identify known metabolites while highlighting potential new bioactive compounds within molecular families. Unmatched or structurally distinct features suggest novel metabolites, contributing to the study’s novelty and potential antibacterial activity.
The results of this study reveal that the actinobacteria isolated from the Thai mangrove ecosystem belong to the Streptomyces genus (Table 3, Figure 5). The search for novel Streptomyces species has increasingly shifted toward underexplored environments such as mangroves [54]. However, research on their diversity in these ecosystems remains limited, with only 19 novel species reported between 2009 and 2019 across China, Malaysia, India, and Thailand. Given that mangrove-derived actinobacteria are prolific producers of bioactive compounds, these findings underscore their potential as a valuable source of novel bioactive compounds with possibly new mechanism of action to combat AMR [55]. Although the identified bioactive compounds demonstrate strong antibacterial activity in vitro, their effectiveness in clinical applications remains uncertain. Further pharmacokinetic and toxicity studies are essential to evaluate their safety, stability, and therapeutic potential for in vivo use.
5. Conclusion
AMR is a critical global health threat, emphasizing the urgent need for novel antimicrobial agents. This study aimed to screen actinobacteria isolated from the mangrove ecosystem in Thailand for putative bioactive metabolites with antibacterial activity. We employed a comprehensive workflow integrating bioactivity screening, phylogenetic analysis, and dereplication. 16S rRNA gene sequencing identified the selected actinobacteria as Streptomyces, a prolific producer of bioactive compounds. The metabolomic analysis revealed both known and novel bioactive metabolites, highlighting their potential as antibacterial agents. Our findings underscore antibacterial potential of mangrove-derived actinobacteria, positioning them as a promising source of unique bioactive metabolites. Future research should focus on evaluating these bioactive compounds in animal models to assess their safety and efficacy, as well as exploring their potential synergy with existing antibiotics to combat multidrug-resistant infections and collaborations with pharmaceutical companies for preclinical trials for the most promising bioactive compounds to combat AMR.
Ethics Statement
This study was approved by the Institutional Biosafety Committee, Walailak University. Ethical clearance was obtained from the Ethics Committee on biosafety Walailak University (WU-IBC-66-026).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Samson Cheruiyot Koech and Amit Jaisi performed all the experiments and data analysis. Atchara Paemanee performed the LCMS data acquisition. Samson Cheruiyot Koech, Wasu Pathom-aree, and Amit Jaisi drafted the manuscript. Phitchayapak Wintachai and Chonticha Romyasamit provided the tested pathogens. Wasu Pathom-aree provided the actinobacteria and other resources, data analysis, and conceptualization. Wasu Pathom-aree, Zdenek Kamenik, and Amit Jaisi edited and revised the manuscripts. Amit Jaisi conceived, designed, and acquired funding for the project. All authors read and approved the final manuscript.
Funding
This study was funded by the National Research Council of Thailand, under the Southeast Asia-Europe Joint Funding Scheme for Research and Innovation (Contract No. N10A650701 and N10A660580)and Walailak University international research collaboration scheme (WU-CIA-03506/2024). Zdenek Kamenik was supported by the Ministry of Education, Youth and Sports of the Czech Republic grant Talking microbes - understanding microbial interactions within One Health framework (CZ.02.01.01/00/22_008/0004597) and the project National Institute of Virology and Bacteriology (Programme EXCELES, ID Project No. LX22NPO5103) - Funded by the European Union - Next Generation EU.
Acknowledgments
The authors wish to express their gratitude for the additional partial financial support and scholarships provided by various organizations. Samson Cheruiyot Koech would like to thank Walailak University for Ph.D Scholarships for high potential candidates (Contract No. HP014/2021). Amit Jaisi would like to thank National Research Council of Thailand (Contract No. N10A650701 and N10A660580) and Walailak University international research collaboration scheme (WU-CIA-03506/2024). Zdenek Kamenik was supported by the Ministry of Education, Youth and Sports of the Czech Republic grant Talking microbes - understandingmicrobial interactions within One Health framework (CZ.02.01.01/00/22_008/0004597) and the project National Institute of Virologyand Bacteriology (Programme EXCELES, ID Project No. LX22NPO5103) - Funded bythe European Union - Next Generation EU.
Supporting Information
Figure 1S: Agar overlay assay of selected actinobacteria active against methicillin resistant Staphylococcus aureus PW01.
Figure 2S: Agar overlay assay of selected actinobacteria active against Acinetobacter baumannii PW01.
Figure 3S: Agar overlay assay of selected actinobacteria active against Klebsiella pneumonia PW01.
Figure 4S: Dehydroxynorcardamine (MS spectrum (A), and MS/MS fragmentations with sample query (Black) and GNPS database (Green).
Figure 5S: Desferrioxamine G, MS spectrum (A), and MS/MS fragmentations with sample query (Black) and GNPS database (Green).
Figure 6S: Kanchanamycin-C, MS spectrum (A), and MS/MS fragmentations with sample query (Black) and GNPS database (Green).
Figure 7S: Bisucaberin, MS spectrum (A), and MS/MS fragmentations with sample query (Black) and GNPS database (Green).
Figure 8S: Desmethylenylnocardamine MS Spectrum (A) and MS/MS fragmentations with sample query (Black) and GNPS database (Green).
Figure 9S: Ferrioxamine-B, MS spectrum (A), and MS/MS fragmentations with sample query (Black) and GNPS database (Green).
Figure 10S: Deferoxamine, MS spectrum (A), and MS/MS fragmentations with sample query (Black) and GNPS database (Green).
Figure 11S: Ferrioxamine-E, MS spectrum (A), and MS/MS fragmentations with sample query (Black) and GNPS database (Green).
Figure 12S: Desferrioxamine-E MS spectrum (A) and MS/MS fragmentations with sample query (Black) and GNPS database (Green).
Figure 13S: Elaiophylin, MS spectrum (A), and MS/MS fragmentations with sample query (Black) and GNPS database (Green).
Figure 14S: Ikarugamycin epoxide MS spectrum (A) and MS/MS fragmentations with sample query (Black) and GNPS database (Green).
Figure 15S: microbeMASST for desmethylnocardamine (CCMSLIB00004698381).
Figure 16S: microbeMASST for desferrioxamine E (CCMSLIB00004695117).
Figure 17S: microMASST for ikarugamycin epoxide (CCMSLIB00011906150).
Figure 18S: microbeMASST for desferrioxamine G (CCMSLIB00009918935).
Figure 19S: microbeMASST for ferrioxamine-E (CCMSLIB00005723618).
Figure 20S: microbeMASST for deferioxamine (CCMSLIB00005435927).
Open Research
Data Availability Statement
The data that support the findings of this study are available within the article and in the Supporting Information of this article.




