Incretin Hormone Levels (GLP-1 and GIP) in Children and Adolescents With Type 1 Diabetes: A Systematic Review
Abstract
Background: Incretin hormones like glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are regulators of insulin and glucagon secretion, and thereby, glucose metabolism. The hormones also have other valuable antidiabetic actions, like decreased appetite and reduced gastric emptying, causing blood glucose levels to decrease. Their role in children with type 1 diabetes (T1D) is not well defined and evidence is scarce. The aim of this systematic review was to compare fasting and postprandial incretin levels in children and adolescents with T1D versus healthy controls and to assess their relationship with glycemic outcomes and disease duration in T1D.
Methods: A search of MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) identified 2694 studies, of which 10 met the inclusion criteria. Study quality was assessed using Joanna Briggs Institute (JBI) appraisal tools. Meta-analysis using a random effects model estimated pooled Hedges’ g and 95% prediction intervals (PIs) for incretin levels.
Results: The 10 included studies had moderate risk of bias and varied in design (sample sizes: 7–257). No consistent differences in GLP-1 levels were observed between T1D and healthy controls, except during ketoacidosis. Pooled fasting GLP-1 and GIP levels were 8.03 and 9.85 pmol/L, respectively; postprandial levels were 18.98 (GLP-1) and 44.79 pmol/L (GIP). We found a significant heterogeneity in study designs and measurement methods.
Conclusions: We found no significant difference in incretin levels between the three small and heterogeneous studies that compared T1D children to a healthy control group. A wide range of factors seems to influence the incretin levels in children with T1D. Disease duration, remission status, and meal size together with composition of nutrients in the meal were investigated in relation to GLP-1 and GIP levels in the included studies. Larger studies are needed to better understand the associations between incretin levels and metabolic outcomes in children and adolescents with T1D.
1. Introduction
Basic and clinical research on the incretin system has been one of the most significant and rapidly advancing areas in diabetes research in recent years [1]. Incretin hormones are naturally occurring gut hormones secreted from the intestines upon intake of nutrients via the oral route [2]. In a healthy person, the incretin hormones stimulate insulin secretion from the pancreatic beta cells in a glucose dependent manner. The incretin effect is the phenomenon where oral glucose elicits higher insulin secretion than does intravenous glucose, despite similar levels of glycemia [3]. The incretin hormones involves both glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) and their effect are thought to account for up to 70% of the enhancement in insulin secretion via the incretin effect in healthy individuals [2]. GLP-1 additionally inhibit glucagon release from the alpha cells [1–3] and have other valuable antidiabetic actions, like decreased appetite and reduced gastric emptying, causing blood glucose levels to decrease [4]. It is previously described that incretins play an important part in the glucose tolerance of healthy individuals and individuals with type 2 diabetes [2], but less is known about the role in type 1 diabetes (T1D)—especially in children. T1D is characterized by a gradual loss of beta cell mass and function and ultimately loss of insulin secretion. In addition, glucagon is found to be inappropriately increased which, in combination with lack of insulin, results in hyperglycemia [5]. This “double-trouble scenario” is a fundamental problem for the glycaemic excursions in individuals with T1D [6]. The inhibition of glucagon and potential increase in glucose sensitivity by GLP-1, as seen in youth with obesity [7, 8], is also of potential interest in the context of improved blood glucose management in T1D patients [9]. It has, therefore, been speculated whether GLP-1, as a glucagon suppressor and insulin enhancer, could be a potential treatment target in patients with T1D [10]. In a small retrospective study in newly diagnosed adults with T1D, receiving a GLP-1 receptor agonist (GLP-1 RA) within 3 months from the diagnosis were associated with reducing premeal insulin in all patients along with increased C-peptide levels and improved glycaemic outcome during the first-year postdiagnosis compared to a historic cohort [11]. Though the design of the abovementioned study were suboptimal to suggest a causal relationship, it seems possible that there is a dysfunctional response in hormones secreted from both the alpha and beta cells of the inflamed pancreas in patients with T1D. We, therefore, find it relevant to investigate the levels of these other co-regulatory hormones like GLP-1 and GIP and to further examine if incretin hormone levels could be compromised—or inappropriate—for the pediatric and adolescent patient group with T1D compared to healthy peers. The aim of this systematic review was to compare fasting and postprandial incretin levels (GLP-1 and GIP) in children and adolescents with T1D versus healthy controls and to assess their relationship with glycemic outcomes and disease duration in T1D.
2. Methods and Materials
This systematic review was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) [1] with registration ID: CRD42023410404 and was reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [2].
