Antioxidative Stress Pathways of Amorphophallus oncophyllus Tuber Extract as a Gastroprotector Against Indomethacin-Induced Rat Gastric Ulcers
Abstract
Gastric ulcers can result from oxidative stress, which generates free radicals. Amorphophallus oncophyllus (A. oncophyllus) has been reported to possess strong antioxidant activity. This study aims to evaluate the antioxidative stress potential of A. oncophyllus tuber extract in preventing indomethacin-induced gastric ulcers in rats. In this study, 40 male rats were divided into five groups. The negative control group received 0.5% carboxymethyl cellulose (CMC) solvent, while the positive control group was given a single dose of indomethacin at 50 mg/kg BW. The remaining three groups were designated as treatment groups, where rats were administered A. oncophyllus tuber extract orally at doses of 100, 200, and 400 mg/kg BW, once daily for 14 days. On the 14th day, the treatment groups received a single dose of indomethacin at 40 mg/kg BW, 1 hour after administering the A. oncophyllus tuber extract. Four hours following indomethacin administration, the rats were euthanized for further analysis. Gastric tissues were collected for histological analysis and quantification of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPx) levels. Phytochemical screening of A. oncophyllus tuber extracts showed the presence of saponins, flavonoids, alkaloids, phenolics, and tannins. Antioxidant activity was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) test, which had an IC50 value of 157.21 μg/mL. A single dose of indomethacin at 50 mg/kg BW caused the formation of hemorrhagic lesions in the gastric mucosa, elevated MDA levels, and decreased SOD and GPx levels and led to necrosis of gastric mucosal epithelial cells. In contrast, the administration of A. oncophyllus tuber extract at a dosage of 400 mg/kg BW showed significant improvements in gastric ulcers, including reduced MDA levels, increased SOD and GPx levels, and decreased necrosis of gastric mucosal epithelial cells compared to the positive control group. However, no significant effects were observed at dosages of 100 mg/kg BW and 200 mg/kg BW. In conclusion, the findings indicate that A. oncophyllus tuber extract possesses strong antioxidant activity and has the potential as a gastroprotective agent for mitigating indomethacin-induced gastric ulcers in rats.
1. Introduction
Gastric ulcers are a common gastrointestinal disorder, affecting approximately 4.6 million people worldwide each year. Notably, this condition is associated with a fatality rate of one per 10,000 reported cases [1, 2]. Several etiological factors have been extensively linked to the development of peptic ulcers, including alcohol consumption, the use of nonsteroidal anti-inflammatory drugs (NSAIDs), psychological stress, and infection with Helicobacter pylori [3–7].
Oxidative stress, defined as an imbalance between the production of reactive oxygen species (ROS) and the body’s antioxidant defense mechanisms, plays a crucial role in various pathological conditions [8, 9]. Indomethacin is a NSAID known to induce oxidative stress through several mechanisms, including inhibiting cyclooxygenase enzymes and disrupting mitochondrial function. As a result, increased ROS production and reduced antioxidant capacity are observed following indomethacin exposure. This imbalance can cause cellular damage, trigger inflammation, and contribute to the development of conditions such as gastric ulceration [10–12]. The radicals mentioned above have been shown to play a significant role in microvascular injury and cellular necrosis. Oxidative stress disrupts the ability of antioxidants to neutralize ROS within gastric cells [13, 14]. Antioxidants, including enzymatic defenses (superoxide dismutase [SOD], glutathione peroxidase [GPx], and catalase), and nonenzymatic defenses (vitamin E), help mitigate oxidative stress by scavenging ROS and restoring redox balance [15–17]. The interplay between indomethacin-induced oxidative stress and the antioxidant defense system is critical for understanding both the drug’s therapeutic effects and its adverse consequences. An imbalance in this system may contribute to lipid peroxidation, leading to the destruction of proteins and DNA within gastric cells. This damage can ultimately result in cell death through apoptosis or necrosis [8, 18, 19].
As a direct consequence of lipid peroxidation, the measurement of malondialdehyde (MDA) concentrations serves as a reliable indicator of lipid peroxidation. The increase in MDA levels reflects the heightened peroxidation of lipid membranes, which is a well-established marker of oxidative stress [20–22]. In the context of indomethacin-induced injury, evidence suggests that the administration of antioxidants or the inhibition of free radical generation can have a protective effect on the gastric mucosa. Therefore, it is logical to use antioxidants as protective agents in such situations [10, 11, 13–15]. The cost-effectiveness, widespread availability, and lack of adverse effects make natural antioxidants a promising option [10, 12, 19].
Extensive research has been conducted to understand the processes underlying the pathogenesis of peptic ulcer disease. However, the etiology of peptic ulcers remains unclear due to the complexity of the condition. To elucidate the mechanisms of action of antipeptic ulcer medications, the rodent model of indomethacin-induced gastric ulcers is commonly used. These models have proven useful in evaluating the therapeutic efficacy of pharmaceutical interventions for peptic ulcers [20–22].
