Pharmacokinetic Properties of Once-Weekly Insulin Icodec in Children and Adolescents With Type 2 Diabetes
Abstract
Background: Young people with type 2 diabetes (T2D) often need basal insulin for diabetes management. Insulin icodec is a novel basal insulin designed for once-weekly administration.
Methods: This study investigated the pharmacokinetic properties of icodec in children and adolescents with T2D aged 10 to < 18 years. Eighteen insulin-treated (daily basal insulin dose ≥ 0.2 U/kg) participants (11 females/7 males; mean ± standard deviation body mass index 38.5 ± 7.1 kg/m2, HbA1c 7.5 ± 1.3%) received a single subcutaneous injection of icodec (5.6 U/kg). Blood samples for pharmacokinetic analysis were drawn frequently until 35 days postinjection. Observed single-dose pharmacokinetic profiles were extrapolated to steady state using pharmacokinetic modeling.
Results: One-week icodec pharmacokinetic profiles observed after a single dose and extrapolated to steady state suggest that icodec exposure covers the full weekly dosing interval in children and adolescents with T2D. After a single dose, geometric mean total exposure was 819∙105 pmol∙h/L [coefficient of variation (CV) 28.2%] and maximum concentration (Cmax) was 4.22∙105 pmol/L (CV 25.8%). Median time to maximum concentration (tmax) was 21 h (range 12–27 h). When extrapolated to steady state, geometric mean total exposure was 771∙105 pmol∙h/L (CV 29.9%) and maximum concentration was 6.35∙105 pmol/L (CV 27.3%). Median tmax was 15 h (range 13–19 h). In total, six adverse events (AEs) were reported in two participants. All AEs were mild and recovered/resolved. No serious or severe AEs, hypersensitivity or injection site reactions, or hyperglycemic episodes (plasma glucose > 14.0 mmol/L and blood ketones > 1.5 mmol/L) were reported. One level 1 hypoglycemic episode (plasma glucose < 3.9 and ≥ 3.0 mmol/L) was reported.
Conclusion: Icodec was safe and well-tolerated in the current study in children and adolescents with T2D. The pharmacokinetic properties of icodec support its potential for once-weekly administration in children and adolescents with T2D.
Trial Registration: ClinicalTrials.gov identifier: NCT05790681
1. Introduction
The incidence of type 2 diabetes (T2D) in youth is increasing worldwide [1]. Projections estimate that there will be between 30,000 and 84,000 youths with T2D in the USA by 2050 [2]. The Treatment Options for Diabetes in Adolescents and Youth (TODAY) study has shown that early tight glycemic control in youth-onset T2D can improve long-term glycemic control and reduce the risk of diabetes-related complications [3]. Glycemia is, however, above target in many young people with T2D when treated with lifestyle intervention alone or with oral glucose-lowering medication [4].
Insulin is highly effective in lowering blood glucose levels and remains part of treatment algorithms for youth onset T2D [5, 6]. However, the complexity of treatment and the need for daily injections are factors known to raise the burden of insulin therapy in T2D [7, 8]. Adherence to diabetes medication has been shown to decline over time in youth with T2D [9], and adherence is suboptimal in insulin-treated young adults with youth onset T2D [10]. The treatment burden for young people with T2D who require basal insulin may be mitigated by decreasing the frequency of basal insulin injections from once or twice daily to once weekly [11]. Improved adherence to treatment with once-weekly versus daily dosing has recently been observed for the subcutaneous injection of glucagon-like peptide-1 (GLP-1) receptor agonists in adults with T2D [12].
Insulin icodec is a new basal insulin developed for once-weekly administration [13]. Following subcutaneous dosing, absorption of icodec occurs into the circulation, where icodec links to albumin, thereby, basically creating a depot of inactive icodec that equilibrates with an active, but considerably smaller free pool [14]. Free icodec is able to stimulate the insulin receptor, albeit with a low binding affinity, giving rise to slow clearance and extended glucose-lowering [14]. In adults with T2D, icodec was shown to have pharmacokinetic and pharmacodynamic properties suitable for once-weekly administration [14–16]. The efficacy and safety of once-weekly icodec were demonstrated in phase 3a trials in adults with T2D [17–21]. Until now, no studies have investigated insulin icodec in the pediatric population.