2.1. Search Method
We conducted a literature search using the electronic databases: MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL). The search was done on May 25, 2023 and updated on January 10, 2024. The main concepts were children and adolescents, T1D mellitus, and GLP-1, GIP, and incretin hormones. All concepts were searched with medical subject headings (e.g., MeSH) and free text words. The search string was built in MEDLINE and was subsequently translated to the other databases. No restrictions were imposed on the search regarding language or date of publication. The search string was evaluated by testing if 10 key articles within the field was retrieved. All search strings from the databases are provided in Appendix S1. As part of the search strategy, citation tracking was conducted to find all references and all articles citing the articles included in the review after closure of the study selection process. Citation tracking was run in the software tool citationchaser [3] and identified articles were screened for inclusion. The search strategy was developed by an information specialist (Tue Helms Andersen) and peer reviewed by another information specialist.
2.2. Inclusion and Exclusion Criteria
Human trials with measurements of fasting and/or postprandial GLP-1 and GIP levels in children and adolescents with T1D were considered. Measurements of GLP-1 could be given as both the total and/or active form and could be measured in both plasma and serum. Only articles available in full text that met the following criteria were included: (a) participants diagnosed with T1D, (b) participants < age of 18 years, (c) participants did not have severe comorbidity (>two chronic diseases), and (d) participants did not take any medications affecting gut hormones, except insulin. Exclusion criteria were: (a) protocol articles, (b) conference abstracts, and (c) review articles.
2.3. Study Selection Process
Identified studies were independently evaluated for inclusion by two researchers (Trine Witzner Hessel Lawaetz and Zerina Kücük1) and any discrepancies were resolved through discussion with the participant of a third researcher (Jesper Johannesen). All identified records were imported into Eppi Reviewer 6 [4] and duplicates were removed. The remaining records were independently screened on title and abstract and then retrieved in full text and reviewed against inclusion and exclusion criteria by both reviewers (Trine Witzner Hessel Lawaetz and Zerina Kücük1).
2.4. Data Extraction and Synthesis
Data extractions were done by both reviewers (Trine Witzner Hessel Lawaetz and Zerina Kücük1). From eligible studies, number of participants, mean, standard deviation (SD) of fasting and/or postprandial GLP-1 and GIP concentrations (total and/or active) were extracted. In case of missing data, corresponding authors were contacted every 2 weeks up to a total of three times for clarification. If GLP-1 and GIP values were given in units other than pmol/L, they were converted. Units presented in mass concentration was converted to molar mass using the molecular weight of 3300 g/mol for GLP-1 and 5100 g/mol for GIP. If GLP-1 and GIP values were only given in a figure or plot, the mean and SD were manually gathered by using an online plot digitizer tool [5]. Only studies comparing GLP-1 and GIP concentrations between cases and controls were included in the meta-analysis. Absolute values with no controls were presented visually as single-arm presentation plots.
2.5. Risk of Bias Assessment
Joanna Briggs Institute (JBI) critical appraisal tools [6] were used to assess the quality of included studies. The two reviewers (Zerina Kücük1 and Trine Witzner Hessel Lawaetz) assessed all 10 studies, that were then categorized into following distinct study designs: randomized controlled trials (as either baseline values or cross over studies), cross-sectional studies, or cohort studies.
Depending on study design, checklists with specifically tailored questions, were answered for all 10 studies to evaluate the methodological quality and potential biases inherent in that specific study design. The checklists consisted of a different set of questions and these were answered with either “yes,” “no,” “unclear,” or “unapplicable.” A total score was calculated from the number of “Yes” divided by the total number of questions and presented in percent (%).
2.6. Statistical Analysis
R 4.3.0 [7] was used to conduct the statistical analysis. We used the R packages meta [8] and dmetar [9] according to the “Doing Meta-Analysis with R: A Hands-On Guide” [10]. The codes and outputs were revised by a statistician (Andreas Kryger Jensen). The random effects model was chosen for the meta-analysis and forest plot due to large heterogeneity among selected studies. Each study’s effect size underwent weighting through the inverse variance approach. The confidence intervals (CIs) around the pooled effect were calculated using the Knapp–Hartung adjustments [10]. The restricted maximum likelihood estimator (REML) was used to calculate the variance of study heterogenity [10]. Both 95% CIs and prediction intervals (PIs) were computed. Single-arm Forest plots were performed showing point estimates with CIs to give an overview of the absolute values for the fasting and postprandial levels of GLP-1 and GIP in the studies that did not have a control group to compare with.
