The Unexplored Biodiversity of ‘Glacier Fleas’ (Hexapoda: Collembola): Taxonomy, Distribution and Ecology in the European Alps and Apennines
Abstract
Springtails (Hexapoda: Collembola) are unique among Alpine arthropods for including cryophilic species, able to live in contact with glacial ice, the so-called ‘glacier fleas’. Despite being historically recorded, their taxonomy and distribution are largely unknown. In this article, we present the first comprehensive study of ice-dwelling springtails (family Isotomidae) of the European Alps and Apennines. Morphological and molecular analyses of two mitochondrial genes (cox1 and 16S) were performed after an extensive field sampling across 48 European sites. Five new species were identified and described on the Alps: Desoria orobica sp. nov., Vertagopus glacialis sp. nov., V. psychrophilus sp. nov., V. glacieinigrae sp. nov. and V. fradustaensis sp. nov., together with the already known D. saltans and V. alpinus. The evidence for two further new species was also reported, with the first occurrence of Gnathisotoma bicolor for the Alpine chain. Desoria calderonis occurs on the only glacier of the Apennines. Among the new species, V. glacialis and V. psychrophilus exhibit a wide range distribution, while the other species show a narrow endemic distribution. The study highlighted the unexplored diversity of Alpine ‘glacier fleas’ and their ecological and biogeographic interest, together with the conservation concern in the context of the present warming cycle.
1. Introduction
The cryosphere, especially mountain glaciers, faces significant threats from climate change [1], undergoing profound transformations that impact biotic communities [1–3]. As a consequence of intense melting, many glaciers are increasingly covered by stony debris [4–6]. By the end of the 21st century, most glaciers in the Alps are expected to disappear [7–9]. Permafrost and related landforms (for example, rock glaciers) are also experiencing heavy degradation, for instance through the thickening of the active layer and the reduction of ice volume [10].
Glacial and periglacial landforms host a unique biodiversity of highly specialised and often endemic organisms, adapted to low temperatures [11]. The potential disappearance of these habitats poses a significant risk to this biodiversity, underscoring the urgency for their study and conservation [2, 3, 12–14]. Studying glaciers and rock glaciers, as well as their associated biodiversity, is a necessary prerequisite for future monitoring and management actions [2, 15].
Springtails (Hexapoda: Collembola) are edaphic arthropods that colonise a wide spectrum of habitats, from Equatorial forests to cold biomes [16]. They are among the few terrestrial animals able to colonise Antarctica [17] and,among Alpine arthropods, they are unique for including cryophilic, ice-dwelling species, that is thriving in direct contact with glacial ice [2, 14, 18]. Such species belong to the family Isotomidae [11, 18, 19] which represent ‘true ice-dwelling’ cryophilic species [11]. Such species should be distinguished from ‘firn- and debris-dwelling’ cryophilic species that occupy iceless snow patches and the habitat at the margin of the ice. Similarly to snow-dwelling springtails [20], the main period of activity of ice-dwelling springtails occurs during the winter months [21], when they occupy the microhabitat at the interface between ice and snow [11]. Only when snow starts melting, they move up to the snow surface and, during summer, after snow disappearance, they move on the ice and into the ice interstices, down to a depth of about 30 cm [11]. Some species are also obligate ice dwellers, unable to survive elsewhere.
The physiological and behavioural ecology adaptation of springtails to survive under freezing conditions has been the object of many studies (for example, [11, 22–28]). Steinböck [29] demonstrated that ice-dwelling species have extreme adaptations to cold conditions, falling into a chill coma only at −16°C, whereas less adapted, non-glacial species become inactive at −6°C. Furthermore, Schaller and Zinkler [30] found that ice-dwelling springtails have comparatively high respiration rates close to 0°C, demonstrating active metabolism around the freezing point.
Springtails are key components of glacier food webs [31, 32], feeding primarily on in-blown organic material, like pollen granules [21, 28], and on biofilm [32] and serving as prey for numerous predators, like spiders and ground beetles [21, 33, 34].
In high mountain areas, glaciers and permafrost-related landforms (for example, rock glaciers) probably act for ice-dwelling species like islands of ice in a matrix of rocky grounds, prairies, woods and other ice-free landforms, like the so called ‘sky islands’ [35]. In this situation, the patchily distributed ice-dwelling springtail populations are good candidates for studying the biogeography and ecology of Alpine glacial environments [18].
Desor [36] cited for the first time Desoria saltans Nicolet, 1841, noteworthy for its showy, swarming and large assemblages on Alpine glaciers. For the following years, this species was considered the only Alpine ice-dwelling springtail, the so-called ‘glacier flea’. Haybach [37] described a new species of ice-dwelling Isotomidae—Vertagopus helveticus Haybach, 1980—on the Morteratsch glacier (Bernina, Switzerland), under supraglacial stony debris. This species is taxonomically related to Vertagopus alpinus Haybach, 1972, which had been discovered a few years before in the snowy and high-altitude habitats of Grossglockner, close to the Pasterze glacier (Austria). Only recently another ice-dwelling Desoria species has been described from the last remaining glacier of the Apennines (Abruzzo, Italy), D. calderonis Valle, 2021 [18], while Vertagopus nunataki Potapov & Kremenitsa, 2020 was described from Caucasus [38], although this latter was collected close to a glacier rather than on it.
Thus, in Europe, at least two genera of ice-dwelling Isotomidae are currently thought to be associated with the supraglacial habitat, Desoria Nicolet in Desor, 1841 and Vertagopus Bagnall, 1939. A third one, Gnathisotoma Cassagnau, 1957, is a debris-dwelling cryophilic springtail known to occur in Europe on Pyrenees and Cantabrian mountains and, possibly, a key genus of ice-dwelling alpine springtail evolution [39].
The diversity and distribution of ice-dwelling springtails in the European Alps, however, have been relatively understudied, with existing research suggesting that their diversity is likely underestimated [11, 18, 24].
This article aims to address this gap by presenting the first comprehensive description of ice-dwelling springtails of the European Alps and Apennines employing an integrative taxonomy approach (using morphology and DNA barcode, integrated with biogeographical and ecological information), based on extensive field sampling in 48 sites on Alps, Apennines and Pyrenees.
2. Material and Methods
2.1. Study Area
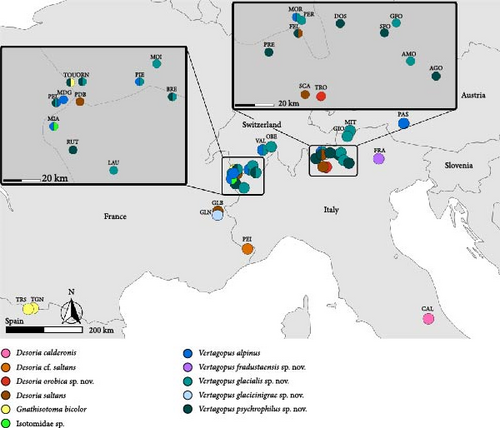
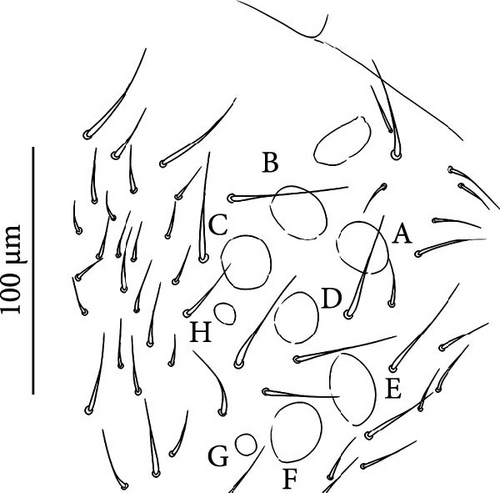
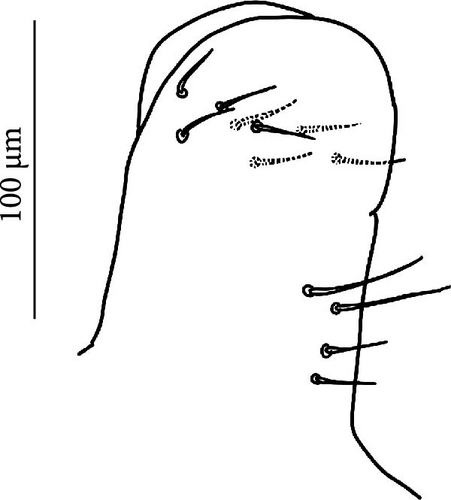
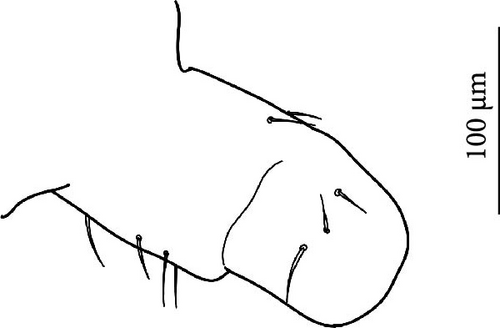
The sampling was performed in 48 sites in the European Alps (45), Apennines (1) and Pyrenees (2) (Table A1, Figures 1A–H, 2). In the European Alps, we included a wide spectrum of glacier size throughout the Alpine chain. Desoria calderonis specimens were sampled by Valle et al. [18] on the Calderone glacier, the last glacier of the Apennines (Table A1).