The main objective of the current study was to investigate the pharmacokinetic properties of once-weekly icodec in children and adolescents with T2D aged 10 to <18 years, as observed after a single dose and extrapolated to steady state. Furthermore, the pharmacokinetics of icodec shown in the current study in children and adolescents with T2D were compared with those previously seen in adults with T2D.
2. Materials and Methods
2.1. Ethics
The study was approved by the health authorities (US Food and Drug Administration) and by local institutional review boards (WCG Institutional Review Board, Princeton, NJ, ID 20223823; Pennington Biomedical Research Center Institutional Review Board, Baton Rouge, LA, ID 2022-044-PBRC; Cincinnati Children’s Hospital Medical Center Institutional Review Board, Cincinnati, OH, ID 2022-0898; Johns Hopkins Medicine Institutional Review Board, Baltimore, MD, ID IRB00357803; and Baptist Health Institutional Review Board, Jacksonville, FL, ID 22-176). It was carried out in line with the Declaration of Helsinki [22], Good Clinical Practice [23], and regulatory guidance on clinical studies in the pediatric population [24, 25]. Prior to any study-related activities, the children (10–11 years) provided written or oral informed assent, while the adolescents (12 to <18 years) provided written informed assent. Age-specific assent forms were used (one for 10–11 years and one for 12 to <18 years). Parents or legally accepted representatives (LARs) of the children and adolescents provided written informed consent. Participants were compensated for their participation in the study.
2.2. Study Design and Participants
This was a one-period, single-dose study (Figure 1) conducted at seven clinical study sites in the USA: Yale New Haven Hospital, New Haven, CT; Pennington Biomedical Research Center, Baton Rouge, LA; Children’s Hospital Los Angeles, Los Angeles, CA; Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Johns Hopkins Hospital, Baltimore, MD; Nemours Children’s Health, Jacksonville, FL; and the University of Virginia School of Medicine, Charlottesville, VA.

To be eligible for participation in the study, individuals were 10 to <18 years of age, had a diagnosis of T2D at least 30 days before screening, and glycated hemoglobin (HbA1c) ≤ 10.0% (86 mmol/mol). Eligible participants were treated with basal insulin, premixed insulin, or continuous subcutaneous insulin infusion (CSII), with or without bolus insulin or noninsulin glucose-lowering medication. Their current daily basal insulin dose was at least 0.2 U/kg body weight and stable for at least 30 days before screening (dose adjustments of ± 25% were allowed). Screened individuals with any disorder that could jeopardize participant safety or interfere with study procedures as assessed by the investigator, including hypoglycemic unawareness or recurrent severe hypoglycemia, those with a diagnosis of type 1 diabetes or maturity onset diabetes of the young, and pregnant females were considered ineligible for participation. There were no inclusion or exclusion criteria related to body weight or body mass index.
2.3. Procedures and Assessments
The study comprised a screening visit (3–21 days prior to dosing), a treatment visit followed by a 5-week observation period including three posttreatment assessment visits, and an end-of-study visit.
At the treatment visit, participants were admitted to the clinical study sites to receive a single dose of icodec (5.6 U/kg body weight; 700 U/mL; Novo Nordisk, Bagsværd, Denmark). Since the accumulation ratio for icodec exposure from single dose to steady state is approximately 2, an icodec single dose of 5.6 U/kg body weight corresponds to a once-weekly dose of 2.8 U/kg body weight at steady state, equivalent to a daily dose of 0.4 U/kg body weight. Thus, the chosen icodec dose corresponds to recommendations for the basal insulin starting dose in the pediatric population of 0.25–0.5 U/kg body weight per day [5, 6] and is also in line with the median daily insulin dose in youth with T2D in the USA of 0.5 U/kg body weight [26]. The icodec single dose was administered subcutaneously into a lifted skin fold of the thigh by trained site staff using a 3 mL PDS290 prefilled pen-injector (Novo Nordisk) at 18 : 00 h ± 5 h. Participants were discharged from the clinical study sites at 48 h after icodec administration but could remain at the clinical study site for safety reasons if deemed necessary by the investigator. During the 48-h in-house period, the participants were served meals and snacks appropriate for their body weight and appropriate for individuals with diabetes. As the main aim of this study was to assess icodec pharmacokinetics, there were no food or snack restrictions during the study. After the in-house period, participants were followed during outpatient visits until 5 weeks postdose.