3. Results
3.1. Study Selection and Study Quality
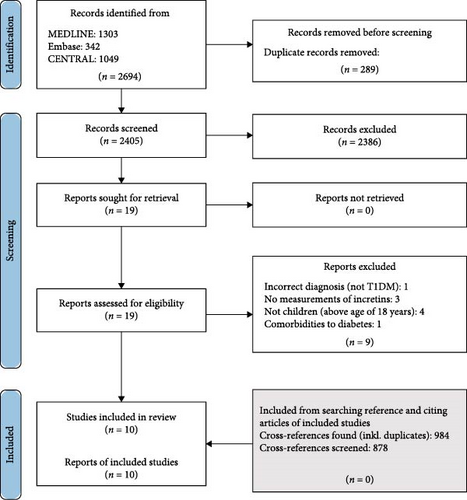
A flow diagram of the study screening and selection is provided in Figure 1. The systematic literature search identified 2694 studies, of which 2405 remained for screening on title and abstract after removal of duplicates. The interrater reliability (IRR) percentage agreement was 98.39%. Only 19 studies fulfilled inclusion criteria on title and abstract and were retrieved in full text. Based on the listed inclusion and exclusion criteria, nine studies were excluded from full text screening and 10 studies remained for analysis in our systematic review. Four articles were excluded as they had a mix of children under the age of 18 and young adults. The authors were contacted, but they were not able to separate the patient groups on age. Finally, 878 studies were further screened for eligible cross-references of included studies, however, none were eligible. Main findings in the 10 studies are shown in Table 1.
| Study | Publication year | Study design description | n | Girls (in %) | Mean age | Study type | Disease duration (years) | TDD (units/kg/day) | HbA1c (mean ± SD in %) | BMI | ROBa (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tell et al. [12] | 2022 | MMTT | 40 | 60 | 16.1 | Randomized cross over | 7.4 ± 4.6 | 0.89 ± 0.03 | 8.3 [7.5–9.1] | 24 [19.9–26.6] | 85 |
| Harray et al. [13] | 2022 | Clamp + low protein meal | 11 | 55 | 16.5 | Randomized cross over | 6.9 ± 5.1 | 0.8 ± 0.3 | 6.9 ± 0.8 | 0.2b | 63 |
| Harray et al. [13] | 2022 | Clamp + high protein meal | 11 | 55 | 16.5 | Randomized cross over | 6.9 ± 5.1 | 0.8 ± 0.3 | 6.9 ± 0.8 | 0.2b | 63 |
| Harray et al. [13] | 2022 | Clamp + high fat low protein meal | 10 | 40 | 16.1 | Randomized cross over | 8.2 ± 4.9 | 0.7 ± 0.2 | 7.1 ± 0.8 | 0.6b | 63 |
| Harray et al. [13] | 2022 | Clamp + low fat high protein meal | 10 | 40 | 16.1 | Randomized cross over | 8.2 ± 4.9 | 0.7 ± 0.2 | 7.1 ± 0.8 | 0.6b | 63 |
| Lodefalk et al. [14] | 2010 | Solid–high fat meal | 7 | 57 | 16.4 | Cross-sectional study | 3.7 ± 1.2 | 0.8 ± 0.2 | 7.3 ± 0.7 | 23.2 ± 3.4 | 46 |
| Lodefalk et al. [14] | 2010 | Solid–low fat meal | 7 | 57 | 16.4 | Cross-sectional study | 3.7 ± 1.2 | 0.8 ± 0.2 | 7.3 ± 0.7 | 23.2 ± 3.4 | 46 |
| Akinci, Aydın, and Özerol [15] | 2009 | Ketoacidosis | 24 | 50 | 10.5 | Cohort study | 2.15 ± 0.38 | — | 13.2 ± 4.1 | 14.93 ± 0.48 | 60 |
| Akinci, Aydın, and Özerol [15] | 2009 | 96 h after keto | 24 | 50 | 10.6 | Cohort study | 2.15 ± 0.38 | — | 13.2 ± 4.1 | — | 60 |
| Akinci, Aydın, and Özerol [15] | 2009 | After normal weight | 24 | 50 | 10.6 | Cohort study | 2.15 ± 0.38 | — | 13.2 ± 4.1 | 16.31 ± 0.46 | 60 |
| Huml et al. [16] | 2011 | No stimulation | 19 | 34 | 13.4 | Cross-sectional study | 6.5 ± 2.2 | 0.92 ± 0.3 | 7.2 ± 0.28 | 19.49 ± 4.34 | 63 |
| Pörksen et al. [17] | 2007 | MMTT | 257 | 51 | 9.05 | Cohort study | 0.08 | — | 11.2 ± 2.1 | 16.5 ± 3.2 | 60 |
| Pörksen et al. [17] | 2007 | MMTT | — | — | — | Cohort study | 0.5 | — | — | — | 60 |
| Pörksen et al. [17] | 2007 | MMTT | — | — | — | Cohort study | 1 | — | — | — | 60 |
| Ho et al. [18] | 2019 | No stimulation | 21 | 67 | 11.9 | RCT baseline | 4.7 ± 3.07 | 0.92 ± 0.3 | 8.08 ± 0.91 | — | 85 |
| Kaas et al. [19] | 2011 | MMTT | 213 | — | — | Cohort study | 0.08 | — | — | — | 60 |
| Kaas et al. [19] | 2011 | MMTT | 127 | — | — | Cohort study | 0.5 | — | — | — | 60 |
| Kaas et al. [19] | 2011 | MMTT | 189 | — | — | Cohort Study | 1 | — | — | — | 60 |
| Uslu et al. [20] | 2016 | OGTT | 25 | 36 | 9.04 | Cross-sectional study | — | — | 12.2 ± 2.34 | 16.27 ± 3.04 | 63 |
| Uslu et al. [20] | 2016 | OGTT | 25 | 36 | 9.04 | Cross-sectional study | — | — | 12.2 ± 2.34 | 16.27 ± 3.04 | 63 |