Herbal remedies are widely used for the prevention and treatment of various diseases, owing to the diverse phytochemical constituents found in herbal preparations, such as saponins, flavonoids, alkaloids, phenolics, glycosides, and tannins. Alkaloids, flavonoids, and tannins are the primary antioxidants identified in herbal medicines, according to previous research [23, 24]. Herbal antioxidant remedies, such as apple, Arthrospira maxima, cinnamon, and Ginkgo biloba are commonly used in this regard. These natural treatments are often employed as alternative exogenous antioxidants to protect rodents from indomethacin-induced gastric ulcers [10, 19, 20, 25]. The use of alternative remedies that harness the antioxidant properties of herbal is considered a promising approach for developing new pharmaceuticals aimed at preventing and treating gastric ulcers. This view is supported by numerous studies that consistently show these herbal antioxidants have no significant side effects [8, 20].
Available evidence suggests that Amorphophallus oncophyllus (A. oncophyllus) exhibits antioxidant properties [26–28]. Its biocompatibility, biodegradability, and excellent gelling and film-forming abilities contribute to the widespread use of Amorphophallus species as dietary supplements and additives in various industries, including food, cosmetics, biotechnology, and pharmaceuticals. Several studies have shown that A. oncophyllus possesses significant biological properties, including antioxidant, anti-inflammatory, antiasthma, anticough, antidiabetic, and anticancer effects [26–29]. Based on this, it was hypothesized that the tuber extract of A. oncophyllus could have a protective effect against gastric ulcers induced by NSAIDs through the modulation of oxidative stress–related processes. The objective of this study was to investigate the potential gastroprotective properties of the extract derived from the tuber of A. oncophyllus against indomethacin-induced stomach ulcers in rats, with a focus on its antioxidative stress mechanisms.
2. Materials and Methods
2.1. Preparation of A. oncophyllus Tuber Extracts by the Maceration Method
One hundred grams of dried powder samples of A. oncophyllus tubers were subjected to maceration in 1000 mL of ethanol for 3 days, with intermittent stirring, at ambient temperature. The extract underwent filtration using a glass funnel and Whatman filter paper number 1. The filtrate was obtained and subjected to concentration using a rotary evaporator (Büchi Labortechnik, Germany) under reduced pressure and controlled temperature within the range of 40°C–50°C to get the concentrated extract. The extracts were subsequently subjected to a drying process using a water bath set at a temperature of 40°C and were thereafter allowed to air dry at ambient temperature until achieving total dryness. During the extraction process, 1 kg of A. oncophyllus powder yields approximately 48.7 g of the extract. The desiccated extracts were preserved in hermetically sealed vials at ambient temperature until subsequent use [28, 29].
2.2. Qualitative Phytochemical Screening
The qualitative determination of phytochemical components in A. oncophyllus tuber extracts, such as alkaloids, terpenoids, steroids, saponins, flavonoids, and phenolics, was conducted using established standard procedures [8, 30].
2.2.1. Mayer’s and Dragendorff’s Test for Alkaloids
Three milliliters of NH3 were added to 100 mg of the extract, and the mixture was allowed to stand for 2 hours, forming two distinct layers. Subsequently, a volume of 5 mL of chloroform was introduced. The layers that had been disintegrated were divided into three different test tubes. Subsequently, the first, second, and third tubes were supplemented with Mayer’s, Wagner’s, and Dragendorff’s reagents, correspondingly. The precipitates observed in the experiment exhibited favorable results for alkaloids, displaying colors ranging from white, and yellow, to reddish-brown [8, 30].
2.2.2. Shinoda Test for Flavonoids
The sample (100 mg) was solubilized in methanol, followed by the addition of a mixture of Mg2+ powder and HCl solution in methanol (1:1). The presence of flavonoids can be inferred when seeing a red or purple tint [8, 30].
2.2.3. Liebermann–Burchard Test for Terpenoid and Steroid
The Liebermann–Burchard reagent was introduced to a solution containing 100 mg of the extract that had been dissolved in methanol. The presence of purple or red suggested the identification of terpenoids, whereas the presence of green or blue indicated the presence of steroids [8, 30].
2.2.4. Foam Test for Saponin
The sample (100 mg) was dissolved in methanol, subjected to heat, and violently agitated. The observation of foam development, with a duration of 30 min, served as an indication of the existence of saponins [8, 30].
2.2.5. Ferric Chloride Test for Tannins
Tannins were detected by adding 100 mg of the extract to 5% FeCl3 (5 drops). The presence of a dark blue or black color indicated the presence of tannins [8, 30].