The participants’ prestudy insulin treatment was washed out prior to icodec administration. The last dose of basal insulin was taken at least 48 h before the icodec dose for insulin degludec and insulin glargine U300, at least 24 h before for insulin glargine U100 and insulin detemir, and at least 12 h before for Neutral Protamine Hagedorn insulin or premixed insulin. For participants using CSII, the pump was stopped at least 6 h before the icodec dose. For participants using bolus insulin (fast-acting insulin analogs or regular human insulin), the last bolus was taken at least 6 h before the icodec dose. Treatment with noninsulin glucose-lowering medication could be continued throughout the study. In case of hyperglycemia during the prestudy insulin washout period or during the 48-h inhouse stay following icodec administration, participants could receive bolus doses of insulin aspart (NovoLog; 100 U/mL; Novo Nordisk) as determined to be necessary by the investigator and administered subcutaneously into a lifted skinfold on the abdomen using a 3 mL FlexPen prefilled pen-injector (Novo Nordisk). Based on frequent blood glucose measurements during the 48-h in-house period, while taking the type of prestudy insulin treatment into account, the investigator guided the participants and their parents or LARs on how to safely return to their prestudy insulin treatment.
Sampling of blood for pharmacokinetic evaluation was carried out predose and 1, 3, 6, 12, 15, 18, 21, 24, 27, 30, 36, 42, 48, 96, 168, 504, and 840 h postdose. The concentration of icodec in serum was measured by a validated icodec specific immunoassay, as previously described [16].
The safety of icodec was evaluated based on recording of adverse events (AEs). Hypoglycemic episodes were classified as—level 1 [hypoglycemia alert value; plasma glucose < 3.9 mmol/L (<70 mg/dL) and ≥ 3.0 mmol/L (≥54 mg/dL)], level 2 [clinically significant hypoglycemia; plasma glucose < 3.0 mmol/L (< 54 mg/dL)], and level 3 [no specific plasma glucose threshold; severe cognitive impairment requiring external assistance for recovery] [27]. Hyperglycemic episodes [defined as plasma glucose > 14.0 mmol/L (> 250 mg/dL) and blood ketones > 1.5 mmol/L], clinical laboratory safety measurements, vital signs, physical examinations, and electrocardiograms, were also recorded. AEs, hypoglycemic episodes, and hyperglycemic episodes were collected throughout the study until the end-of-study visit and were defined as treatment-emergent if they occurred from icodec administration until 1 week postdose. Clinical laboratory safety measurements vital signs, physical examinations, and electrocardiograms were carried out at the screening and end-of-study visits.
2.4. Statistics
The primary endpoints were the total exposure (AUC0−∞,SD), the maximum observed concentration (Cmax,SD), and the time to maximum observed concentration (tmax,SD) after a single dose of icodec. The study was descriptive in nature, and the number of required participants was set to 16 without performing any formal sample size calculation. In a single-dose study with the once-daily basal insulin degludec in children and adolescents with type 1 diabetes, where participants received a fixed dose of insulin degludec (0.4 U/kg, corresponding to 2.8 U/kg for a once-weekly insulin), the observed standard deviations for log transformed AUC0−∞,SD and Cmax,SD were 0.43 and 0.35, respectively [28]. Assuming that the standard deviation for the log transformed AUC0−∞,SD was 0.40 in the current study, a total of 16 evaluable participants would imply that the 95% confidence interval (CI) for the geometric mean AUC0−∞,SD would range from 0.77 to 1.30 times the geometric mean with 90% probability. This was considered sufficiently accurate to fulfill the objective of the study. To ensure at least 16 evaluable participants, a total of 18 individuals were included in the study.
SAS Version 9.4 (SAS Institute, Cary, NC, USA) and R Version 4.3.1 were used to carry out the statistical procedures. AUC0−∞,SD, Cmax,SD, and tmax,SD were derived as previously described [16] and presented by summary statistics. Safety parameters were also evaluated using summary statistics. For AEs, hypoglycemic episodes, and hyperglycemic episodes, only treatment-emergent events were summarized.