| Fredheim et al. [21] | 2015 | MMTT | — | — | — | Cohort study | 0.08 | — | 9.0 ± 3.5 | — | 60 |
| Fredheim et al. [21] | 2015 | MMTT | — | — | — | Cohort study | 0.25 | — | 7.1 ± 3.39 | — | 60 |
| Fredheim et al. [21] | 2015 | MMTT | — | — | — | Cohort study | 0.5 | — | 7.4 ± 4.15 | — | 60 |
| Fredheim et al. [21] | 2015 | MMTT | 129 | 49 | 10.0 | Cohort study | 1 | — | 7.7 ± 3.44 | 18.6 ± 2.9 | 60 |
| Fredheim et al. [21] | 2015 | MMTT | 40 | — | — | Cohort study | 5 | — | 8.4 ± 3.14 | — | 60 |
- Abbreviations: MMTT, mixed meal tolerance test; OGTT, oral glucose tolerance test; SD, standard deviation.
- aRisk of bias assessment score (Yes/total in %).
- bPresented as BMI z-score.
The questions from the JBI quality assessment of the 10 included studies is demonstrated in Appendix S2. We ranked the study quality into high risk (below 50%), medium (50%–70%), or low risk (70%–100%) of bias, like done in other studies using JBI. Checklists for each study in the four different study design groups can be found on the JBI Critical Appraisal Tool website and citings [6, 11]. In general, a moderate risk of bias was seen in the included studies. One study was found to have a high risk of bias (46%). Seven were recorded as medium risk of bias (60%–63%) and two studies were found to have a low risk of bias with a score of 85% (Table 1).
3.2. Variation in Study Design of the Included Studies
As seen in Table 1, there was large difference in the study designs. The number of participants varied from seven to 257 children with T1D. Only three studies included healthy children as comparisons. Mean age ranged from 9.04 to 16.4 years. The disease duration ranged from 1 month to 8.2 ± 4.9 years. Nine studies included metabolic parameters BMI, TDD, and HbA1c, but only four studies included them all. Three studies were cross-sectional, two were randomized cross over trials, one had baseline placebo arm values from an RCT, and three were cohort studies. One study measured active GLP-1 (GLP-1 (7–36) or (7–37) amide [20]) compared to the other cohorts measuring total GLP-1. For the postprandial measurements four studies used mixed meal tolerance test (MMTT) as stimulation method; one study used oral glucose tolerance test (OGTT) and three studies used solid meals (Table 1). For Harray et al. [13], the solid meals with different macronutrient compositions were given during euglycemic clamp. The postprandial sampling time point of GLP-1 and GIP was set to 90 min after a meal (T90) for comparison; however, two studies only had measurements after 30 min (T30) [20] and 120 min (T120) [12], respectively. All GLP-1 and GIP analysis were done on plasma except Ho et al. [18] that used serum and Tell et al. [12] that did not mention it in the article. Most studies presented their concentrations in pmol/L, but some presented them in pg/mL even though the numeric numbers were similar to the other studies and did not differ by a factor 1000 as the unit would apply [16]. The authors were contacted to confirm the units and as they did not reply, we assumed that it should have been in pmol/L. This study was included in the meta-analysis (standardized mean difference (SMD)), but not in the fasting single-arm presentation due to uncertainty in the validity of the absolute value units. The adding of DPP-4 to the samples and the preanalytical handling of the samples prior to analysis were only clearly described in two articles (Table 2). The company names of the assays used for quantifying the concentration of GLP-1 and GIP varied in all studies and all were using either enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay (RIA) measurement techniques.