2.3. Antioxidant Activity Test With 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)
The DPPH assay [27], using 95% ethanol as the solvent, a solution of DPPH with a concentration of 0.006% weight/volume (w/v) was prepared. To create a stock solution with a concentration of 1 mg/mL, the ethanol extract derived from A. oncophyllus tubers was combined with 95% ethanol. The experiment utilized freshly prepared DPPH solution, which was then transferred to test tubes. Then, samples were placed in separate test containers, and a series of dilutions (10, 20, 40, 80, and 160 g) were performed. After 30 min of incubation in the absence of light, the absorbance was measured using a UV–Vis spectrophotometer with a wavelength of 517 nm. The measurements were conducted with at least three duplicates. Using the same volume without extract, a control sample was prepared, and 95% ethanol was used as a blank solution. The following equation was used to determine the DPPH free radical scavenging efficiency: we calculate the percentage of the DPPH scavenging effect using the formula (Ao − A1)/Ao × 100. Here, Ao represents the absorbance seen in the control, and A1 represents the absorbance observed in the presence of the sample (A. oncophyllus tuber extract) [31].
2.4. Experimental Animals
For experimental purposes, male Wistar albino rats weighing around 200–250 g (2.5–3 months) were acquired from Gadjah Mada University in Yogyakarta, Indonesia. The subjects were contained within plastic enclosures situated in a climate-controlled chamber, where the temperature was consistently regulated at 26 ± 2°C. In addition, the subjects were exposed to alternating periods of light and darkness, each lasting 12 h. The rats were provided with tap water and were given unrestricted access to typical commercial rat food.
2.5. Ethical Approval
The present investigation was carried out in the Department of Pharmacology, Faculty of Veterinary Medicine, Airlangga University. The Ethical Clearance Committee for Preclinical Research, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia, approved all operations conducted in this study (ethical clearance no. 88/FKH, UA/6/2023).
2.6. Experimental Design
Forty male rodents were categorized into five distinct groups for this study. The initial cohort served as the experimental control and received a 0.5% sodium carboxymethyl cellulose (Na CMC) vehicle dose daily. The second group served as the positive control and received a single oral dose of indomethacin at 40 mg/kg body weight [28, 29]. The three remaining groups were classified as treatment groups and received oral doses of 100, 200, or 400 mg/kg BW of A. oncophyllus tuber extract once daily for 14 consecutive days [24, 30]. On the 14th day of the experiment, the treatment groups were additionally administered 40 mg/kg body weight of indomethacin orally, 1 hour after the administration of the A. oncophyllus tuber extract. The rats were euthanized 4 hours after receiving indomethacin orally. Consequently, stomach tissues were obtained to conduct histological evaluations and measure the levels of MDA, SOD, and GPx. The stomach tissues were subsequently deposited in a 10% neutral buffered formalin solution to facilitate histological examination.
2.7. Macroscopic Evaluation of Gastric Ulcer
- -
Control gastric ulcer is the total ulcerated area in a group not receiving the treatment (control group).
- -
Treated gastric ulcer is the total ulcerated area in a group receiving the treatment.
2.8. Measurement of MDA Level
The quantification of MDA was conducted in the supernatant of the homogenized stomach tissue by employing the thiobarbituric acid (TBA) technique, which is often used to assess the development of MDA. The measurement of MDA concentration was conducted at a wavelength of 532 nm, and the calculation was performed utilizing the absorbance coefficient of the MDA–TBA complex. The expression of 8 MDA was quantified as nanomoles of MDA per gram of tissue [8, 9].
2.9. Measurement of Antioxidant Enzymes
The rat stomach tissue designated for enzyme testing was rapidly thawed from a temperature of −70°C to the surrounding room temperature for 5 min. Following this, the tissue underwent hand homogenization using a cold phosphate buffer solution with a pH of 7.4. Undesirable particulate matter was removed using the process of centrifugation, employing the Centrifuge 5415 R, a product of Eppendorf AG located in Hamburg, Germany. The centrifugation was conducted at a force of 3500 g for 10 min. The liquid portions were gathered and afterward employed for the evaluation of enzyme activity and protein content [8, 9].
The evaluation of SOD activity was performed using a SOD detection reagent according to the manufacturer’s instructions (USA, Cat. no. 706002, Cayman Chemicals). This study employed a diagnostic assay to determine the presence of superoxide radicals, which are generated by the enzymatic activity of xanthine oxidase and hypoxanthine. The term “one unit of SOD” refers to the quantity of enzyme required to catalyze the 50% dismutation of the superoxide radical. Utilizing a plate reader, absorbance within the wavelength range of 440–460 nm was measured. The results are presented in micromoles per milligram of protein tissue for gastric tissue [8, 9].
The GPx activity was measured using a commercially available GPx detection reagent in conformance with the manufacturer’s recommended protocols (USA, Cat. No. 703102, Cayman Chemicals). A coupling reaction with glutathione reductase was utilized to evaluate the GPx activity indirectly. The reduction of hydroperoxide by GPx results in the formation of oxidized glutathione, as described in the preceding chemical reaction. The absorbance was measured using a plate reader set to 340 nm at a specific wavelength. The GPx activity in stomach tissue was determined by quantifying the GPx content in micromoles per milligram of protein tissue [8, 9].