2.2. Sampling Activity
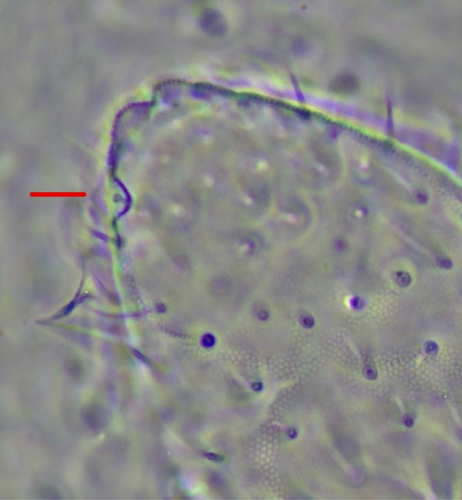
The sampling activity (Figures 1A–H) was performed during the summers of 2020–2023, in the period July–September, on glaciers and rock glaciers in the European Alps and Apennines (Table A1). Snowfields in extinct glacial sites were also included (i.e., areas formerly occupied by glaciers where perennial or semi-perennial snow cover is still present; Figure 1D). Specimens were searched in the supraglacial habitats, in contact with the ice. Sampling consisted of hand-catching where springtails made consistent assemblages on snow or under the stones on bare ice (Figure 1C,E,F), whereas the flotation method [40, 41] was used to collect the specimens occurring inside the supraglacial stony debris (i.e., frontal or medial moraines, dirt cones) (Figure 1A,B,D). For rock glaciers, it was not possible to reach the ice because of the deep debris layer, and only sub-surface interstitial habitats were inspected. Gnathisotoma specimens of the Pyrenees were sampled with the flotation method in the stony debris at the margin of snow patches in relict glacial areas (Table A1).
2.3. Morphological Analysis
2.3.1. Specimen Preparation and Conservation
Specimens were preserved in 96% ethanol at −20°C. For slide preparation, they were passed in boiling alcohol to remove fats and cleared by a short immersion in 10% KOH solution. Then, specimens were passed in chloralphenol and finally mounted on permanent slides using Swann medium as a preservative solution [42]. Other specimens were initially cleared through a short immersion in 10% KOH solution and then mounted on depression slides using lactic acid as a preservative solution for better observing the body shape and other diagnostic parts. The specimens fixed in ethyl alcohol are deposited at the Science Museum of Bergamo, Italy. Holotypes and paratypes of new species and material examined for known species are lodged in the following collections: Senckenberg Museum of Natural Sciences, Görlitz, Germany (SMNG), Science Museum of Bergamo, Italy (MCSNBG), MUSE—Science Museum of Trento, Italy (MUSE). Other topotypic material is deposited in thesecollections.
2.3.2. Species Identification
Identification was performed through the keys reported in Potapov [43]. Morphological observations and pictures were made with light microscopes Leica DM2500 with phase and DIC contrasts and drawing arm, a Nikon Eclipse Ci and Carl Zeiss Axiolab 5 with phase contrast, a Carl Zeiss AXIO Zoom V16 stereomicroscope and a Quanta400 (FEI) scanning electron microscope.
2.4. Genetic Analysis
2.4.1. DNA Sequencing
Whole genomic DNA was extracted from 6 to 10 specimens (entire organism) from each of the sampled species found in each site, using the Wizard® SV Genomic DNA Purification System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Due to limited sample availability, a smaller number of individuals was extracted for some localities (Table A1). DNA extraction of individuals from ORN and PEL was performed on the body while the head was prepared in slides, to verify, by comparing morphology and genetics (i.e., the cox1 and/or 16S sequence or amplification efficacy with specific primers)—the efficacy of head diagnostic characteristic for discriminating among V. alpinus, V. glacialis and V. psychrophilus based on the presence/absence of seta among OMMA A, B and C, D and of seta e7 on labium. Overall, 17 specimens were analysed, 7 V. glacialis (ORN20, 27; PEL 20,29,31,34,36), 6 V. psychrophilus (ORN 21,22,23,24,26,31) and 4 V. alpinus (PEL 20,24,26,28).
For each specimen, we amplified and sequenced two mitochondrial markers. The cytochrome c oxidase subunit 1, 5P fragment (cox1) was amplified using the primers 5′ GGTCAACAAATCATAAAGATATTGG 3′ (LCO1490, forward) and 5′- AAACTTCAGGGTGACCAAAAAATCA - 3′ (HCO2198, reverse) [44]. The mitochondrial ribosomal large subunit RNA (16S) was amplified using the primers 5′- CCGGTCTGAACTCAAATCATGT - 3′ (LR-J-12887M, forward) and 5′- CGACTGTTTAACAAAAACAT - 3′ (LR-N-13398M, reverse) (Simon et al. [45], modified after Zhang et al. [46]).
Due to the non-specific amplification for cox1 in D. orobica sp. nov. (TRO), G. bicolor (TGN and TOU_14), V. alpinus (MIA_3-4, MDG, and PAS) and V. glacieinigrae sp. nov. (GLN), we designed a different primer pair for this locus and employed it for populations of these four species. Specifically, a new forward primer (LCO_ALPI; 5′ TCAACAAACCACAAAGATATTGG 3′) was obtained from the correction of LCO1490 using the BOLD sequence of Desoria tigrina (OD987622.1). Two new HCO primers were specifically designed using a partial sequencing of the neighbour fragment of cytochrome c oxidase subunit 1 achieved through the partial amplification of the adjacent locus through the primers COIf (5′ - CCTGCAGGAGGAGGAGATCC - 3′, forward) COIa (5′- AGTATAAGCGTCTGGGTAGTC - 3′, reverse; [47]) for D. orobica sp. nov. (TRO), G. bicolor (TGN and TOU_14), V. alpinus (MIA_3–4, MDG, and PAS) and V. glacieinigrae sp. nov. (GLN). As such, two distinct reverse primers were obtained: HCO_PAS (5′- TATACTTCAGGRTGGCCAAAAAATCA - 3′), used with LCO_ALPI to amplify and sequence V. alpinus (PAS, MIA, MDG) and V. glacieinigrae sp. nov.; and HCO_TRO (5′- TAYACTTCTGGGTGCCCAAAAAATCA - 3′), employed with LCO_ALPI to amplify and sequence D. orobica sp. nov. (TRO) and G. bicolor and (TGN, and TOU_14).
PCRs were prepared with AmpliTaq Gold 360 Master Mix in a 10-μL reaction volume containing: 2 μL of whole genomic DNA and 0.5 μL of both forward and reverse primers (10 μM). Amplifications were run with the following conditions for each of the 35 cycles: a denaturation step at 95°C for 1 min, an annealing step at 50°C for 1 min and an elongation step at 72°C for 90s. An additional initial denaturation step was set at 95°C for 5 min as well as a final extension step at 72°C for 7 min [18, 47]. For TGN cox1 amplification, PCR cycles were extended up to 40 cycles. For D. calderonis (CAL), the 16S marker was sequenced from the same nine specimens of the original description [18].
PCR products were then purified using the kit Wizard SV Gel and PCR Clean-up (Promega, Madison, WI, USA), sequenced on both strands with a 3730 x l DNA Analyzer (Applied Biosystems) at the BMR Genomics laboratory (Padova, Italy) and assembled using Benchling [48]. Final corrected sequences were deposited in Genbank (NCBI) under accession numbers: PP441687–PP441920 (cox1) and PP465209–PP465442 (16S) (see Table A3 for details).
2.4.2. Phylogenetic Analysis
The final datasets included 232 sequences for both the cox1 and the 16S markers. To understand the phylogenetic relationships of the sampled species, three outgroups (Cryptopygus terranovus, Folsomia candida as in Valle et al. [18] replacing Parisotoma notabilis, for which both cox1 and 16S from the same specimens are not available, with Folsomotoma octooculata due to its proximity with studied ingroups [49]) were added to these two datasets and independently aligned. The cox1 dataset was aligned using macse v. 2.07 (-gc_def 5 [50],) to preserve codon structure, while the 16S dataset was aligned using MUSCLE [51] as implemented in Aliview v. 1.28 [52]. The two datasets were then trimmed in Gblocks (-t = c -b1 = $var -b2 = $var -b3 = 6 -b4 = 10 -b5 = h; where $var = number of sequences/2 + 1; [53]) and concatenated through catsequences (https://github.com/ChrisCreevey/catsequences). Sequences were collapsed into haplotypes using DNAcollapser as implemented in the FaBox web server (https://birc.au.dk/~palle/php/fabox/dnacollapser.php). The resulting final matrix was 985 nucleotides in length and accounted for 111 haplotypes (including the outgroups). Haplotype sequence headers were renamed according to all the haplotype site names. The alignment, along with the scheme partition file divided into blocks by gene and codon position (only for the cox1), was used for an IQTREE2 v. 2.2.0 (-B 1000 -m MFP+MERGE; [54]) analysis, with model estimated using the BIC criterion GTR+F+I+G4 for all the three partitions.
To unravel the possible phylogenetic relationships with other species belonging to the subfamily Isotominae, the published dataset used in Valle et al. [18] cox1 fragment only was downloaded from BOLD using the bold R package [55]. The longest sequence for each species studied in this work was added to the Valle et al. [18] dataset. The final trimmed alignment accounted for 97 sequences with 657 nucleotides in length. The matrix was partitioned as Valle et al. [18]- that is excluding the third position — and run in IQTREE2 with previously reported parameters. Both phylogenetic trees were visualised in iTOL v.6 [56].
2.4.3. Genetic Distances
To quantify genetic distances within and among species in the genera of interest, all Desoria and Vertagopus COI-5P sequences were downloaded from BOLD using the bold R package [55] and aligned through macse v.2.07, as previously described. Unaligned sequences, possibly belonging to another cox1 fragment and erroneously deposited in BOLD as COI-5P, were removed. Similarly, all cox1 sequences of Alpine springtail species were aligned with macse v.2.07, as previously described. Both matrices were separately imported in R v.4.3.2 [57]. Genetic distances were separately calculated for both matrices with the ape package [58] using the dist.dna function (model = ‘raw’, pairwise.deletion = T) and plotted with ggplot2 [59].
2.4.4. Species Delimination Analyses
Two methods of species delimitation were employed to assess species number and species boundaries within the COMBINED dataset. In detail, the glacial springtail phylogenetic tree was processed with bPTP (bayesian Poisson Tree Processes; available at: https://species.h-its.org/) under default parameters and applying a burn-in of 0.25 [60]. The corresponding sequence alignment was analysed in ASAP (assemble species by automatic partitioning; available at: https://bioinfo.mnhn.fr/abi/public/asap/) using default parameters [61]. The best partition was identified according to the lowest asap-score.
