Incidence Trends of Type 1 Diabetes in Estonian Children (1983–2022) and the Impact of the COVID-19 Pandemic
Abstract
Background: The annual incidence rate (IR) of childhood-onset type 1 diabetes mellitus (T1DM) among Estonian children under 15 years of age was 12.6 cases per 100,000 between 1983 and 2006, with the highest IR occurring in the 10–14-year-old age group. Notably, the 0–4-year-old age group saw the most significant annual increase, at 9.3%.
Objective: This study aims to determine the incidence and trends of T1DM among Estonian children from 1983 to 2022, focusing on the period from 2007 to 2022. Additionally, the study evaluates the impact of the COVID-19 pandemic on the incidence of T1DM in this population.
Subjects and Methods: This retrospective cohort study gathered data from 2007 to 2022 using population-based registries from Estonia’s paediatric medical centers. Preceding data were sourced from previous publications. The subjects were divided into three age groups, and the study period was segmented into five time periods, each spanning 8 years.
Results: From 2007 to 2022, 1104 new T1DM cases were diagnosed in children under 15 years in Estonia, with a crude IR of 32.9 per 100,000 persons per year (95% confidence interval [CI] 30.9–34.8). The highest incidence was in the 10–14-year age group (40.5 per 100,000 per year) (95% CI 36.7–44.3). On average, the incidence grew by 1.6% during this time period for all age groups combined. The most significant growth was in the 5–9-year-old group, with an annual increase of 2.9% (relative risk [RR] 1.23, 95% CI 1.0–1.5). The highest IR ever recorded in Estonia was in 2021, during the highest fatality year of COVID-19, at 45.8 per 100,000 (95% CI 26.9–54.8).
Conclusions: The incidence of childhood-onset T1DM in Estonia continues to rise, with a notable spike in 2021 during the peak of the COVID-19 pandemic.
1. Introduction
Type 1 diabetes mellitus (T1DM) incidence rates (IRs) in children under 15 years remain among the highest in Europe compared to other parts of the world [1]. In 2021, Europe’s average IR was 24.7 per 100,000 persons, a much higher number than, for example, that of the West Pacific at 0.1 per 100,000 persons [2]. Patterson [3] found that between 1989 and 2013, the amount of new T1DM cases increased on average by 3.4% per year in children across 26 European countries. The United States has been experiencing a slower growth rate of 1.8% per annum compared to Europe, while China’s IR is growing more rapidly at 12% per annum [3]. However, in some Western countries, the T1DM growth rates are decelerating, suggesting a plateau trend [4–6].
Previously in Estonia, the epidemiology of childhood onset T1DM has been studied in three 8-year time periods [7, 8]. In 1983–1990, the IR was 10.1 per 100,000 per year; in 1991–1998, it was 12.2 per 100,000 per year; and in 1999–2006, it was 16.9 per 100,000 per year. Between the first two time periods (1983–1990 and 1991–1998), the IR grew 2.6% annually, whereas between the second two time periods (1991–1998 and 1999–2006), the growth rate almost doubled, with 4.8% annually. During the first two time periods, the incidence was consistently the highest among children aged 10–14 years. Additionally, the incidence increased most rapidly in the youngest age group at 15.1% growth annually. However, during the third time period (1999–2006), it was the 5–9-year-olds who had the highest IR at 21.2 per 100,000 and the greatest growth rate with 12.7% increase annually.
The impact of the COVID-19 pandemic on the incidence of T1DM in children is inconsistent worldwide. Ireland reported a 21% increase in the IR [9], whereas Denmark saw no changes [10] during the pandemic. However, a multicentre study found a significant increase in the incidence of diabetic ketoacidosis (DKA) in children across 13 Western countries during the COVID-19 pandemic [11].
The aim of this retrospective cohort study was to calculate the incidence trends of type 1 diabetes among Estonian children from 1983 to 2022, split into five 8-year time periods, with special emphasis on 2007–2022. Additionally, there was an interest to analyse the impact of the COVID-19 pandemic on the incidence of childhood-onset diabetes in Estonia.