2.10. Histopathological Examination
Following the conclusion of the investigation, the stomach tissues of all rats were subjected to fixation in a 10% formalin buffer solution and subsequently embedded in paraffin. A histological sample of 4 μm in thickness, obtained from the stomach tissue, was subjected to staining using hematoxylin and eosin. A light microscope was employed for the histopathological examination of the stomach tissue, focusing on identifying stomach cell necrosis. A scoring system was used to evaluate stomach histopathology as follows: no necrosis = 0, score of necrosis = 1 (necrosis < 25%), score of necrosis = 2 (necrosis 26%–50%), score of necrosis = 3 (necrosis 51%–75%), and score of necrosis = 4 (necrosis > 75%) [11, 32].
2.11. Statistical Analysis
The data are given in the form of means accompanied by their corresponding standard deviations. A post hoc analysis was conducted using a one-way analysis of variance (ANOVA), followed by statistical comparisons across the groups using the least significant difference (LSD) test. The statistical package program used for this analysis was SPSS Version 17.0.
3. Results
3.1. Phytochemical Tests of A. oncophyllus Tuber Extract
The phytochemical analysis of the tuber extract of A. oncophyllus using different organic solvents demonstrated the existence of alkaloids, flavonoids, steroids, triterpenoids, saponins, and tannins, as indicated in Table 1. The possible antioxidant properties of the A. oncophyllus tuber extract can be inferred from the presence of phytochemical components, including alkaloids, flavonoids, and tannins.
| No | Phytochemical compounds | A. oncophyllus tuber extract |
|---|---|---|
| 1 | Alkaloid | + |
| 2 | Flavonoid | + |
| 3 | Tannin | + |
| 4 | Saponin | + |
| 5 | Steroid | + |
- Note: Contains the substance examined (+).
3.2. Determination of A. oncophyllus Tuber Extract for Antioxidant Activity
The determination of the antioxidant activity of the tuber extract of A. oncophyllus was conducted using the DPPH assay. The selection of this particular approach was based on its attributes of simplicity, ease of use, and efficiency in quantifying antioxidant activity. Furthermore, it should be noted that DPPH necessitates minimal quantities of antioxidants derived from natural substances.
The antioxidant activity of the tuber extract of A. oncophyllus was evaluated using the DPPH test at various doses (10, 20, 40, 80, and 160 μ/mL), and the results are shown in Table 2. The antioxidant activity percentages of the A. oncophyllus tuber extract at the above concentrations were 1.66%, 10.71%, 17.69%, 55.13%, and 55.13%, respectively. The findings from the antioxidant activity assessment utilizing the DPPH technique indicated the presence of antioxidant activity in the extract derived from A. oncophyllus. The IC50 value obtained was 157.21 μg/mL.
| A. oncophyllus tuber extract | |||
|---|---|---|---|
| Concentration (μg/mL) | Absorbance | Antioxidant activity (%) | |
| 10 | 0.817 | 1.66 | IC50 = 157.21 μg/mL |
| 20 | 0.725 | 10.71 | |
| 40 | 0.685 | 17.69 | |
| 80 | 0.569 | 31.65 | |
| 160 | 0.3989 | 55.13 | |
3.3. Effect of A. oncophyllus Tuber Extract on Indomethacin-Induced Gastric Ulcer
The induction of stomach mucosal hemorrhagic lesions was considerably increased following the oral administration of indomethacin, particularly when pretreated with a solution containing 0.5% Na CMC (Figure 1). Administration of A. oncophyllus tuber extract at a dosage of 400 mg/kg BW demonstrated a substantial (p < 0.05) reduction in gastric damage, although dosages of 200 mg/kg BW or 100 mg/kg BW did not exhibit the same effect. This reduction in gastric injury was observed in a dose-dependent manner when compared to the group treated with indomethacin, as depicted in Table 3 and Figure 1. The tuber extract of A. oncophyllus demonstrated gastroprotective properties when administered at dosages of 100 mg/kg BW, 200 mg/kg BW, and 400 mg/kg BW, resulting in corresponding reductions of indomethacin-induced stomach ulceration by 8.2%, 21.6%, and 74.42%.