| Study | Publication year | Stimulation method | Max time | Plasma/serum | DPP4 inhibitor | Assay type | Assay compary/group name producing GLP-1/GIP assay |
|---|---|---|---|---|---|---|---|
| Tell et al. [12] | 2022 | MMTT | T120 | Unknown | Unknown | ELISA | Mercoda |
| Harray et al. [13] | 2022 | Solid | T90 | Plasma | Unknown | RIA | Millipore |
| Lodefalk et al. [14] | 2010 | Solid | T90 | Plasma | Unknown | RIA | J.J Holst group referenced |
| Akinci, Aydın, and Özerol [15] | 2009 | No stim | — | Plasma | Yes | ELISA | Millipore |
| Huml et al. [16] | 2011 | Solid | T90 | Plasma | Yes | RIA | LINCOplex |
| Pörksen et al. [17] | 2007 | MMTT | T90 | Plasma | Unknown | RIA | J.J Holst group referenced |
| Ho et al. [18] | 2019 | No stim | — | Serum | Unknown | ELISA | Millipore |
| Kaas et al. [19] | 2011 | MMTT | T90 | Plasma | Unknown | RIA | J.J Holst group referenced |
| Uslu et al. [20] | 2016 | OGTT | T30 | Plasma | Unknown | RIA | Linco Research |
| Fredheim et al. [21] | 2015 | MMTT | T90 | Plasma | Unknown | RIA | J.J Holst group referenced |
- Abbreviations: ELISA, enzyme-linked immunosorbent assay; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; MMTT, mixed meal tolerance test; OGTT, oral glucose tolerance test; RIA, radioimmunoassay.
3.3. GLP-1 and GIP Levels in Children With T1D Compared to Healthy Controls
Three studies measured GLP-1 levels in children with T1D compared to healthy controls. There were 68 in the T1D group and 61 children in control groups. The standard mean difference was 0.42 (−0.91, 1.75), Tau2 was 1.41% and I2 was 89%, (p < 0.01; Figure 2).

Akinci, Aydın, and Özerol [15] looked at GLP-1 levels in children with T1D when admitted with ketoacidosis, when ketonemia resolved and when the weight gain started, compared to healthy children as controls. They found that the GLP-1 levels were significantly higher when admitted with diabetic ketoacidosis compared to the healthy controls, but there were no significant difference in the levels after 8 and 96 h, when ketonemia had resolved [15]. Uslu et al. [20] compared active GLP-1 during an OGTT. They found that fasting and postprandial levels of GLP-1 in 25 children with T1D were not different compared to the 22 healthy controls at identical conditions [20]. Huml et al. [16] measured postprandial GIP and GLP-1 levels amongst other hormones in 19 children with T1D and 21 controls matched on age, sex, and BMI. They found that GIP levels in children with T1D were higher (p < 0.01) compared to healthy children, but the GLP-1 levels were not significantly different [16].
3.4. Fasting GLP-1 and GIP Levels in Children With T1D
Three studies have investigated fasting levels of GLP-1 (Figure 3) with a total number of 68 participants. Two studies measured GIP within a total of five different conditions and were, therefore, presented as individual measurements (Figure 4). The total number of participants in the five studies were 28. The pooled effect sizes/means across all fasting studies measuring total fasting GLP-1 were 8.03 pmol/L (4.52, 11.53 pmol/L). The pooled estimate across the studies measuring total fasting GIP were 9.85 pmol/L (5.60, 14.09 pmol/L).
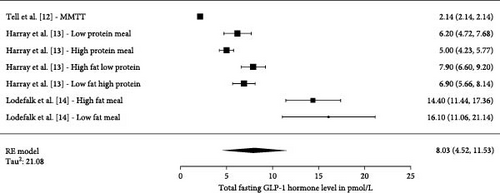
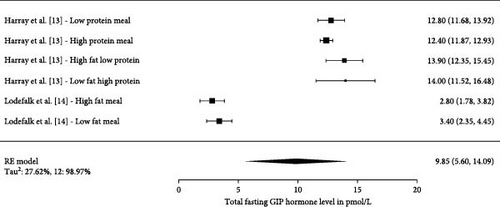
3.5. Postprandial Incretin Levels in Children With T1D
Five studies had 15 measurements on postprandial GLP-1 values (Figure 5). The total number of participants in the five studies were 667. The pooled estimate across all studies measuring total postprandial GLP-1 was 18.98 pmol/L (15.02, 22.94 pmol/L), Tau2 were 57%, and I2 were 99.99%. Three studies had 11 measurements on postprandial GIP values (Figure 6). The pooled estimate across all studies measuring total postprandial GIP was 44.79 pmol/L (35.37, 54.20 pmol/L), Tau2 was 243.7%, and I2 was 98.99%.