2. Methods
2.1. Data Collection
All children under 15 years who are diagnosed with T1DM in Estonia are referred to a paediatric endocrinologist either at the Children’s Clinic of Tartu University Hospital or Tallinn Children’s Hospital for the confirmation of diagnosis and further management. The incidence data from 2007 to 2022 was collected from these Estonian hospital registries. A previous study by Teeäär [8] confirms the accuracy of our sample. Using a mark–recapture method, they compared the number of insulin prescriptions issued by the Estonian Health Insurance Fund with the number of diagnosed T1DM cases from the registries of the aforementioned hospitals. The comparison found that these registries covered at least 98% of all children in Estonia with insulin prescriptions.
The remaining data from 1983 to 2006 were collected from research published in the journals Diabetologia and Pediatric Diabetes [7, 8].
2.2. Statistics
The study sample was divided into three age groups (0–4.9, 5.0–9.9 and 10.0–14.9 years old), in keeping with other relevant research papers on this topic [4–6, 8]. The data were grouped into five 8-year time periods (I 1983–1990; II 1991–1998; III 1999–2006; IV 2007–2014; and V 2015–2022). Annual population data collected from the Estonian State Agency of Statistics [12] were used to produce IR calculations.
The definition this paper uses for crude IR is the number of new T1DM cases divided by the number of Estonian children in that age group, per 100,000 persons, per year. This was calculated for each of the three age groups during the five time periods, as well as, for the group as a whole (0–14.9-year-olds). Growth rate is defined as the percentage of change in T1DM IR within a specific time period. The 95% confidence intervals (CIs) were calculated using Wald’s method and normal approximation of Poisson distribution of the cases. The Poisson regression model was used to analyse T1DM incidence trends and IR differences for the time period of 1999–2022 among the different age groups. Relative risk (RR) was calculated to evaluate the probability of type 1 diabetes incidence across different age groups and time periods. A p-value of less than 0.05 was considered to be statistically significant.
Our data were processed using RStudio 2023.12.1 and programme SAS 9.4.
3. Results and Discussion
3.1. Results
Between 2007 and 2022, 1104 new cases of T1DM were diagnosed in children under 15 years of age, in Estonia. The crude IRs per 100,000 persons varied over this study period as shown in Table 1.
| Year | Incidence rate (per 100,000 persons per year) | 95% confidence interval |
|---|---|---|
| 2007 | 37.7 | 29.1–46.2 |
| 2008 | 29.1 | 21.6–36.6 |
| 2009 | 31.4 | 23.6–39.2 |
| 2010 | 38.9 | 30.4–47.5 |
| 2011 | 22.9 | 16.4–29.5 |
| 2012 | 24.7 | 17.9–31.5 |
| 2013 | 28.0 | 20.8–35.2 |
| 2014 | 35.4 | 27.4–43.5 |
| 2015 | 27.1 | 20.1–34.1 |
| 2016 | 31.5 | 24.0–39.1 |
| 2017 | 35.9 | 27.9–43.9 |
| 2018 | 31.9 | 24.4–39.4 |
| 2019 | 34.9 | 27.0–42.7 |
| 2020 | 32.0 | 24.5–39.5 |
| 2021 | 45.8 | 36.9–54.8 |
| 2022 | 37.6 | 29.5–45.7 |
On average, the crude IR of T1DM in children under 15 years, during 2007–2022, was 32.9 per 100,000 per year (95% CI 30.9–34.8), 31.0 (95% CI 28.3–33.7) for the period 2007–2014 and 34.6 (95% CI 31.9–37.4) during 2015–2022. The IR in different age groups are shown in Table 2.