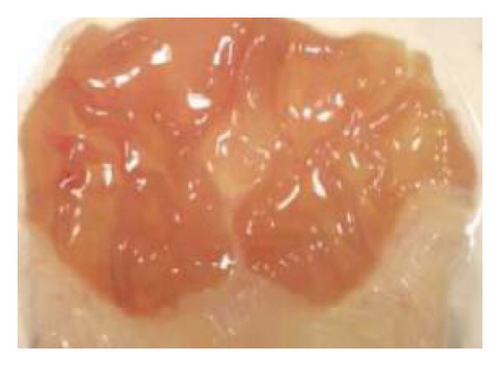
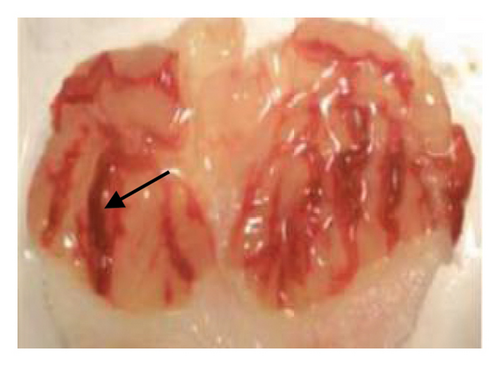
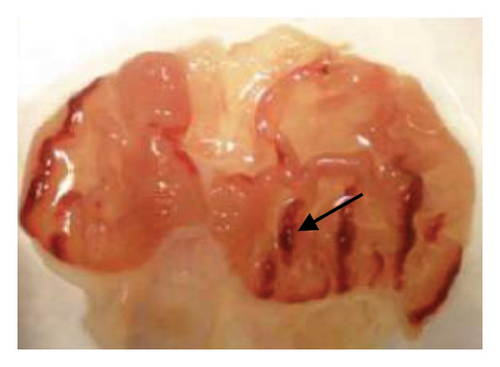
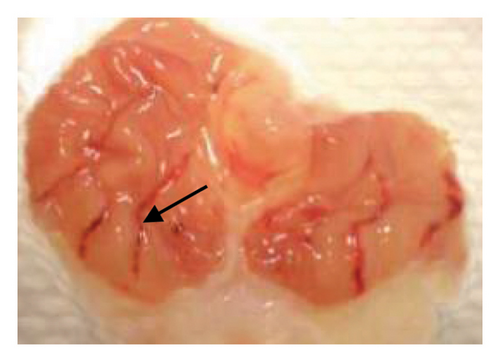
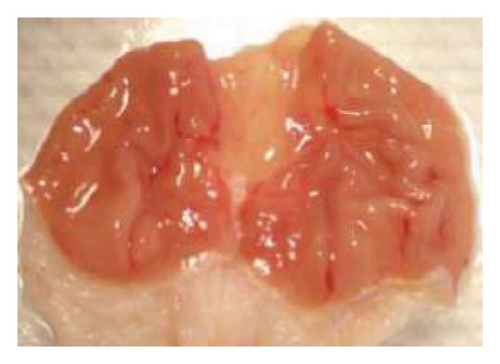
| Groups | Gastric ulcer | Relieve gastric ulcers (%) |
|---|---|---|
| Control | 0a ± 0 | — |
| Indomethacin 100 mg/kg BW | 4.3b ± 0.61 | — |
| A. oncophyllus 100 mg. BW | 3.8b ± 0.58 | 11.63 |
| A. oncophyllus 200 mg. BW | 3.1b ± 0.68 | 27.91 |
| A. oncophyllus 400 mg. BW | 1.1c ± 0.39 | 74.42 |
- Note:a,b,cDifferent superscripts within each column indicate a significant difference between the means (p < 0.05).
3.4. Effect of A. oncophyllus Tuber Extract on the Levels of MDA in Indomethacin-Induced Gastric Ulcers
The assessment of MDA production serves as a means to quantify the extent of oxidative stress, specifically stomach damage resulting from heightened ROS generation. Table 4 presents the findings about the effectiveness of A. oncophyllus tuber extract in modulating MDA levels within stomach tissue. The gastric tissue of control rats exhibited substantially lower levels of MDA compared to rats that were orally administered indomethacin (p < 0.05). Conversely, the administration of A. oncophyllus tuber extract resulted in a dose-dependent reduction of MDA levels in the gastric tissue.
| Groups | Means ± standard deviation MDA (nmol/mg tissue) |
|---|---|
| Control | 22.83a ± 1.86 |
| Indomethacin 100 mg/kg BW | 36.14b ± 2.76 |
| A. oncophyllus 100 mg.kg BW | 37.23b ± 3.21 |
| A. oncophyllus 200 mg.kg BW | 30.82b ± 2.67 |
| A. oncophyllus 400 mg.kg BW | 25.12c ± 2.42 |
- Note:a,b,cDifferent superscripts within each column indicate a significant difference between the means (p < 0.05).