2.5. Nomenclatural Acts
The new names contained in this article are available under the International Code of Zoological Nomenclature. This work and the nomenclatural acts it contains have been registered in ZooBank. Zoobank Life Science Identifier (LSID) for this publication is: urn:lsid:zoobank.org:pub:CF4D6E35-869A-4677-B8C7-3577F6C95144. The LSID registration and any associated information can be viewed in a web browser by adding the LSID to the prefix ‘https://zoobank.org/’
3. Results
Ice-dwelling springtails were found in 32 sites of the 45 sites in the investigated European Alps (Table A1, Figure 2), and on the only glacier of the Apennines. All the ice-dwelling springtails were found on clean-ice glaciers and debris-covered glaciers, and not on snow patches and rock glaciers (Table A1), where only ground-dwelling species were found. In most of the cases, one single species was found on each glacier, but in eight sites two or three species were found (Table A1, Figure 2).
3.1. Morphological Analyses
Morphological analyses allowed the identification of eight distinct species on the European Alps, three already known - Desoria saltans, Vertagopus alpinus and Gnathisotoma bicolor, while five were undescribed so far: D. orobica sp. nov., V. glacialis sp. nov., V. psychrophilus sp. nov., V. glacieinigrae sp. nov. and V. fradustaensis sp. nov. (Table A1). Species descriptions and morphological notes are reported at the end of the results.
3.2. Genetic Analysis
The phylogenetic tree based on combined sequences of cox1 and 16S (Figure 3) is well structured, with long internal nodes and clusters characterised by a limited differentiation in the terminal ones. Internal nodes are usually supported by medium–high bootstrap values (99–100 for basal nodes, 83–98 for the other internal nodes, except for one with 51). Clusters are uniform, and there is a complete correspondence of these groups with species as morphologically identified. The basal branch separates Desoria saltans and D. cf. saltans from all other ice-dwelling springtails. G. bicolor and D. orobica constitutes two well separate clades, while D. calderonis, V. glacieinigrae, V. alpinus, Isotomidae sp., V. psychrophilus, V. glacialis constitute the apical branch of the tree. Two previously known species D. saltans and V. alpinus display intra-specific variability. Within G. bicolor, the individuals sampled in the Alps are reciprocally monophyletic with those from the Pyrenees. For Fellaria (FEL2), Miage (MIA1,5), Peirabroc (PEI4) and Scais (SCA1-5), we have a few specimens with only genetic data: SCA1-5 and FEL2 sequences correspond to D. saltans, PEI4 sequence is close to D. saltans but with a distance of 12.4%–13.6% (thus called Desoria cf.saltans), while MIA1,5 sequences were not closely related to any other known sequences and was nominated Isotomidae sp. (Figure 3).

Species delimitation analyses, performed using bPTP and ASAP, produced identical results identifying 17 different partitions with medium/high bPTP bayesian support (Figure 3). Eight out of 11 species (D. cf.saltans, D. orobica, D. calderonis, V. glacieinigrae, Isotomidae sp., V. psychrophilus, V. fradustaensis and V. glacialis) were retrieved as separate partitions that perfectly match the morphological and phylogenetic results. Three species (D. saltans, G. bicolor and V. alpinus, as identified based on morphology and phylogeny) were further split in 2–5 partitions each. These do not contrast with species attribution but may suggest additional internal diversification within named species (Figure 3).
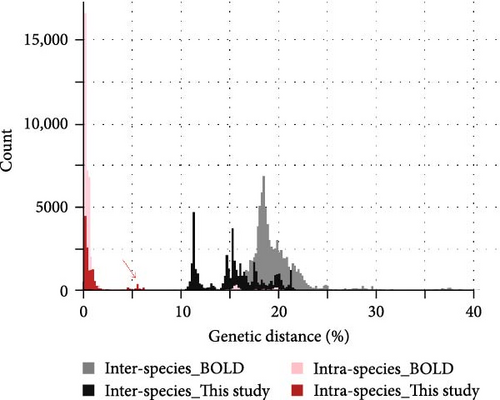
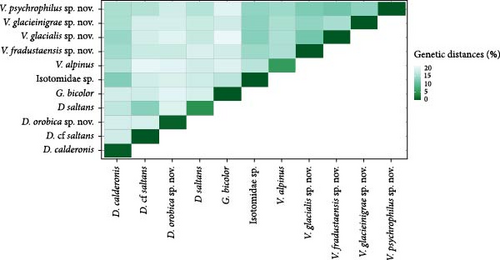
Genetic distances from cox1 reference sequences identified a distribution that spans between 0 and 1.8% for intra-specific comparisons and a spectrum higher than 14.2% (with a long tail in the upper range) for inter-specific comparisons (Figure 4). Some outliers of intra-specific diversity were identified around 14.5–20% and probably arise from unexpected divergence within named BOLD species that probably represent undescribed cryptic diversity. Within our dataset, intra-specific divergence ranges between 0 and 2.2%, while inter-specific divergence is higher than 10.6% in all inter-specific comparisons, showing a good correspondence to BOLD divergences and support for the species distinctions (Figure 4). A limited number of species show very high intra-specific divergence (around 4.4%–6%; red arrow in Figure 4): they correspond to D. saltans, G. bicolor and V. alpinus that display variability within species (visible also in the phylogenetic tree and in the species delimitation analyses, Figure 3). Overall, the level of differentiation, both within and among clusters, is fully in line with that observed for named species of the same genera in the BOLD database, supporting the subdivision of specimens collected among clusters. The distribution of intra- and inter-specific distances for cox1 visualised as a heatmap (Figure 5) shows the high intra-specific variability of D. saltans and V. alpinus, and identifies a close relationship among all Vertagopus species, including the unidentified Isotomidae sp. samples and D. calderonis.
Based on genetic analysis and morphologic description, paraphyly of Desoria is evident, from which a node emerges for Gnathisotoma, and another one includes the five Vertagopus species found in this work.
The phylogenetic investigation of the collocation of the new species described within the Isotominae tree is not possible because of the difficulty of collecting genetic information for such a large group of species. However, a preliminary investigation performed with the available data was conducted comparing our sequences (cox1 only) with the BOLD database, currently the most complete available dataset (Figure S1). Although the tree shows low bootstrap values and a polyphyletic distribution of the genera, the glacial species found in this work emerged as three separate clusters. This suggests that the ice-dwelling Vertagopus species, D. calderonis and D. orobica represent a monophyletic group, separated from D. saltans and G. bicolor.
From the specimens of V. alpinus, V. glacialis sp. nov. and V. psychrophilus sp. nov. for which the barcode was obtained after removing the head for morphological identification, it is possible to affirm that e7 seta on labium is always present in V. alpinus and V. psychrophilus, while on V. glacialis it could be missing in either one or both sides of the labium. The seta among ommatidia is absent in V. alpinus and V. glacialis sp. nov. and present in V. psychrophilus sp. nov.
3.3. Species Descriptions
From both morphological and phylogenetic analyses, Vertagopus glacialis, V. psychrophilus, V. glacieinigrae and V. fradustaensis (Figures 6A,B,C,F) resulted to be species new for science belonging to the genus Vertagopus genus, while Desoria orobica (Figure 6I) is a new species morphologically belonging to the Desoria nivalis complex. For the already described D. saltans, Gnathisotoma bicolor, V. alpinus and V. helveticus (Figures 6D,E,H,L), we present taxonomic, morphological and ecological notes for the Alpine populations. Genetic evidence indicates that Desoria cf. saltans found on Peirabroc (‘PEI4’; Maritime Alps) and Isotomidae sp. on Miage (‘MIA1,5’; Mont Blanc) could likely represent undescribed species; however, an insufficient number of specimens was found for their proper description.










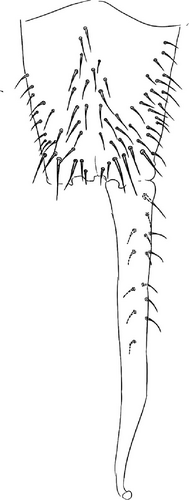
4. Vertagopus psychrophilus Valle sp. nov. Figures 1F, 6B, and 7A–I


4.1. Material Examined
Holotype: ITALY • ♂; Lombardia, Valfurva (Sondrio province), European Alps, Ortles-Cevedale massif, Sforzellina Glacier (‘SFO’); under stones on bare ice; on supraglacial snow and in supraglacial debris, 46°20′58.7”N, 10°30′44.1”E, 2860 ma.s.l.; 5 Aug. 2020, B. Valle leg.; B. Valle det., collected with flotation method; Genbank (NCBI) PP441886-63 (cox1), PP465408−15 (16S); (SMNG-APT-AA04468).
Paratype: ITALY • 2 ♂ 6 ♀; same collection data as for holotype; (MCSNBG-205 to 208, SMNG-APT-AA04469 to 72); ITALY • 1 ♂ and 2 ♀; Lombardia, Valdidentro (Sondrio province), European Alps, Dosdè Glacier (‘DOS’); under stones on bare ice; 46°23′34.8”N, 10°12′58.9”E, 2760 ma.s.l.; 5 Aug. 2020, R. Ambrosini, R.S. Azzoni leg.; B. Valle det., collected by hand; Genbank (NCBI) PP441719-28 95 (cox1), PP465241−50 (16S); (SMNG-APT-AA04473 to 75). ITALY • 1 ♂ and 2 ♀; Trentino, Stenico (Trento province), European Alps, Brenta Dolomites, Agola Glacier (‘AGO’); on supraglacial snow and ice, 46°08′57.9”N, 10°51′27.0”E, 2580 ma.s.l.; 10 Aug. 2020, M. Gobbi leg.; B. Valle det., collected by hand; Genbank (NCBI) PP441687-95 (cox1), PP465209-17 (16S); (MUSE-INV-c001 000414 to 416).
4.2. Description
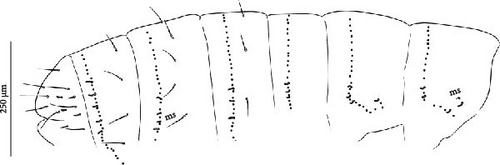
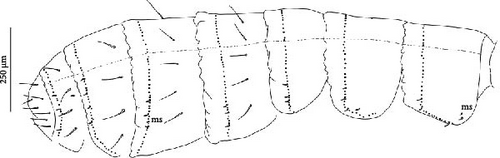
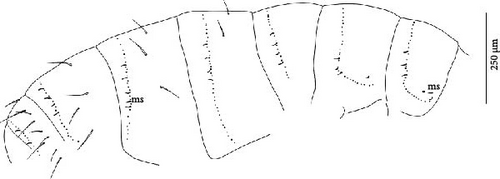
Body: Mean body length (with antennae) 1.9 (standard deviation: 0.3 mm on 11 specimens, see Table A2). Colour totally black, also juvenile, but the basis of the furca can be paler (Figure 6B). Cuticle granulation fine and regularly distributed; all dorsal tergites clearly separated from each other (Figures 7D, S3A). Abd. III and IV of approximately the same width.