| Time period | Age group (years old) | Incidence rate (per 100,000 persons per year) | 95% confidence interval |
|---|---|---|---|
| 2007–2014 | 0–4.9 | 26.2 | 22.1–30.3 |
| 5.0–9.9 | 31.1 | 26.4–35.8 | |
| 10.0–14.9 | 36.7 | 31.3–42.0 | |
| 2015–2022 | 0–4.9 | 21.5 | 17.6–25.3 |
| 5.0–9.9 | 38.2 | 33.3–43.2 | |
| 10.0–14.9 | 43.9 | 38.4–49.3 | |
| 2007–2022 | 0–4.9 | 23.9 | 21.1–26.7 |
| 5.0–9.9 | 34.9 | 31.4–38.4 | |
| 10.0–14.9 | 40.5 | 36.7–44.3 | |
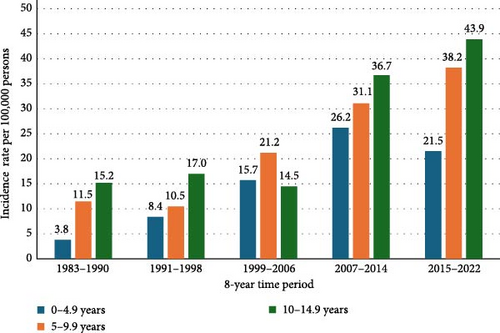
With each consecutive 8-year time period interval, there was a significant increase in the incidence of T1DM (Figure 1). The IR of 34.6 per 100,000 per year in 2015–2022 was higher than the IRs in the previous time periods in this study: 10.1 per 100,000 per year in 1983–1990 (RR 3.46, 95% CI 3.00–3.99, p < 0.0001), 12.2 per 100,000 per year in 1991–1998 (RR 2.85, 95% CI 2.48–3.27, p < 0.0001), 16.9 per 100,000 per year in 1999–2006 (RR 2.05, 95% CI 1.79–2.36, p < 0.0001) and 31.0 per 100,000 per year in 2007–2014 (RR 1.12, 95% CI 0.99–1.26, p = 0.066). During the whole observation period of 1983–2022, the incidence of T1DM grew from 10.1 to 34.6 per 100,000 person-years. Therefore, during this 40-year time period, the relative crude incidence increased by 346%, that is, 10.8% annually.

The growth rate within the three age groups over the five consecutive 8-year time periods are shown in Figure 2. When comparing the last two time periods (2007–2014 and 2015–2022), significant growth was observed only in 5–9-year-old children, growing from 31.1 to 38.2 per 100,000, resulting in an increase of 2.9% annually (RR 1.2, 95% CI 1.01–1.5, p = 0.043). During this time, the oldest age group experienced a slight, but not statistically significant, increase in IR with 36.7 to 43.9 per 100,000 per year (RR 1.2, 95% CI 1.0–1.4, p = 0.064). On the other hand, the youngest age group’s IR declined, although not significantly, with 26.2 to 21.5 per 100,000 per year (RR 1.2, 95% CI 0.96–1.5, p = 0.10).
In the year of 2021, Estonia experienced the highest number of total deaths due to a SARS-CoV-2 infection, highlighting the severity of the pandemic during this time [13]. Therefore, we chose 2021 as the pandemic peak in Estonia for this study. During this year, the crude IR of T1DM in Estonian children was 45.8 (95% CI 36.9–54.8) per 100,000 persons. Compared to the previous year of 2020, the IR had increased by 43.2% (RR 1.4, 95% CI 1.1–1.9, p = 0.021). This is the highest increment of annual IR between any 2 consecutive years observed in this study. To further investigate the impact of the COVID-19 pandemic on T1DM rates, we compared the incidence during 2021 to the last two time periods (2007–2022), during which the average IR was 32.9 per 100,000 (95% CI 30.9–34.8). The results confirm a significantly increased incidence during the pandemic peak (RR 1.4, 95% CI 1.1–1.7, p = 0.0014).