3.5. Effects of A. oncophyllus Tuber Extract on the Levels of SOD and GPx in Indomethacin-Induced Gastric Ulcers
Table 5 displays the levels of SOD and GPx in gastric tissues across various treatment groups. SOD and GPx are endogenous antioxidant enzymes that effectively neutralize the presence of free radicals, thereby preventing the development of oxidative stress. After oral administration of indomethacin, the concentrations of SOD and GPx in the gastric tissue of rats were significantly decreased compared to the control group (p 0.05). In contrast, administration of A. oncophyllus tuber extract caused an indomethacin-induced dose-dependent increase in the levels of SOD and GPx in the gastrointestinal tissue of rats. The administration of 400 mg/kg body weight of A. oncophyllus tuber extract significantly increased the levels of SOD and GPx in the gastric tissue of indomethacin-induced rats (p 0.05).
| Groups | Means ± standard deviation | |
|---|---|---|
| SOD levels (U/mg protein) | GPx levels (U/mg protein) | |
| Control | 6.98a ± 0.44 | 59.0a ± 2.7 |
| Indomethacin 100 mg/kg BW | 3.76b ± 0.39 | 39.3b ± 2.3 |
| A. oncophyllus 100 mg.kg BW | 3.70b ± 0.35 | 38.2b ± 1.9 |
| A. oncophyllus 200 mg.kg BW | 3.95b ± 0.32 | 40.6b ± 1.7 |
| A. oncophyllus 400 mg.kg BW | 5.10c ± 0.29 | 45.9c ± 2.1 |
- Note:a,b,cDifferent superscripts within each column indicate a significant difference between the means (p < 0.05).
3.6. Effects of A. oncophyllus Tuber Extract on Indomethacin-Induced Gastric Ulcers in the Histopathological Study
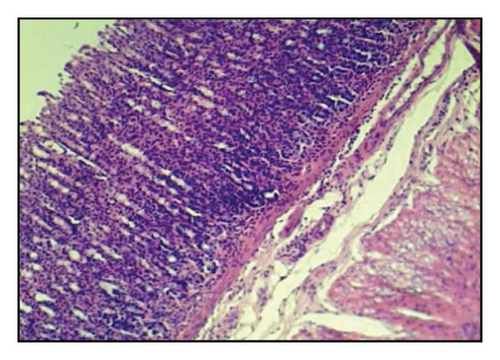
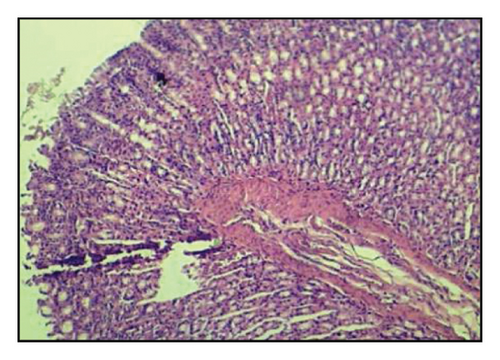
Table 6 and Figure 2 show that the histopathological examination was performed with the aid of light microscopy. A microscopic examination revealed that the negative controls contained normal gastric epithelium that appeared intact and neatly arranged. When indomethacin was administered to rats, epithelial cells appeared damaged and detached from the surface, and lost clear cell boundaries were observed in the necrotic epithelial cells of stomach tissues. The administration of 400 mg/kg BW of A. oncophyllus tuber extract before treatment significantly inhibited the necrosis of mucosal epithelial cells in rats treated with indomethacin. At concentrations of 200 mg/kg or 100 mg/kg BW, however, no significant effects were observed. As shown in Figure 2, the level of necrosis prevention attained by the 400 mg/kg BW dose was comparable to that of the control group. The results of the study suggest that the administration of A. oncophyllus tuber extract may mitigate the gastrointestinal side effects of indomethacin.
| Group | Means ± SD |
|---|---|
| Necrosis | |
| Control | 0.33a ± 0.52 |
| Indomethacin 100 mg/kg BW | 2.50b ± 0.55 |
| A. oncophyllus 100 mg.kg BW | 2.33b ± 0.52 |
| A. oncophyllus 200 mg.kg BW | 2.17b ± 0.41 |
| A. oncophyllus 400 mg.kg BW | 1.17c ± 0.75 |
- Note:a–cDistinct superscripts in each column indicate a significant difference from the mean (p < 0.05).