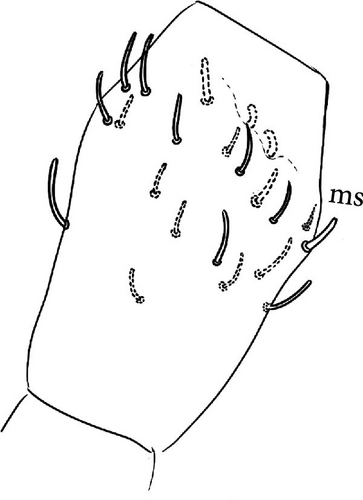
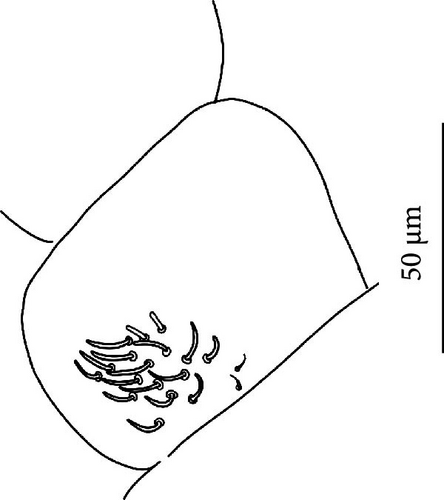
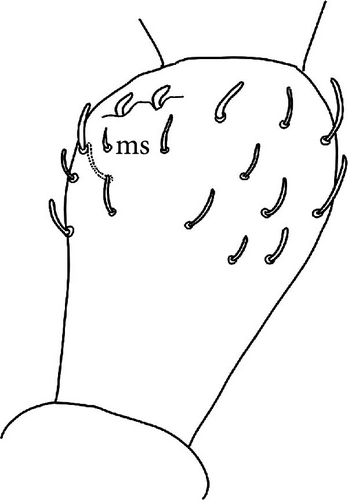
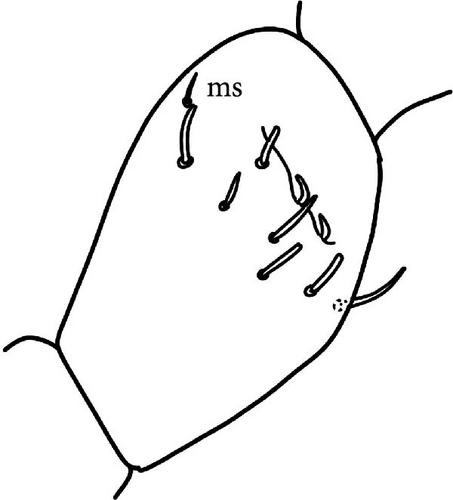
Chaetotaxy: Terga consist of micro-, meso- and macrosetae, these latter well differentiated on last abdominal tergites (Abd. IV–VI, in median position) but not well distinguished from ordinary setae on other tergites (Figure 7D). No setae in the ventral side of Th. II–Th. III. All setae smooth. Macrosetae on Abd. V 0.6 times the median length of tergite and 1.4 times as long as the inner edge of Claw III (Table A2). Sensory chaetotaxy constituted by ms-setae, accp-, al- and as-setae. Only Th. II and Abd. III have ms-setae (formula 10/001). Dorsal s-setae constituted by single al-seta on Th. II and Th. III, single as-seta on Abd. V and by accp-setae normally set within p-row (Figure 7D). The number of accp-setae can be expressed as (2)3, (2)3/ (2)3, (2)3, 3, 4(5), 3(4).
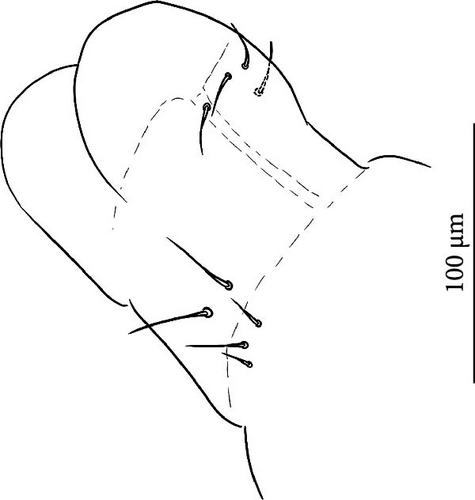
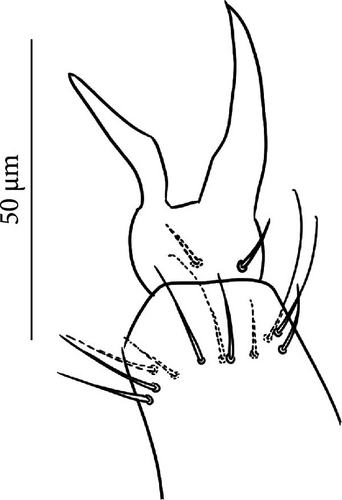
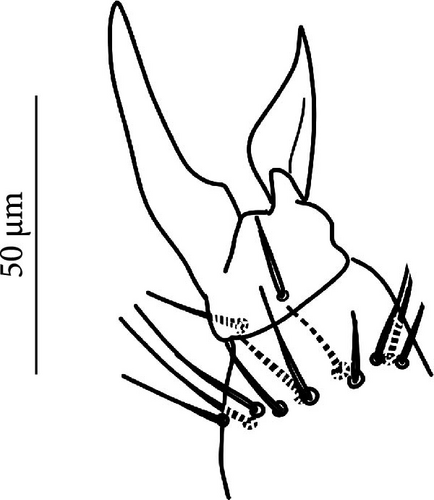
VT with 0 + 0 anterior, 4 + 4 latero-distal and (4)5-6 posterior setae with 2 in apical transverse row (Figure 7F). Retinaculum with 4 teeth and 4-7 setae.
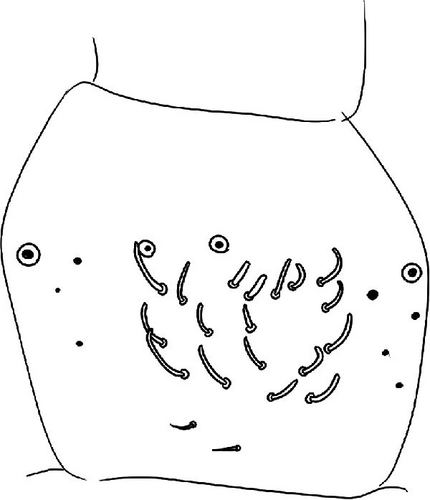
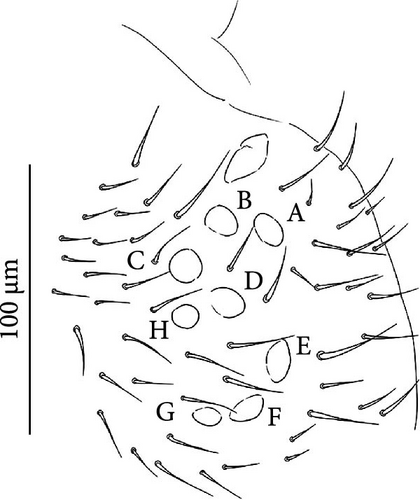
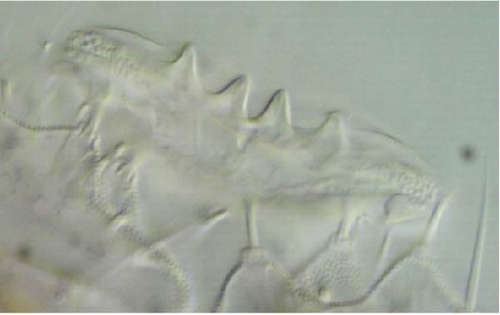
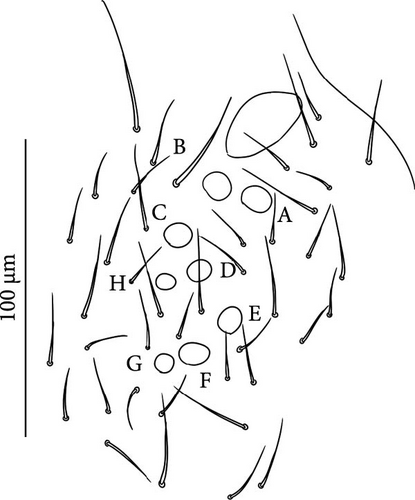
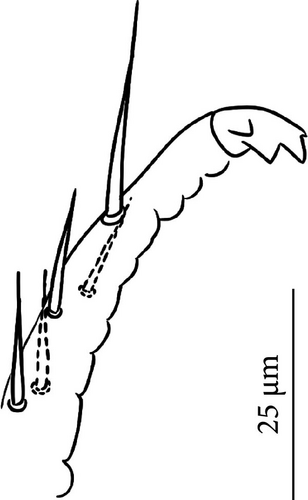
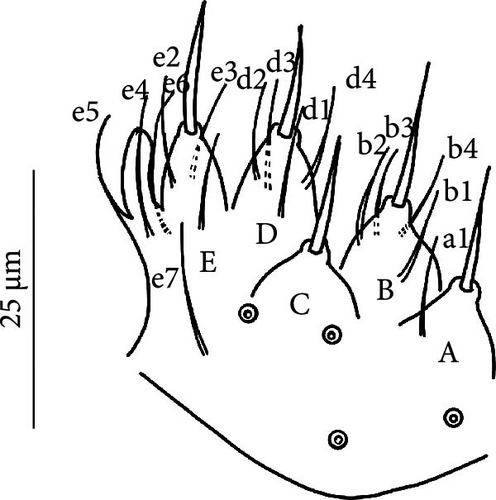
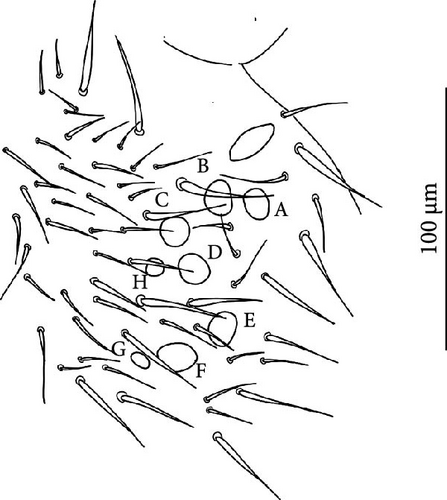
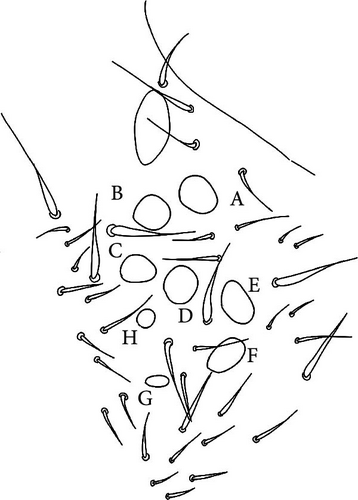
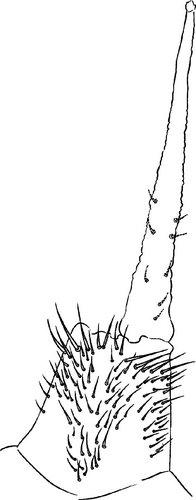
Head: Antennae slightly longer than cephalic diagonal (D/A = 0.38, Antennae = 0.43 mm; Table A2). Ratio among Ant. I/Ant. II/Ant. III/Ant. IV is 1/1.5/1.4/2.7 (Table A2). There are often cases of asymmetry among s-setae between antennae of the same specimen. Ant. I has 12–23 s-setae in ventro-lateral position, (1)-2-(3) of them are shorter and in distal position; two microsetae in ventro-proximal position (Figure 7A). Ant. II 4–6 s-setae in distal position. Ant. III has a sensory field that includes two inner s-setae of AO III, one lateral ms-seta and about 11–15 s-setae (Figure 7B). Ant. IV has one simple small subapical, peg-shaped organite and a bifurcate pin-like seta (Figure 7H). Eye spots strongly dark pigmented with 8 + 8 ocelli (G and H smaller; Figures 7C, S3B). Presence of a seta among ommatidia A, B and C, D (Figure 7C); sometimes there is asymmetry and only one side has the seta among ommatidia (important to check several specimens). PAO elongated about two times as long as the diameter of the nearest ocellus (Figure 7C). Prelabral setae 4. Labral formula as 5,5 and 4 and 4 sharp apical folds (Figures 7I, S3I). Maxillary palp bifurcated and maxillary outer lobe with four sublobal hairs. Labial palp with 5 papillae and a total of 16 guard setae [62] distributed as: A1, B1–4, C0, D1–4, E1–7 (like in Figure S3I). Hypostomal papilla with H shorter than h1/h2. Proximal (px), basomedian (bm) and basolateral (bl) fields of labium with 4, 4 and 5 setae, respectively, and 4–8 postlabial setae. Mandible with well-developed molar plate.
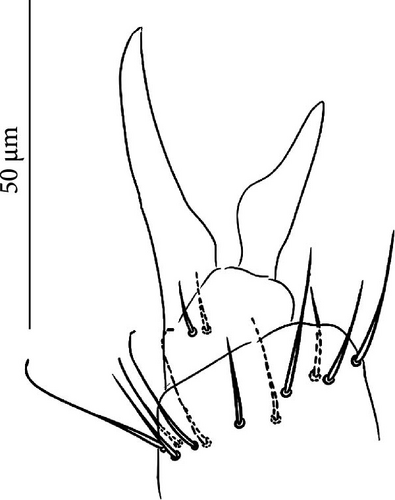
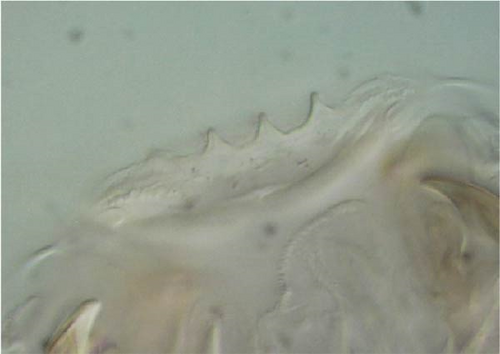
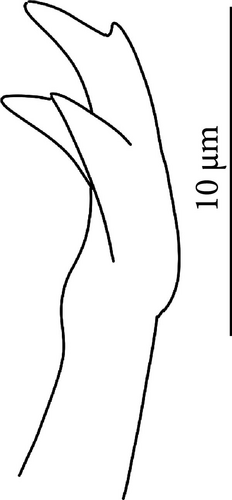