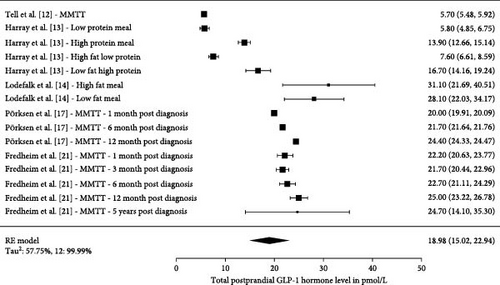
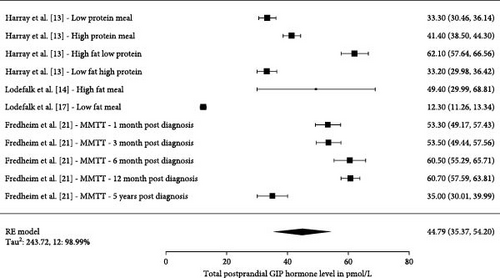
Harray et al. [13] studied the postprandial effect on GLP-1, GIP, and glucagon levels during a cross over study, where they used an insulin clamp technique to determine the insulin requirements to obtain euglycemia during meals, being classified as: low protein, high protein, low fat high protein, or high fat low protein. They found that the area under the curve (AUC) for GLP-1 were higher for the high protein meal compared to low protein meal. GLP-1 AUC were also higher for low fat high protein meal compared to the high fat low protein meal [13] (Figure 5). Lodefalk et al. [14] looked at meal stimulation from a high-fat or low-fat meal and found that the AUC for GLP-1 were significantly larger for the high-fat meal compared to the low-fat meal after 120 min. GIP had significantly larger peak values and AUC after 120 and 240 min when stimulated by a high fat meal [14]. Pörksen et al. [17] and Fredheim et al. [21] investigated the postprandial glucagon levels and their relationship with incretin levels at different visits during the first year following the T1D diagnoses. Only results from Pörksen et al. [17] were included in the single-arm presentation (Figure 5) as GLP-1 and GIP levels in the study by Kaas et al. [19] was divided into patients being in remission versus nonremission at three different visits. In the study by Kaas et al. [19], they found that GLP-1 levels increased by 24% from 1 month after diagnosis to the 12 months visit and that GLP-1 was positively associated with glucagon release in response to the liquid mixed meal. Fredheim et al. [21] investigated a different cohort in a similar set-up, where newly diagnosed children with T1D were tested with a MMTT used to investigate c-peptide, as a surrogate for their endogenous insulin production and their beta cell function. They were tested at three visits within the first year including a 5-year follow-up. They found that total GLP-1 levels increased in the first year, but after that it was not significant when looking at the full 5-year follow-up period. In the same study, it was seen that “a doubling in GLP-1 was associated with a 33% increase in glucagon” and that GIP levels decreased by 34% from 1 month to 5 years.
4. Discussion
Few studies have investigated GLP-1 and GIP levels in children with T1D. Three studies measured GLP-1 and had a healthy control group for comparisons. These studies were small in sample size and heterogeneous in design (various baseline settings, meal stimulation method, fasting or postprandial state, active or total hormone levels, and laboratory methods used). One study demonstrated significantly higher GIP levels in their T1D cohort compared to the control cohort. However, no difference in GLP-1 levels between T1D children and controls could be demonstrated in this small meta-analysis.
Adult studies investigating GLP-1 can, to some extent, be used to examine the incretin effect of GLP-1 and GIP in health and disease [22]. However, there is not a linear relationship between adults and children as many nonlinear changes take place in childhood and in adolescents such as change in body composition, neurological maturation, sex hormonal changes, body proportions, among others physiological fluctuations. We, therefore, found it important to investigate the responses of the different hormones in studies only involving children with T1D.
The pooled estimate across all our included studies in children measuring total postprandial GIP was 44.79 pmol/L and the pooled estimate was 18.98 pmol/L for total GLP-1. The high Tau and Tau2 in the analyses indicate that there are great variability and dispersion among the true effects. This is emphasizing that the studies in the single-arm presentations contributing to a high degree of uncertainty related with the estimate. The I2 was also very high in all studies possible due to the known differences in study designs, study population, and not due to random error or other study parameters. When comparing adult levels of total concentrations obtained in a study where a 566 kcal meal was given, the postprandial levels were approximately 100–110 pmol/L for GIP and 20–25 pmol/L for GLP-1 [23] for comparison. This is close to the GLP-1 levels in our studies, the results from the individual studies in this review still highlight how the between study absolute values can vary significantly.