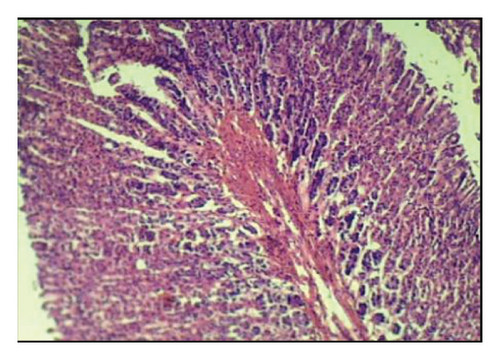
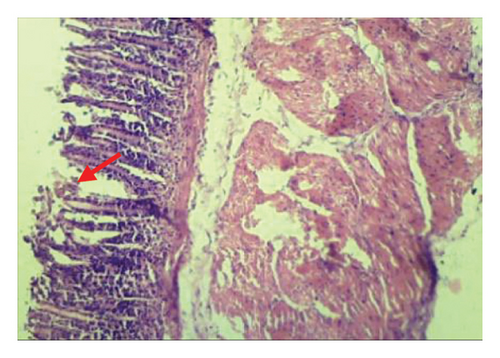
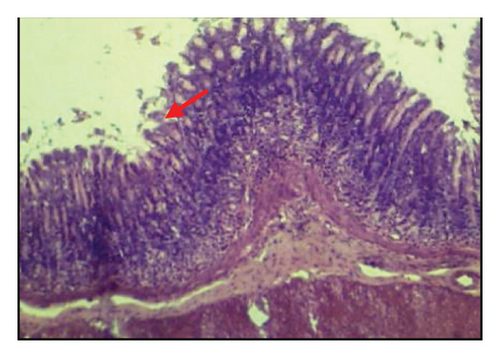
4. Discussion
Throughout history, herbal remedies have been extensively employed for the treatment of several ailments. The escalation in undesirable drug-induced side effects, such as the occurrence of ulcers, resulting from some drugs, has emerged as a significant impetus for individuals to explore alternative therapeutic approaches to discover more effective and secure methods for the prevention and treatment of ulcers. Medicines generated from plants have the potential to be more cost-effective, less toxic, or devoid of poisonous properties [8, 19, 20]. In recent years, there has been a growing interest in herbal medicine, leading to extensive research on its phytochemical screening to reveal the presence of alkaloids, flavonoids, saponins, tannins, and steroids. Prior research has established that alkaloids, flavonoids, and tannins are the primary antioxidants found in herbal products [23, 24].
The assessment of the antioxidant efficacy of the tuber extract of A. oncophyllus was conducted utilizing the DPPH assay. This particular approach was selected based on its simplicity, ease of use, and efficiency in evaluating antioxidant activity. Moreover, it should be noted that DPPH necessitates minimal quantities of antioxidants sourced from natural substances [31]. The findings from the DPPH method-based antioxidant activity test indicated the presence of antioxidant activity in the extract derived from the tuber of A. oncophyllus. The IC50 value for this extract was determined to be 157.21 μg/mL. The presence of alkaloids, flavonoids, and tannins in the A. oncophyllus tuber extract enables it to function as a scavenger of free radicals. This is due to the ability of the hydroxyl groups present in these compounds to donate hydrogen, preventing their conversion into radicals. According to several reports, herbal medications with antioxidant properties or the ability to block the production of free radicals are essential in safeguarding against stomach cell damage generated by indomethacin [19, 20, 25].
Indomethacin is frequently prescribed to treat pain, fever, and inflammation. However, prolonged use of indomethacin can result in the development of gastric ulcers due to an increase in the production of free radicals and a decrease in the levels of naturally occurring antioxidants [19, 20]. This study intended to evaluate the antioxidant activity of A. oncophyllus tuber extract in mitigating gastric tissue injury caused by indomethacin treatment in rats. This study demonstrates a significant decrease in SOD and GPx levels following indomethacin injection. In addition, levels of MDA increased significantly. Compared to the control group, it was discovered that indomethacin caused stomach cell necrosis in the histopathology group as well. The results of this study indicate that indomethacin administration results in an excessive production of ROS, which has detrimental effects on gastrointestinal tissues. It has been observed that prolonged exposure to indomethacin results in increased levels of MDA or lipid peroxidation, suppression of SOD and GPx activity, and the initiation of oxidative damage in the gastric mucosa. It has been discovered that indomethacin induces the production of ROS, including hydroperoxides, singlet oxygen, and hydrogen peroxide, leading to elevated levels of MDA as lipid peroxidation metabolites [19, 21, 22].
MDA levels in the gastrointestinal tissues of rats treated with indomethacin were significantly higher than in the control group, according to the findings of the present study. These results suggest that the administration of indomethacin to rats led to an increase in oxidative stress. Therefore, the observed decrease in MDA in the stomach tissues of groups treated with A. oncophyllus tuber extract versus those treated with indomethacin suggests a decrease in lipid peroxidation. The occurrence of oxidative stress and tissue injury generated by indomethacin can be attributed to the heightened generation of free radicals, leading to a direct depletion of antioxidant reserves [21, 22]. The administration of indomethacin can result in a substantial increase in lipid peroxidation, which can have detrimental effects on cytoplasmic membranes and mitochondria. The discharge of lipid hydroperoxides into the bloodstream as a result of this tissue injury indicates the presence of oxidative stress. This investigation revealed that the A. oncophyllus tuber extract possesses potent antioxidant properties and functions as a free radical scavenger. Rat stomach tissue levels of MDA were disrupted by indomethacin, but the extract effectively reduced these levels. The administration of A. oncophyllus tuber extract at a dose of 400 mg/kg mitigated the increase in MDA levels caused by indomethacin in rats. The results of this study suggest that the tuber extract of A. oncophyllus can reduce gastrointestinal cell injury caused by indomethacin, most likely due to its antioxidant properties. The antioxidant defense mechanism reduces ROS and scavenges free radicals responsible for gastrointestinal system injury. As a consequence, it inhibits lipid peroxidation, which can be measured by MDA levels [18, 19]. The decrease in levels of MDA under gastrointestinal conditions induced by indomethacin demonstrates that the extract derived from A. oncophyllus tubers can mitigate oxidative stress by reducing lipid peroxidation. Similarly, the administration of vitamins C and E has been found to increase the antioxidant capacity and prevent lipid peroxidation in rats with indomethacin-induced stomach cell damage. The findings of this study suggest that the antioxidant actions of vitamins C and E primarily target the lipid components of cellular structures [15–17].