2.5. Pharmacokinetic Modelling
Each participant’s serum icodec concentration-time profile was simulated at steady state based on the observed single-dose data using a one compartment pharmacokinetic model, as previously described [29]. Once-weekly dosing of icodec at 5.6 U/kg for 10 weeks was simulated, and total exposure, maximum concentration, and time to maximum concentration at steady state (AUCτ,SS, Cmax,SS, and tmax,SS) were derived for each participant and summarized by descriptive statistics.
3. Results
3.1. Participant Disposition and Baseline Characteristics
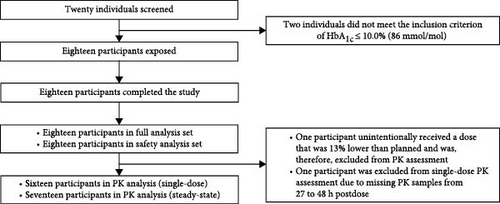
In total, 20 individuals were screened between 25 April 2023 and 13 December 2023, and 18 were exposed to a single dose of icodec (Figure 2). All participants completed the study. Baseline characteristics are provided in Table 1.
| Parameter | N = 18 |
|---|---|
|
|
- Note: Data are mean ± standard deviation unless otherwise stated.
- Abbreviations: BMI, body mass index; CSII, continuous subcutaneous insulin infusion; GLP-1, glucagon-like peptide-1; HbA1c, glycated hemoglobin; N, number of participants; SGLT2, sodium-glucose cotransporter-2.
3.2. Pharmacokinetics
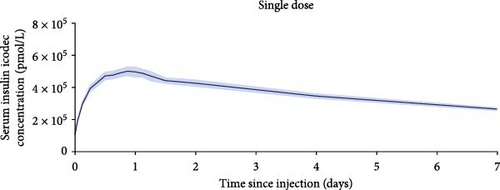
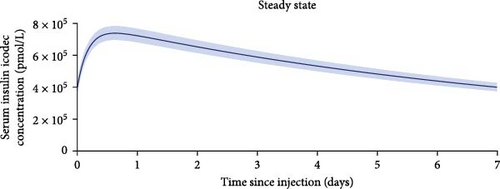
One-week pharmacokinetic profiles of icodec observed after a single dose and extrapolated to steady state are provided in Figure 3 and suggest that icodec exposure covers the full 1-week dosing interval at steady state in children and adolescents with T2D. Total exposure, maximum concentration, and time to maximum concentration after a single dose and at steady state are presented in Table 2.
| Endpoint | Children and adolescents | Adults |
|---|---|---|
|
|
|
- Note: Data are for a fixed icodec dose level of 5.6 U/kg. Adult data are from a previous study with similar overall methodology [16] under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/). AUC and Cmax endpoints are shown as geometric mean (CV%), and tmax endpoints are shown as median (minimum–maximum). Children and adolescents: N = 16 for SD endpoints and N = 17 for SS endpoints. Adults: N = 23.
- Abbreviations: AUC, area under the curve; Cmax, maximum concentration; CV%, coefficient of variation in percent; N, number of participants; ND, not determined; SD, single dose; SS, steady state; T2D, type 2 diabetes; τ, dosing interval of 1 week; tmax, time to maximum concentration; U, units.
- aSince extrapolation to SS has in theory no effect on total exposure, AUCτ,SS was not simulated in the study in adults but considered to be similar to the observed AUC0−∞,SD. In the current study in children and adolescents, where fewer pharmacokinetic blood samples were taken and more data points were missing, it was chosen to simulate AUCτ,SS. The difference between the observed AUC0−∞,SD and the simulated AUCτ,SS is due to the approximation of the model to the observed data and the difference in number of participants for SD (N = 16) and SS (N = 17).
3.3. Safety
In total, six AEs were reported in two participants. All AEs were mild and were recovered/resolved at the end of the study. Of the AEs, four were evaluated by the investigator as possibly related to study product (ageusia, decreased appetite, headache, and nasal congestion), and two were evaluated as unlikely related (headache and oropharyngeal pain). There were no serious or severe AEs, hypersensitivity or injection site reactions, or hyperglycemic episodes. One level 1 hypoglycemic episode was reported one day after icodec administration. There were no clinically significant findings in safety laboratory parameters, vital signs, physical examination, or electrocardiogram.
4. Discussion
The main findings of the present study were that icodec exposure observed after a single dose and extrapolated to steady state covered the full one-week dosing interval and that icodec was safe and well-tolerated in children and adolescents with T2D.