Furca: Furca almost as long as the antenna (see Table A1; Figure 7G); ratio of mucro/dens/manubrium = 1/25/16. Maximum number of ventral setae on manubrium about 38–40 (less in juveniles, and it is possible to confuse them with species A), including ventral–apical setae ((8)−10) that are usually larger than the others (Figure 7G) except for 1/2 + 1/2 shorter setae in apical–medial position; about 40 dorsal setae. Dens with dorsal crenulations, more than 90 ventral and 12–14 dorsal setae. Mucro quadridentate with apical tooth much smaller than subapical one (Figure S3C).
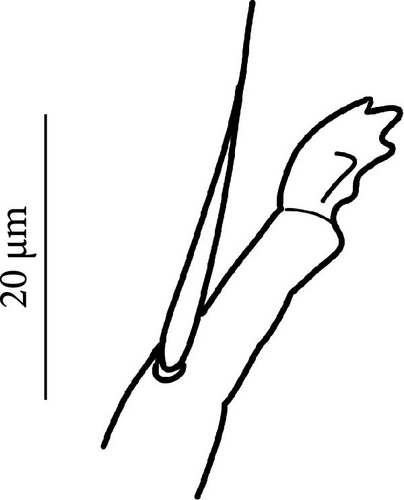
Legs: Eleven setae in the distal apical ring of Tita; 2-3-3 very weakly clavate tenent hairs (Figure 7E), shorter than the inner edge of Cl. Claw of normal shape without lateral and inner teeth; empodium sometimes with a small inner tooth; pretarsus with a pair of setae (Figures 7E, S3E).
Distribution and habitat: V. psychrophilus has been found in 10 Alpine glaciers from Western to Eastern Alps. Most records are in Southern Rhaetian Alps in Italy, three are in Graian Alps and one in Pennine Alps (Figure 2; Table A1 for more information). In general, past records of D. saltans should be revised and could probably be attributed to V. glacialis and V. psychrophilus (see notes in D. saltans).
Ecology: V. psychrophilus is a strictly ice-dwelling springtail that can be found on bare ice under stones, within supraglacial continuous stony debris (like medial moraines), on supraglacial snow (Figure 1F), and under stones on bare ice (Figure 1C). Valle et al. [41] noted its distribution on supraglacial debris and its rapid disappearance just moving from the ice front (as ‘Vertagopus sp. nov.’). Its life cycle and behaviour on the ice may be similar to that of D. saltans (see notes for this species); however, V. psychrophilus is probably more linked to stony microhabitat on the ice.
Some past ecological studies on D. saltans or ice-dwelling species in general could be referred to this species, but it is not verifiable if morphological notes are not reported.
Etymology: the epithet refers to the ecology of the species that is a strictly ice-dwelling cryophilic species.
Remarks: V. psychrophilus belongs to this new group of closely related, strictly ice-dwelling Vertagopus species that includes also V. glacialis, V. glacieinigrae and V. fradustaensis. These species are close to V. alpinus for the absence of setae on the anterior side of the VT and a small apical tooth on mucro but differ in the presence of only weakly clavate tenent hairs (strongly clavate in V. alpinus). Among the group of strictly ice-dwelling Vertagopus species, V. psychrophilus is differentiated from V. glacialis by a higher number of anterior setae on Man (on average 38–42, 18−22 in V. glacialis), a higher number of accp-setae (usually 3 from Th. II to Abd. III, less in V. glacialis), the presence of e7 seta on labium (that could be absent in V. glacialis) and the presence of a seta among ommatidia A, B and C, D (usually absent in V. glacialis). V. psychrophilus is differentiated from V. glacieinigrae for having Abd. V-VI well separated by a narrowing (they are not clearly separated in V. glacieinigrae) and from V. fradustaensis from the higher number of anterior setae on Man (38–40 in V. psychrophilus, 26–30 in V. fradustaensis).
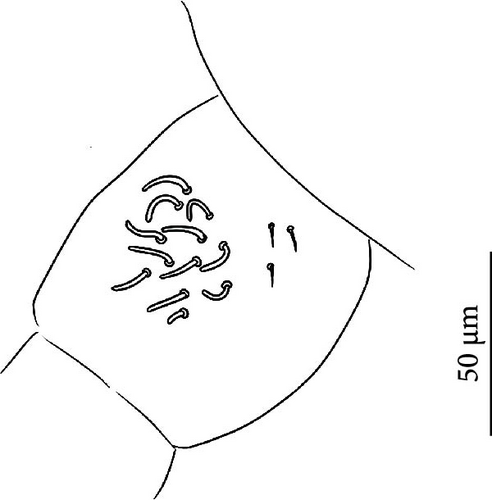
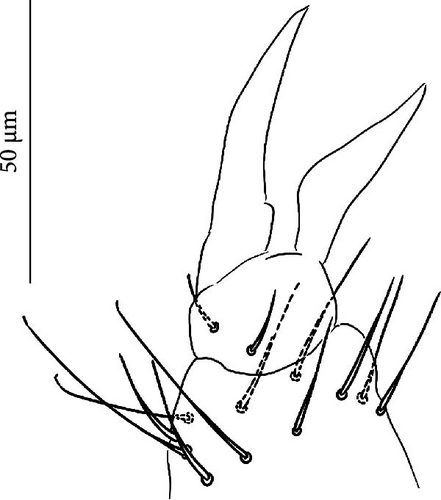
5. Vertagopus glacialis Valle sp. nov. Figures 1C,H, 6A, 8A–I
5.1. Material Examined
Holotype: ITALY • ♀; Lombardia, Valfurva (Sondrio province), Ortles-Cevedale massif, European Alps, Forni Glacier (‘GFO’); under stones on bare ice; 46°23′42.95”N, 10°35′24.79”E, 2630 ma.s.l.; 3 Aug. 2020; B. Valle leg.; B. Valle det., collected by hand; Genbank (NCBI) PP441735−41 (cox1), PP465257−63 (16S); (SMNG-APT-AA04476).