Healthy gastric cells have innate enzymatic defense mechanisms that protect them from the detrimental effects of free radicals and ROS. The enzymatic defense system consists of the enzymes SOD and GPx. These enzymes effectively eliminate and neutralize ROS, such as superoxide, hydrogen peroxide, and hydroxyl radicals [13–15]. Cells can tolerate ROS in the presence of moderate oxidative stress. As oxidative stress intensifies, ROS and oxidative agents can interact with numerous cellular components, including DNA, lipids, proteins, and cell membranes. This interaction ultimately results in necrosis and apoptosis. As antioxidant enzymes, SOD and GPx protect cellular components from oxidative damage caused by ROS [8–10]. In the present study, treatment with indomethacin decreased the enzymatic activity of SOD and GPx in the gastrointestinal tissue of rats. The observed decrease in SOD and GPx activities following administration of indomethacin is consistent with previous research indicating that indomethacin induces oxidative stress by inhibiting the enzymatic activity of these antioxidants. A. oncophyllus tuber extract increased the activities of SOD and GPx in the gastrointestinal tissue of indomethacin-treated rats in a dose-dependent manner. The observed improvement can be attributed to the A. oncophyllus tuber extract’s ability to inhibit the formation of free radicals. A. oncophyllus tuber extract is effective at scavenging oxygen-derived free radicals, which inhibits the production of free radicals and provides gastrointestinal protection. Similar results have been observed in herbal antioxidants such as apple, Arthrospira maxima, cinnamon, and Ginkgo biloba, which can also be used as protection against indomethacin-induced gastric ulcers by decreasing MDA levels and increasing antioxidant activity [10, 19, 20, 25].
Indomethacin administration has been linked to histological changes in gastric tissue, according to prior research [11–13]. In the present study, histological examination of stomach sections from the group exposed to indomethacin revealed necrosis in the gastric mucosal epithelial cells, as opposed to the control group. Rats pretreated with A. oncophyllus tuber extract at a dose of 400 mg/kg body weight demonstrated a significant reduction in the extent of necrosis observed in the gastrointestinal mucosal epithelial cells. The presumed gastroprotective effect of A. oncophyllus tuber extract is attributable to its antiradical and antioxidant properties.
Additional research into the protective effects of A. oncophyllus tuber extract against stomach damage caused by indomethacin has the potential to substantially influence the development of clinically effective methods for treating patients with indomethacin-induced gastric ulcers. While the study was conducted using a rat model, which provides valuable insights due to physiological similarities with humans, this also represents a key limitation. The results obtained from animal models may not fully translate to human conditions due to species-specific differences in metabolism, immune responses, and behavior. In addition, the controlled experimental environment may not capture the complexity of human lifestyles or environmental exposures. However, this model still offers a strong foundation for understanding underlying mechanisms and generating hypotheses for future human studies. Further research, including clinical trials, is necessary to confirm the applicability of these findings to human health.
5. Conclusion
In conclusion, the results of the study indicate that the antioxidant effect of A. oncophyllus tuber extract can protect against gastric damage in rats induced by indomethacin, through decreasing MDA levels, increasing SOD and GPx levels, and significant differences in histological parameters. A. oncophyllus tuber extract can be used as a natural medicine to prevent gastric injury caused by NSAIDs such as indomethacin.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Rochmah Kurnijasanti, Mohd. Rais Mustafa, and Sri Agus Sudjarwo designed the experiments. Rochmah Kurnijasanti performed all experiments and analyzed the data. Giftania Wardani assisted in reagent preparation and data analysis. Sri Agus Sudjarwo supervised all experiments. Rochmah Kurnijasanti, Mohd. Rais Mustafa, and Sri Agus Sudjarwo analyzed data and wrote and edited the manuscript.
Funding
This research was supported by a grant from Universitas Airlangga, Surabaya, Indonesia.
Acknowledgments
This research was supported by a grant from Universitas Airlangga, Surabaya, Indonesia.
Open Research
Data Availability Statement
The article includes data that are used to support the study’s conclusions.