In this section, we have discussed various physiological circumstances and other factors mentioned in the articles that might influence the measured levels of GLP-1. It is well known that factors such as nutrient composition, degradation by dipeptidyl peptidase (DPP-4) as well as the expression and sensitivity of the incretin receptors play a key role in the incretin response, meaning that the incretin plasma levels alone do not reflect the sole activity of the hormone. Clinical conditions and altered metabolic physiology in children during sample collection of GLP-1 and GIP also affect the incretin levels. Stinson et al. [24] recently reported that fasting GLP-1 levels were higher in overweight children and adolescents when compared to controls with normal weight and that one SD increase in fasting GLP-1 was associated with insulin resistance, hyperglycemia, and other cardiometabolic parameters. In a different study by the same group, they found that children and adolescents with obesity and insulin resistance had elevated fasting concentrations of both glucagon and GLP-1. They had a higher glucagon response—but a significantly lower GLP-1 response—during an OGTT compared to normal weight children and obese children with normal insulin sensitivity. Indicating that GLP-1 and glucagon might play a role in insulin sensitivity in health and disease. In the study by Akinci, Aydın, and Özerol [15], they found a significant difference in the level of GLP-1 depending upon clinical presentation at onset: when admitted to hospital in diabetes ketoacidosis (DKA) compared to when being in a their “stable state.” The authors measured GLP-1 at three different time point and found that the GLP-1 levels had decreased significantly, when ketonemia resolved after the insulin infusion was started, but without consuming any food orally, and therefore, speculated if it was the exogenous insulin that decreased the GLP-1 levels, and therefore, had an inhibitory effect on GLP-1 secretion [15].
The influence of nutrient composition of a meal on incretin hormones was likewise a theme in the included articles and might also play an important role. It is well established that carbohydrates are strong stimulators of incretins when ingested orally [25, 26]. Less is known about fat and protein as stimulants. The trend in the included studies by Harray et al. [13] demonstrated that the difference in meal composition influence the secreted levels of GLP-1, GIP, and glucagon in children with T1D. They found that adding protein to the meal, with or without fat, led to an increase in GLP-1 levels compared to when having meals low in protein. Another study in adults with T1D investigated the insulin needed in response to a meal high in protein and fat compared to a meal low in protein and fat, when the carbohydrate content was constant. They found “a mean of 50% additional insulin was required for the high-protein meal compared to the low-protein meal” [27]. A similar trend was found by Vilsbøl et al. [28] when evaluating incretin secretion in adults with T1D or type 2 diabetes compared to a control group when having differences in meal sizes. The larger the meal, the larger the insulin and GLP-1 response in all groups [28]. However, the GLP-1 AUCs were lower for T2D and for obese individuals compared to T1D and healthy controls. In this study, the authors did not find a significant difference between patients with T1D and healthy control [28]. Uslu et al. [20] similarly found no statistical difference in secreted GLP-1 levels between children with T1D and healthy children following an OGTT; however, the samples were taken 30 min postprandially and might not have reached peak levels [20] as these are expected approximately 40–50 min postmeal, at least in adults [29].
Preanalytical circumstances might also affect the plasma incretin levels. The adding of DPP-4 to the samples and the preanalytical handling of the samples prior to analysis were described in few of the included articles. The correct sample handling is usually recommended to avoid thawing and refreezing plasma samples before analysis, because this is thought to affect the stability of peptide hormones, and thereby, the incretin levels [30]. However, an article by Albrechtsen et al. [31] found that the levels of GLP-1 were relatively stable when exposed to up to three repeated freeze thaw cycles independent of the assay used when DPP-4 were added. They also demonstrated that temperature, being −20 or −80°C, did not impact the GLP-1 levels significantly independent of assay type [31, 32].
The disease duration of children with T1D seem to affect the levels of GLP-1 in some of the studies included in the review. Postprandial levels of GLP-1 after a mixed meal increased by 24% in the first year in the study by Pörksen et al. [17]. Fredheim et al. [21] found that total GLP-1 increased gradually by 11% from 1 to 12 months postdiagnosis. GIP levels decreased 34% in the same period [21]. Kaas et al. [19] looked at GLP-1 and GIP levels when being in clinical remission according to IDAA1c (insulin dose adjusted HbA1c) and found that GLP-1 were significantly lower in children with T1D in remission compared to those not in remission. They also found that GLP-1 levels associated positively with postprandial glucose and that GLP-1 levels measured at 1 month could predict remission status at 6 months. The increase in GLP-1 plasma levels with disease duration could be explained by the increasing need to enhance the insulin secretion, resulting in increased GLP-1 substance availability in plasma. However the overall effect of the hormone can still be missing as the GLP-1 receptor expression in the pancreas might be low or lost due to decreased beta cell mass [33] or hyperglycemia in itself, as seen in animal studies [34].