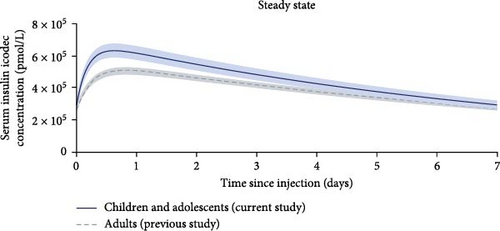
The pharmacokinetics of icodec were previously determined in adults with T2D [16] in a study with similar overall methodology, and where participants received the same single dose of 5.6 U/kg, as in the current study. Pharmacokinetic results from the two studies were, therefore, compared. Figure 4 shows that the shape of the simulated steady-state pharmacokinetic profile in children and adolescents in the present study was essentially similar to that observed in adults. However, the overall exposure level of icodec was slightly higher in children and adolescents than in adults (Table 2). Following absorption into the circulation of a given subcutaneous dose of icodec, its exposure is determined by the clearance. The main route of insulin clearance from the circulation is via binding to the insulin receptor, and subsequent internalization and degradation, which occurs in connection with insulin signaling [30, 31]. Therefore, low insulin sensitivity may lead to higher exposure for a given icodec dose. It is well-known that insulin sensitivity decreases markedly during puberty [32, 33]. Furthermore, mean BMI was somewhat higher in the children and adolescents in the current study (38.5 kg/m2) relative to the BMI in adults in the previous study (30.7 kg/m2) [16], which is also likely to contribute to lower insulin sensitivity in the group of children and adolescents compared to adults [34]. Thus, the slightly higher icodec exposure in the group of children and adolescents in the present study compared to adults in the previous study [16], may potentially be explained by lower insulin sensitivity in the children and adolescents compared to adults. An additional potential explanation could be that during childhood and adolescence, drug absorption and distribution may be affected by continuous growth and developmental changes in various compartments of the body [24, 35]. However, it is important to note that icodec, as with all other insulin products, should be titrated according to individual needs. Therefore, any modestly greater icodec exposure at younger age is considered of limited clinical relevance.
Given the similar pharmacokinetic properties of icodec in children and adolescents versus adults with T2D, in terms of the shape of the pharmacokinetic profile, together with the fact that icodec should be individually titrated, it would be expected that the efficacy and safety profile of icodec in children and adolescents with T2D is comparable to that observed in adults with T2D. In a phase 3a trial in insulin-naïve adults with T2D, HbA1c reduction was superior for once-weekly icodec versus once-daily glargine U100 after 52 weeks of treatment [17]. In another phase 3a trial in insulin-naïve adults with T2D, HbA1c reduction was superior for once-weekly icodec versus once-daily degludec after 26 weeks of treatment [19]. In both trials, the rates of combined level 2 or 3 hypoglycemic episodes were low for all treatments (i.e., less than one hypoglycemic episode per patient-year of exposure) [17, 19]. In a 26-week phase 3a trial in insulin-treated adults with T2D switching from once- or twice-daily basal insulin in a basal-only regimen, once-weekly icodec resulted in superior HbA1c reduction versus once-daily degludec [18]. In a 26-week phase 3a trial in insulin-treated adults with T2D switching from once-daily basal insulin in a basal-bolus regimen, the HbA1c reduction was noninferior for once-weekly icodec versus once-daily glargine U100 [20]. In both these basal switch trials, rates of combined level 2 or 3 hypoglycemic episodes did not differ statisticaly significantly for icodec versus degludec or glargine U100 [18, 20].
In the TODAY 2 observational study, which is a follow-up study to the TODAY clinical trial, individuals with youth onset T2D had a high risk of diabetes-related complications, with the majority affected by young adulthood [36]. These data emphasize the urgent need for improved treatment options in children and adolescents with T2D. Until recently, oral metformin and injectable insulin were the only approved pharmacological treatment options for the management of youth onset T2D. These have now been supplemented with a few alternatives including GLP-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors [5, 6]. The therapeutic options available are, however, still limited [37]. Given the suboptimal treatment adherence in insulin treated young people with T2D [10], once-weekly basal insulin could be a valuable addition to the treatment armamentarium. Compared to once- or twice daily basal insulin, a once-weekly regimen would reduce the number of basal insulin injections from at least 365 to just 52 per year. Relieving the complexity and burden of therapy through fewer injections is likely to increase treatment adherence and improve glycemic control, as previously seen in adults with T2D receiving once-weekly as compared to daily injectable GLP-1 receptor agonist therapy [12]. Thus, in comparison to the current daily basal insulin products, once-weekly icodec could have potential as a simpler treatment option to provide basal insulin coverage in children and adolescents with T2D.