Paratype: ITALY • 2 ♂ and 1 ♀; same collection data as for holotype; (MCSNBG-209 to 211). ITALY • 3 ♀; Alto Adige, Senales (Bolzano province), European Alps, Giogo Alto Glacier (‘GIO’); under stones on bare ice; 46°46′52.1”N, 10°48′20.7”E, 2840 ma.s.l.; 21 Aug. 2020, B. Valle, M. Caccianiga leg.; B. Valle det., collected by hand; Genbank (NCBI) PP441742−50 (cox1), PP465264−72 (16S); (MCSNBG-212 to 214). SWITZERLAND • 2 ♂ and 1 ♀; Hérens, Evolène, European Alps, de Pièce Glacier (‘PIE’), under stones on bare ice, 45°59′59.7”N, 7°28′12.2”E, 2750–2800 ma.s.l.; 17 Aug. 2021, J. Buda leg.; B. Valle det., collected by hand; Genbank (NCBI) PP441859−61,63−69 (cox1), PP465381−91 (16S); (SMNG-APT-AA04477 to 79). SWITZERLAND • 2 ♂; Maloja, Pontresina, European Alps, Bernina massif, Pers Glacier (‘PER’); under stones on bare ice, 46°24′25.9”N, 9°57′32.4”E, 2670–2700 ma.s.l.; 30 Jul. 2021, J. Buda leg.; B. Valle det., collected by hand; Genbank (NCBI) PP441851−58 (cox1), PP465373−80 (16S); (MUSE-INV-c001 000417–418). SWITZERLAND • 1 ♀; Bezirk Westlich Raron, Blatten, European Alps, Lang Glacier (‘VAL’); under stones on bare ice, 46°27′28.3”N, 7°55′55.8”E, 2450–2550 ma.s.l.; 10 Aug. 2021, J. Buda leg.; B. Valle det., collected by hand; Genbank (NCBI) PP441914−20 (cox1), PP465436−42 (16S); (SMNG-APT-AA04480). SWITZERLAND • 1 ♂; Oberland, Guttannen, European Alps, Oberaar Glacier (‘OBE’); under stones on bare ice, 46°32′08.6”N, 8°13′12.4”E, 2380–2440 ma.s.l.; 14 Aug. 2021, J. Buda leg.; B. Valle det., collected by hand; Genbank (NCBI) PP441813−18 (cox1), PP465335−40 (16S); (SMNG-APT-AA04481).
5.2. Description
Body: Mean body length (with antennae) 2.2 mm (standard deviation: 0.2 mm on 29 specimens, Table A2). Colour totally black, also juvenile, but the basis of the furca could be paler (Figure 6A). Cuticle granulation fine and regularly distributed; all dorsal tergites clearly separated from each other (Figures 8D, S4A). Abd. III and IV of approximately same width.
Chaetotaxy: Terga consist of micro-, meso- and macrosetae, these latter well differentiated on last abdominal tergites (Abd. IV–VI, in median position) but not well distinguished from ordinary setae on other tergites (Figures 8D, S4A). No setae in ventral side of Th. II–Th. III. All setae smooth. Macrosetae on Abd. V 0.5 times median length of tergite and 1.2 times as long as the inner edge of Claw III (Table A2). Sensory chaetotaxy constituted by ms-setae, accp-, al- and as-setae. Only Th. II and Abd. III have ms-setae (formula 10/001). Dorsal s-setae constituted by single al-seta on Th. II and Th. III, single as-seta on Abd. V and by accp-setae normally set within p-row (Figure 8D). The number of accp-setae can be expressed as 2, 2/1(2), 1, 1–3, 2–3, 3(4).
VT with 0 + 0 anterior, 4 + 4 latero-distal and (4)5–9 posterior setae with 2 in apical transverse row (Figure 8F).
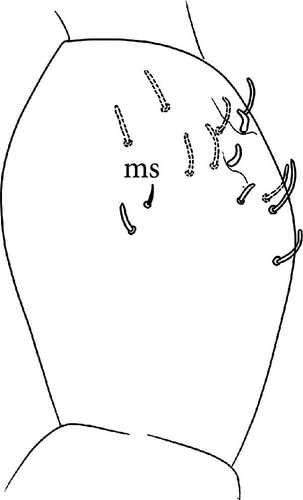
Head: Antennae slightly longer than cephalic diagonal (D/A = 0.42, Antennae = 0.45 mm; Table A2). Ratio among Ant. I/Ant. II/Ant. III/Ant. IV is 1.0/1.6/1.5/3.0 (Table A2). There are often cases of asymmetry among s-setae between antennae of the same specimen. Ant. I has 8–24 s-setae in ventro-lateral position, 1-(2) of them are shorter and in distal position; (1)-2-(3) microsetae in ventro-proximal position (Figure 8A). Ant. II 6 s-setae in distal position. Ant. III has a sensory field that includes 2 inner s-setae of AO III, one lateral ms-seta and about 10–13 s-setae (Figure 8B). Ant. IV with one simple small subapical, peg-shaped organite and a bifurcate pin-like seta (Figure 8G). Eye spots strongly dark pigmented with 8 + 8 ocelli (G and H smaller; Figures 8C, S4B). Usually no setae among OMMA A, B and C, D. PAO elongated (sometimes a weak median constriction is visible) about 1.5 times as long as the diameter of the nearest ocellus (Figure 8C). Prelabral setae 4. Labral formula as 5,5 and 4 and 4 sharp apical folds (Figure 8I). Maxillary palp bifurcated and maxillary outer lobe with 4 sublobal hairs. Labial palp with 5 papillae and a total of 15–16 guard setae [62] are distributed as A1, B1–4, C0, D1–4, E1–6/7 (Figure S4D). Characteristic of this species is the variability in number of guard setae. Usually, e7 is not present but it could be present (like in Figure 8E) and frequently the same individual has one labium with e7 and the other one without (see remarks). Hypostomal papilla with H shorter than h1/h2. Proximal (px), basomedian (bm) and basolateral (bl) fields of labium with 4, 4 and 5 setae, respectively. 4–7 postlabial setae. Mandible with well-developed molar plate.
Furca: Furca almost as long as antenna; ratio of mucro/dens/manubrium = 1/24/16 (Figure 8G. Table A2). Maximum number of ventral setae on manubrium about 18–20-(28), including ventro-apical setae (8) larger than the others (Figure 8G) except for 1(3) + 1(3) shorter setae in apical–medial position; about 50 dorsal setae on Man. Dens with dorsal crenulations, more than 60 ventral and 14–22 dorsal setae. Mucro quadridentate with apical tooth much smaller than subapical one (Figure S4C). Retinaculum with 4 teeth and 5–7 setae.
Legs: Eleven setae in the distal apical ring of Tita; 2–3–3 very weakly clavate tenent hair, shorter than the inner edge of Cl (Figures 8E, S4E). Claw of normal shape without lateral and inner teeth; empodium sometimes with a small inner tooth (Figures 8E, S4E); pretarsus with a pair of setae.
Distribution and habitat: V. glacialis has been found on several Alpine glaciers from Western to Eastern Alps. V. glacialis is present in the Rhaetian, Bernese, Pennine and Graian Alps (Figure 2, Table A1 for more information).
According to the distribution of this species, some past records — ‘Isotomidae sp.’ in Buda et al. [63] and ‘Desoria glacialis’ in Stoppani [64] on Forni Glacier, and ‘Isotoma sp. G’ in Zettel [24] on Oberaar glacier (Bernese Alps, Switzerland) — could be attributed to this species. Buda et al. [63] provided cox1 sequence that corresponds to that of V. glacialis. Zettel [24] specified that it was a ‘still undescribed species of the fennica-propinqua group’ sensu Gisin (1960); Gisin [65] gives few characteristics for these two species but compatible with V. glacialis (in particular, mucro with four teeth, the apical one very small). In general, many other past records of D. saltans should be revised and could probably be attributed to V. glacialis and V. psychrophilus (see notes for D. saltans).
Ecology: V. glacialis is a strictly ice-dwelling springtail that in summer can be found on bare ice under stones (Figure 1C, H), within supraglacial continuous stony debris (like medial moraines; Figure 1B) and on supraglacial snow (for example, Stoppani [64]: see notes in ‘Distribution and habitat’; Figure 1F). Checking under the debris just in front of the glaciers, this species disappears moving out from the ice (like for V. psychrophilus in Valle et al. [41]). Its life cycle and behaviour on the ice may be similar to that of D. saltans (see notes for this species). However, V. glacialis is probably more linked to stony microhabitat on the ice.
Buda et al. [63] probably studied the distribution of V. glacialis under stones along with a transect starting in the forefield toward the centre of Forni Glacier ablation zone (see notes on distribution). Individuals were more abundant under stones on bare ice than under those belonging to the central moraine; however, the authors probably underestimated abundances inside the moraines since they did not use the flotation method [41]. In fact, we observed high abundances of this species within the supraglacial debris. In Zettel [24], analyses of the thermal hysteresis of a ‘Isotoma sp. G’ attributable to V. glacialis are reported (see notes in ‘Distribution and habitat’). Jaroměřská et al. [66] studied the isotopic composition of an ice-dwelling Isotomidae attributable to V. glacialis.
Etymology: the epithet refers to the typical habitat of the species that are Alpine glaciers.