3.2. Discussion
It is important to follow the epidemiological trends of childhood-onset diabetes in order to make comparisons and find possible triggers or protective factors of the disease. Estonia [14] is a small nation located south of the Gulf of Finland, with a population of 1.4 million inhabitants, of which 67% are ethnically Estonians and 22% Russians. Due to its high IR and small, controlled population size, our sample can be considered an excellent cohort to study this autoimmune disease. Furthermore, Estonia experienced a rapid transition from a poorly developed nation in the 1980–1990s to its current relatively high socioeconomic status. This is important to consider when investigating the aetiopathogenesis of T1D in Estonian children. Finally, results from epidemiological studies like this one can be used by different health boards for disease surveillance, assessing the effectiveness of public health interventions and policies.
The last two time periods of this study (2007–2014 and 2015–2022) had the slowest average rate increase at 1.6% growth annually. This figure is below the European average annual growth rate of 3.4%, calculated from data spanning 1989–2013 [3]. Visual inspection of the data presented in Figure 1 suggests that Estonia is approaching the plateau phenomenon, which is already occurring in our neighbouring country Finland, among others [4–6].
Many previous researchers have attempted to deduct the aetiologies of childhood-onset T1DM. For decades, Finland has led the world in type 1 diabetes IRs, leading to extensive research on the subject. Although we know that genetics play an important role in the development of T1DM, environmental triggers exist as well, for example, the link between an increased BMI and the onset of T1DM [15]. Therefore, one argument for the growing incidence of T1DM is likely due to the rising prevalence of childhood overweight and obesity occurring in Estonia [16]. Furthermore, Finnish researchers proposed that the plateau phenomenon they are experiencing may be due to environmental changes, for example, the addition of the rotavirus vaccine to their national vaccination schedule in 2009 [4, 17]. Viral infections are known triggers for many autoimmune diseases, so this vaccine could be considered a protective factor against T1DM by reducing the number of severe rotavirus infections. The rotavirus vaccine was introduced 5 years later to Estonia, in 2014, which could help explain why we are seeing the plateau phenomenon later than our neighbours in Finland [18]. Finally, Parviainen [4] discovered that the age of onset for T1DM in children is increasing, which could explain the drop in IRs observed in the youngest age group during the last two time periods of this study. Further research including older age groups is therefore required to analyse if similar incidence trends are occurring in Estonia.
During the COVID-19 pandemic, the incidence of T1DM significantly increased in the Estonian paediatric population. A previous study in Finland investigated the possible relationship between a newly diagnosed diabetic child and a recent SARS-CoV-2 infection; a significant correlation was not found. They suggested that the sudden change in incidence trends was due to lockdown restrictions affecting the children’s daily environment rather than the virus trigger itself [19]. The strictest lockdown restrictions in Estonia were implemented during 2020. Next, during 2021, we suffered the most number of deaths per year due to COVID-19. Finally, the highest infection period was in 2022, as the restrictions loosened so that children could return to kindergarten and schools again [13].
In this paper, 2021 is considered the peak pandemic year due to the highest death rates during this time. However, using the year 2021 alone is not sufficient to explain the whole impact of the pandemic on the incidence of T1DM in Estonia. Further research considering the effect of all COVID-19 pandemic years on T1DM IRs is warranted. Additionally, due to the novelty of the COVID-19 pandemic, a longer follow-up period is required for a more accurate estimate.
4. Conclusion
In conclusion, this retrospective cohort study demonstrates that the IR of T1DM continues to rise in Estonian children of all age groups since 1983; however, the increment rate has been declining and a plateau trend hopefully occurs in the near future. A significant increase in the IR of T1DM was seen in Estonian children during the severe COVID-19 peak in 2021 compared to 2007–2022 and 2020 alone. Further research on the factors causing these changes is necessary to protect future diabetic children.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The study was supported by grants from the Estonian Research Council (PRG1428 and PRG1912) and by a grant from the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 874864).
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in Statistics Estonia at https://www.stat.ee/en, reference number 12. The data that support the findings of this study are available from the corresponding author upon reasonable request.