The characteristics of the recruited participants with respect to sex, ethnicity, and race, generally reflect the population of youth with T2D in the USA. It should, however, be noted that the present results only directly apply to the studied population of children and adolescents with T2D and overweight/obesity and may not be generalizable to children and adolescents with T2D with other characteristics than those of the recruited participants. Another limitation of the current study was that participants received only one fixed dose of icodec. However, a multiple-dose design was not considered possible with a once-weekly insulin in this vulnerable population of children and adolescents. Rather, the simple study design and the use of modeling to extrapolate the pharmacokinetic properties of icodec to steady state helped ease the burden on the participants. Likewise, the pharmacodynamics of icodec were not assessed in this study as it would not have been feasible to conduct a glucose clamp in children and adolescents of the duration required to obtain meaningful pharmacodynamic results for a once-weekly insulin. However, the shape of the icodec pharmacokinetic profile was shown to be highly similar between the pediatric population and adults with T2D (Figure 4). This is, therefore, also expected to be true for the glucose-lowering effect profile, which is considered important given that insulin should always be individually titrated. Finally, considering the challenges associated with recruitment of children and adolescents with T2D into clinical trials [38–40], it was positive that recruitment in the current study was completed within nine months. Factors contributing to the relatively rapid enrolment included the wide eligibility criteria, the less burdensome study procedures, and the relatively large number of clinical study sites for the number of participants enrolled.
5. Conclusion
Icodec was safe and well-tolerated in the current study. The pharmacokinetic properties in children and adolescents with T2D were comparable to those previously observed in adults with T2D. The findings of the current study support the potential of icodec to provide basal insulin coverage with once-weekly administration in children and adolescents with T2D.
Disclosure
Results from this study were presented at the 24th Annual Diabetes Technology Meeting, October 15–17, 2024, Burlingame, CA, USA.
Conflicts of Interest
Björg Ásbjörnsdóttir, Mikel Murphy Gomes, Keerthana Udupa, Siri Vinther and Rasmus Ribel-Madsen are employees and shareholders of Novo Nordisk. Paula M Hale is a shareholder of Novo Nordisk and was an employee of Novo Nordisk at the time of study conduct. Michelle Van Name has received research support from MannKind, Novo Nordisk and Provention Bio and consulting honoraria from Novo Nordisk and Soleno Therapeutics. Risa M Wolf has received research support from Eli Lilly and Novo Nordisk.
Author Contributions
Michelle Van Name and Risa M Wolf contributed with study design, data collection, critical manuscript revision and final manuscript approval. Björg Ásbjörnsdóttir, Mikel Murphy Gomes, Keerthana Udupa and Siri Vinther contributed with data analysis, critical manuscript revision and final manuscript approval. Paula M Hale and Rasmus Ribel-Madsen contributed with study design, data analysis, critical manuscript revision and final manuscript approval.
Funding
This research was supported by Novo Nordisk.
Acknowledgments
The authors would like to thank Daniel S Hsia, Lily C Chao, Amy S Shah, Matthew R Benson and Christine M Burt Solorzano for being Principal Investigators in this study. Medical writing support was provided by Carsten Roepstorff, PhD, CR Pharma Consult, Copenhagen, Denmark, funded by Novo Nordisk.
Open Research
Data Availability Statement
Individual participant data will be shared in data sets in a de-identified/anonymized format. Data sets from Novo Nordisk sponsored clinical research completed after 2001 for product indications approved in both the EU and US will be shared. Study protocol and redacted Clinical Study Report will be available according to Novo Nordisk data sharing commitments. The data will be available permanently after research completion and approval of product and product use in both EU and US. There is no end date. Data will be shared with bona fide researchers submitting a research proposal requesting access to data for use as approved by the Independent Review Board according to the Independent Review Board Charter (see novonordisk-trials.com). The data will be made available on a specialized SAS data platform.