Remarks: V. glacialis belongs to the new group of closely related, strictly ice-dwelling Vertagopus species that also includes V. psychrophilus, V. glacieinigrae and V. fradustaensis. These species are close to V. alpinus for the absence of setae on the anterior side of the VT and a small apical tooth on mucro but differ by the presence of only weakly clavate tenent hairs (strongly clavate in V. alpinus). Among the group of strictly ice-dwelling Vertagopus species, V. glacialis is characterised by a reduced number of anterior setae on Man (on average 18–22, 26−40 in the others) reduced number of accp-setae (only 2 on each thoracic terga and 1 on first abdominal terga, less than in other species). Other important features are the absence of seta among ommatidia A, B and C, D absent (Figure 8C) and the absence of e7 seta on labium in some specimens: in 31 specimens checked (3–4 from each population), 18 specimens don’t have e7 (58%), 7 specimens have e7 only on one side (23%) and 6 have e7 on both sides (19%). This characteristic is equally distributed among the populations checked (Table A1).
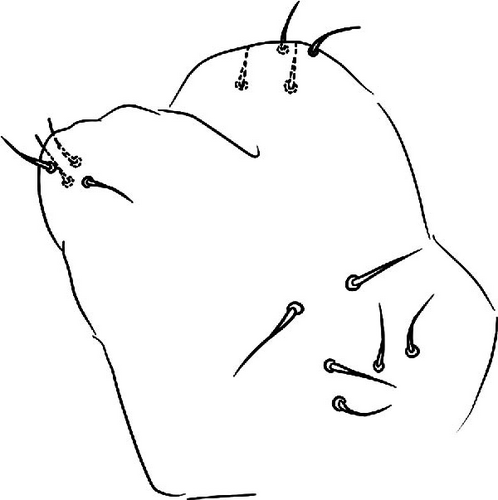
6. Vertagopus glacieinigrae Valle sp. nov. Figures 6C, 9A−I
6.1. Material Examined
Holotype: FRANCE • ♀; Provence-Alpes-Côte d’Azur, Vallouise-Pelvoux, European Alps, Écrins massif, Glacier Noir (‘GLN’); in supraglacial debris 44°55′11.3”N, 6°23′37.8”E, 2240 ma.s.l.; 9 Aug.2021, B. Valle leg.; B. Valle det., collected with flotation method; Genbank (NCBI) PP441759−64 (cox1), PP465281−86 (16S); (SMNG-APT-AA04482).
Paratype: FRANCE • 2 ♂ and 2 ♀; same collection data as for holotype; (SMNG-APT-AA04483 to 86).
6.2. Description
Body: Mean body length (with antennae) 1.7 mm (standard deviation: 0.1 mm on 10 specimens, Table A2). Colour black with appendages a little brownish (Figure 6C); juveniles are brownish. Cuticle granulation fine and regularly distributed; all dorsal tergites separated from each other, but Abd. V-VI not clearly separated by a narrowing (Figure 9D). Abd. III and IV of approximately the same width.
Chaetotaxy: Terga consist of micro-, meso- and macrosetae, the latter well differentiated on last abdominal tergites (Abd. IV–VI, in median position), but not well distinguished from ordinary setae on other tergites (Figure 9D). No setae in the ventral side of Th. II–Th. III. All setae smooth. Macrosetae on Abd. V is equal to the median length of tergite and 2.2 times as long as the inner edge of Claw III (Table A2). Sensory chaetotaxy constituted by ms-setae, accp-, al- and as-setae. Only Th. II and Abd. III have ms-setae (formula 10/001). Dorsal s-setae constituted by single al-seta on Th. II and Th. III, two as-seta on Abd. V and by accp-setae normally set within p-row (Figure 9D). The number of accp-setae can be expressed as 3, 3/3, 3, 3, 5, 3.
VT with 0 + 0 anterior, 4 + 4 latero-distal and 4 posterior setae with 2 in apical transverse row (Figure 9F).
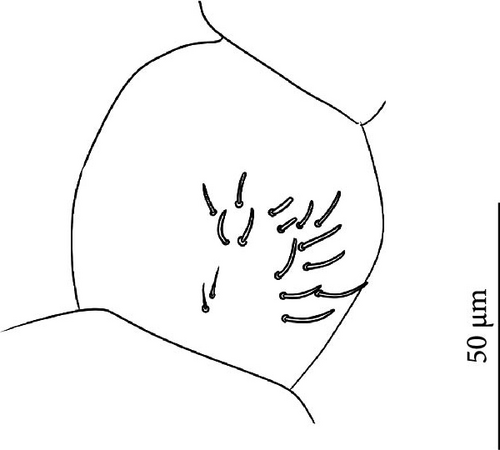
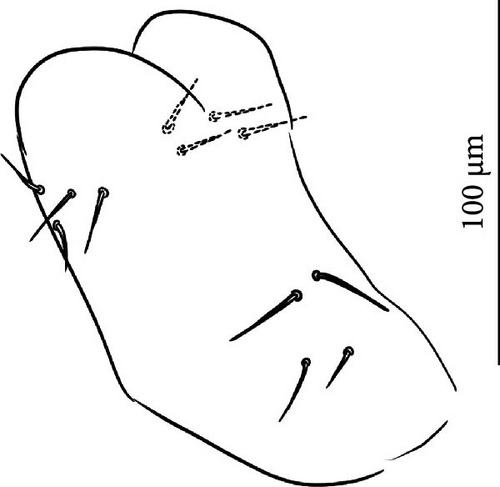
Head: Antennae longer than cephalic diagonal (D/A = 0.33, Antenna = 0.38 mm; Table A2). Ratio among Ant. I/Ant. II/Ant. III/Ant. IV is 1/1.7/1.6/2.9 (Table A2). There are often cases of asymmetry among s-setae between antennae of the same specimen. Ant. I has 8–13 s-setae in ventro-lateral position, 1–3 of them are shorter and in distal position; 2 microsetae in ventro-proximal position (Figure 9A). Ant. II has 6–11 s-setae in distal position. Ant. III has a sensory field that includes 2 inner s-setae of AO III, one lateral ms-seta and about 18 s-setae (Figure 9B). Ant. IV has one big subapical, spherical organite and a bifurcate pin-like seta (Figure 9H). Eye spots strongly dark pigmented with 8 + 8 ocelli subequal and very small (Figure 9C). Presence of a seta among ommatidia A, B, C, D (Figure 9C) PAO little elongated, about three times as long as the diameter of the nearest ocellus (Figure 9C). Prelabral setae 4. Labral formula as 5–5–4 and 4 apical folds squared at the apex (Figure 9I). Maxillary palp bifurcated and maxillary outer lobe with four sublobal hairs. Labial palp with five papillae and a total of 16 guard setae [62] distributed as: A1, B1–4, C0, D1–4, E1–7 (like in Figure 9E). Hypostomal papilla with H shorter than h1/h2. Proximal (px), basomedian (bm) and basolateral (bl) fields of labium with 4, 4 and 5 setae, respectively. 4–5 postlabial setae. Mandible with a well-developed molar plate.
Furca: Furca longer than antenna; ratio of mucro/dens/manubrium = 1/25/13 (Table A2). Ventral setae on manubrium about 36, including ventro-apical setae (8) larger than the others with the exception of 1 + 1 short setae in apical–medial position; more than 40 dorsal setae on Man. Dens with dorsal crenulations, more than 60 ventral and 11–12 dorsal setae. Mucro quadridentate with apical tooth much smaller than subapical one (Figure 9G). Retinaculum with 4 teeth and 5–6 setae.
Legs: 11 setae in the distal apical ring of Tita; 2–3–3 very weakly clavate tenent hairs (Figure 9E), shorter than the inner edge of Cl. Claw of normal shape without lateral and inner teeth; empodium without inner tooth; pretarsus with a pair of setae (Figure 9E).
Distribution and habitat: V. glacieinigrae is currently known only for the type locality, Glacier Noir in French Alps (Figure 2, Table A1).
Ecology: V. glacieinigrae is an ice-dwelling species that inhabits deep supraglacial stony debris (like Figure 1A) of a debris-covered glacier.
Etymology: the epithet literally means ‘of black ice’ and refers both to its occurrence on supraglacial debris of a debris-covered glacier (‘glacier noir’, black glacier, in French) and to the name of the glacier where the species lives (Glacier Noir).
Remarks: V. glacieinigrae belongs to the new group of closely related, strictly ice-dwelling Vertagopus species that also includes V. psychrophilus, V. glacialis, V. fradustaensis. These species are close to V. alpinus for the absence of setae on the anterior side of the VT and a small apical tooth on mucro but differ by the presence of only weakly clavate tenent hairs (strongly clavate in V. alpinus). Among the group of strictly ice-dwelling Vertagopus species, V. glacieinigrae is differentiated from all other species for having a big and spherical organite on Ant. IV (small and peg-shaped in the others) and the PAO 3 times as long as the nearest ocellus (<2 in the others). It is differentiated from V. glacialis even by a higher number of anterior setae on Man (on average 36, 18–22 in V. glacialis) and a higher number of accp-setae (usually 3 from Th. II to Abd. III, less in V. glacialis).
7. Vertagopus fradustaensis Valle sp. nov. Figures 6F, 10A–I

7.1. Material Examined
Holotype: ITALY • ♂; Trentino, Tonadico (Trento province), European Alps, Pale di San Martino, Fradusta Glacier (‘FRA’); under stones on bare ice and in ice fractures; 46°15′06.8” N, 11°52′21.4” E; 2845 ma.s.l.; 8 Aug. 2023; M. Gobbi leg.; B. Valle det., collected by hand; Genbank (NCBI) PP441732−34 (cox1), PP465254−56 (16S). (SMNG-APT-AA04487).
Paratype: ITALY • 5 ♂; same collection data as for holotype; (SMNG-APT-AA04488 to 89, MUSE-INV-c001 000419–421).
7.2. Description
Body: Mean body length (with antennae) 2.0 mm (standard deviation: 0.1 mm on seven specimens, see Table A2). Colour black (also juvenile) but with intermediate cuticle between body segments paler and weakly corrugate (Figures 6F, 10D), at least in specimens preserved in alcohol. Cuticle granulation fine and regularly distributed; all dorsal tergites clearly separated from each other (Figure 10D). Abd. III and IV of approximately the same width.