Fredheim et al. [21] found that during a MMTT at four different visits a doubling in total GLP-1 levels was also associated with a 33% increase in glucagon. They also found that plasma glucagon levels increased 160% over the entire study period of 5 years [21]. This phenomenon of increased glucagon seems appropriate during hypoglycemia, but very inappropriate during hyperglycemias, as an increase in glucagon levels in theory leads to glycogenolysis and gluconeogenesis, which in turn could increase the blood glucose levels even further. Despite being inappropriate, it is a common phenomenon in T1D [35]. A study using a GLP-1 infusion at very high concentrations reduced glucose levels in adults by almost 40% to near normal levels and significantly reduced plasma glucagon concentrations [36]. Fortunately, and in contrast to insulin, the effect of GLP-1 on glucagon depends on the glucose level, as GLP-1 only inhibits glucagon when glucose levels are above fasting levels [37] meaning that it does not affect the positive counterregulatory effect of glucagon seen at hypoglycemia [38]. This arguess for the idea that GLP-1 RA might be beneficial as add on to insulin to reduce both glucose and glucagon in dysregulated patients with T1D. However, as patients with T1D get decreased beta cell mass with increased disease duration the add-on of GLP-1 RA might have limited effect on longstanding T1D with low c-peptide levels [39–42] and might only be beneficial for the patients being early in their disease progression as these patients might still have receptor expression, potentially explaining why this group also seem to be the best therapeutic responders in clinical studies [43, 44].
5. Strengths and Limitations
This is the first review to our knowledge investigating GIP and GLP-1 levels in children with T1D. We did a comprehensive and systematic assessment of the published studies that investigated different clinical, physiological, and methodological settings affecting GLP-1 levels in the T1D patient group. We aimed to investigate if there is a difference in incretin levels between children with T1D and healthy controls and to explore the absolute GLP-1 and GIP levels in children with T1D and we did not find it. There are some obvious limitations to this review. There were very few studies investigating GLP-1 levels in children with T1D and even less measuring GIP. Three of the 10 studies had a healthy control group to compare with. The studies that compared with a healthy control group were small in sample size and measured under different study set-ups. The study set-ups varied according to meal stimulation method, fasting or postprandial state, active or total hormone assays, and laboratory methods used. Preanalytical conditions and the accuracy of the methods used to analyze plasma levels of GLP-1 and GIP are important for the most precise interpretation of the outcomes in the future and to avoid random analytical errors. It is important to recognize that plasma levels represent only one aspect of the overall incretin response and that it is also influenced by factors such as nutrient stimulation, enzymatic degradation, receptor expression, and receptor functionality. Studies and preferably cohort studies should be done to compare incretins and other gut hormones from onset to several years postdiagnosis compared to a healthy pediatric cohort with reporting of incretin levels preferably as z-scores and not just absolute values.
6. Conclusion
We performed a systematic review of available clinical studies on children with T1D measuring GLP-1 and GIP levels. We found no significant difference in incretin levels between the three small and heterogeneous studies comparing T1D children to healthy control groups. A wide range of factors seems to influence the incretin levels in children with T1D including disease duration, remission status, co-regulatory hormones, degradation, and receptor activity together with composition of nutrients in the meal, some of which has been highlighted in the included studies. More and larger studies comparing GLP-1 and GIP levels in children with T1D compared to healthy controls are needed. They should preferably be measured when exposed to similar stimulation methods, clinical and preanalytical conditions, postprandial period, and assay type to make relevant comparisons on the impact of incretins on children with T1D.
Nomenclature
-
- CENTRAL:
-
- Cochrane Central Register of Controlled Trials
-
- DKA:
-
- Diabetes ketoacidoses
-
- GIP:
-
- Glucose-dependent insulinotropic polypeptide
-
- GLP-1:
-
- Glucagon-like peptide-1
-
- HbA1c:
-
- Hemoglobin A1c, glycated hemoglobin
-
- I2:
-
- A between-study heterogeneity measure. Defined as the percentage of variability in the effect sizes, not caused by sampling error
-
- JBI:
-
- Joanna Briggs Institute
-
- MMTT:
-
- Mixed meal tolerance test
-
- OGTT:
-
- Oral glucose tolerance test
-
- PI:
-
- Prediction intervals
-
- PRISMA:
-
- Preferred Items for Systematic Review and Meta-Analysis
-
- PROSPERO:
-
- The International Prospective Register of Systematic Reviews
-
- RoB:
-
- Risk of bias
-
- SMD:
-
- Standardized mean difference
-
- Tau2:
-
- Between-study heterogeneity measure defining the variance of the distribution of the true effect size in this study calculated by restricted maximum likelihood.
Disclosure
The funders had no role in any part of this review.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
All authors are employed at Steno Diabetes Center Copenhagen, a public hospital and research institution under the Capital Region of Denmark, which is partly funded by a grant from the Novo Nordisk Foundation.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