Chaetotaxy: Terga consisting of micro-, meso- and macrosetae, these latter well differentiated on last abdominal tergites (Abd. IV–VI, in median position), but not well distinguished from ordinary setae on other tergites (Figure 10D). No setae in the ventral side of Th. II–Th. III. All setae smooth. Macrosetae on Abd. V 0.6 times the median length of tergite and 1.8 times as long as the inner edge of Claw III (Table A2). Sensory chaetotaxy constituted by ms-setae, accp-, al- and as-setae. Only Th. II and Abd. III have ms-setae (formula 10/0010). Dorsal s-setae constituted by a single al-seta on Th. II and Th. III, a single as-seta on Abd. V and by accp-setae normally set within p-row (Figure 10D). The number of accp-setae can be expressed as (33/33343).
VT with 0 + 0 anterior, 4 + 4 latero-distal and 4–5 posterior setae with 2 in apical transverse row (Figure 10G).
Head: Antennae longer than cephalic diagonal (D/A = 0.8, Antennae = 0.4 mm. Table A2). Ratio among Ant. I/Ant. II/Ant. III/Ant. IV is 1/1.7/1.6/3.1 (Table A2). There are often cases of asymmetry among s-setae between antennae of the same specimen. Ant. I has 11–15 s-setae in ventro-lateral position, two of them are shorter in the distal position; 2 microsetae in ventro-proximal position (Figure 10A). Ant. II has 5–8 s-setae in distal position. Ant. III has a sensory field that includes 2 inner s-setae of AO III, 1 lateral ms-seta and about 9–13 s-setae (Figure 10B). Ant. IV has one simple small subapical, peg-shaped organite and a bifurcate pin-like seta. Eye spots strongly dark pigmented with 8 + 8 ocelli (G and H smaller). Seta among ommatidia A, B and C, D usually present (Figures 10H–I). PAO elongated about 1.5 times as long as the diameter of the nearest ocellus (Figures 10H–I). Prelabral setae 4. Labral formula as 5, 5 and 4 and 4 apical folds squared at the apex (like in Figure 10I of V. glacieinigrae). Maxillary palp bifurcated and maxillary outer lobe with four sublobal hairs. Labial palp with five papillae and a total of 16 guard setae [62] distributed as: A1, B1–4, C0, D1–4, E1–7 (Figure 10E). Hypostomal papilla with H shorter than h1/h2. Proximal (px), basomedian (bm) and basolateral (bl) fields of labium with 4, 4 and 5 setae, respectively, and 7–9 postlabial setae. Mandible with well-developed molar plate.
Furca: Furca longer than antenna (see Table A2); ratio of mucro/dens/manubrium = 1/30/17. Maximum number of ventral setae on manubrium about 26–30 (less in juveniles), including four ventro-apical setae that are usually larger than the others except 1(2) + 1(2) shorter setae in apical–medial position; more than 30 dorsal setae. Dens with dorsal crenulations, more than 80 ventral and with 10- (14) dorsal setae. Mucro quadridentate with apical tooth much smaller than subapical one (Figure 10C). Retinaculum with 4 teeth and 4–8 setae.
Legs: Eleven setae in the distal apical ring of Tita; 2–3–3 very weakly clavate tenent hairs (Figure 10F), shorter than the inner edge of Cl. Claw of normal shape without lateral and inner teeth; empodium sometimes without inner tooth; pretarsus with a pair of setae (Figure 10F).
Distribution and habitat: V. fradustaensis is currently known only for the type locality, Fradusta glacier (‘FRA’; Dolomites, Italy) (Figure 2, Table A1).
Ecology: V. fradustaensis seems to be a strictly ice-dwelling springtail that could be found on bare ice under stones and in ice fractures. Its life cycle and ecology on ice may be similar to that of D. saltans, but ecological studies are needed.
Etymology: the epithet refers to the name of the single glacier where the species was found, Fradusta Glacier in Dolomites Mts, Italy.
Remarks: V. fradustaensis belongs to the new group of closely related, strictly ice-dwelling Vertagopus species that also includes V. psychrophilus, V. glacialis and V. glacieinigrae. These species are close to V. alpinus for the absence of setae on the anterior side of the VT and a small apical tooth on mucro but differ by the presence of only weakly clavate tenent hairs (strongly clavate in V. alpinus). Among the group of strictly ice-dwelling Vertagopus species, V. fradustaensis is differentiated from V. glacialis by a higher number of anterior setae on Man (on average 26–30, 18−22 in V. glacialis), a higher number of accp-setae (3 from Th. II to Abd. III, less in V. glacialis) and for usually having the seta among ommatidia A, B and C, D (Figure 10H). V. fradustaensis is differentiated from V. glacieinigrae for having Abd. V–VI clearly separated by a narrowing (not clear in V. glacieinigrae), a small, peg-shaped organite on Ant. IV (big and spherical in V. glacieinigrae) and the PAO only 1.5 times as long as the nearest ocellus (not 3 times as in V. glacieinigrae). From V. psychrophilus, V. fradustaensis differs in the different pigmentation patterns, being V. fradustaensis characterised by having paler cuticle in the area between body tergites.
8. Desoria orobica sp. nov. Valle B. Figures 1I, 6I, 11A–L

8.1. Material Examined
Holotype: ITALY • ♀; Lombardia, Valbondione (Bergamo province), European Alps, Orobian Alps Trobio Glacier; inside cold, wet and inorganic stony debris at the border of a snow-patch; 46°03′25.5"N, 10°05′30.0"E, 2700 ma.s.l.; July 18, 2020; B. Valle, M. Caccianiga leg.; B. Valle det., collected with flotation method; Genbank (NCBI) PP441909−13 (cox1), PP465431−35 (16S). (SMNG-APT-AA04490).
Paratype: ITALY • 5 ♀; same collection data as for holotype; (SMNG-APT-AA04491 to 92, MCSNBG-215 to 217).
8.2. Description
Body: Mean body length (with antennae) 1.4 mm (standard deviation: 136 mm on 7 specimens, see Table A2). Colour violet-black, with Ant. II–IV yellow, except for a black spot on the top of Ant. IV (Figures 1H, 6I); juveniles have the same pattern but with a paler body. Cuticle granulation fine and regularly distributed; all dorsal tergites clearly separated from each other, except for Abd. V–VI that are only slightly fused (Figures 11D, S5A). Abd. III and IV approximately the same width (Figure 11D).
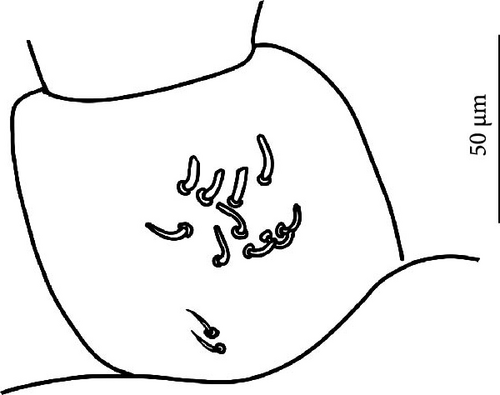
Chaetotaxy: Terga plurichaetotic, consisting of micro-, meso- and macrosetae, the latter well differentiated on last abdominal tergites (Abd. IV–VI, in median position), but not well distinguished from ordinary setae on other tergites (Figures 11D, S5A). No setae in ventral side of Th. II–Th. III. All setae smooth. Macrosetae on Abd. V 0.6 times the median length of tergite and 2 times as long as the inner edge of Claw III (Table A2). Sensory chaetotaxy constituted by ms-setae, accp-, al- and as-setae. Only Th. II and Abd. III have ms-setae (formula 10/0010). Dorsal s-setae constituted by single al-seta on Th. II and Th. III, single as-seta on Abd. V and by accp-setae, normally set within p-row (Figure 11D). The number of accp-setae can be expressed as 34/44442 (Figure 11D).
VT with 1 + 1 anterior, 3 + 3 latero-distal and 4–6 posterior setae with 2 in apical transverse row (Figure 11F).
Head: Ratio among Ant. I/Ant. II/Ant. III/Ant. IV is 1/1.5/1.4/3. Ant. I has 3–7 s-setae in ventro-lateral position and 3(−4) thicker and shorter sens in the most distal part; 2 microsetae in ventro-proximal position (Figure 11A). Ant. II has about 5 s-setae in distal position. Ant. III has a sensory field that includes 2 inner s-setae of AO III, one ms-seta and 6–7 s-setae (Figure 11B). Ant. IV with one simple subapical, peg-shaped organite and a bifurcated pin-like seta (Figure 11I). Eye spots strongly dark pigmented with 6 + 6 ocelli visible (Figures 11C, S4B). PAO elongated, with a weak median constriction, about two times as long as the diameter of the nearest ocellus (Figures 11C, S5B). Prelabral setae 4. Labral formula as 5, 5 anf 4 and 4 apical folds squared at the apex (Figures 11L, S5C). Maxillary palp bifurcated and maxillary outer lobe with 4 sublobal hairs. Labial palp with 5 papillae and a total of 16 guard setae [62] distributed as: A1, B1–4, C0, D1–4, E1–7 (like in Figures 10E, S5). Hypostomal papilla with H shorter than h1/h2. Proximal (px), basomedian (bm) and basolateral (bl) fields of labium with 4, 4 and 6 setae, respectively. 4–6 postlabial setae. Mandible with a well-developed molar plate.
Furca: Furca longer than antennae; ratio of mucro/dens/manubrium = 1/29/15 (Figure 11G). Ventral setae on the manubrium numerous (about 90), including about 10 ventro-apical setae larger than the others (Figure 11G), except for 2 + 2 short apical setae; more than 30 dorsal setae. Dens with dorsal crenulations, more than 80 ventral and 8–11 dorsal setae (Figure 11G). Mucro quadridentate with apical tooth much smaller than subapical one (Figure 11H). Apical seta on dens two times as long as mucro. Retinaculum with 4 teeth and 4–5 setae.
Legs: 11 setae in the distal apical ring of Tita; 2–3–3 very pointed tenent hairs (Figure 11E), shorter than the inner edge of Claw. Claw of normal shape without lateral and inner teeth; empodium without a small inner tooth; pretarsus with a pair of setae (Figures 11E, S5D).
Distribution and habitat: Desoria orobica is currently known only for the type locality, Trobio glacier (‘TRO’; Orobian Alps, Italy) (Figure 2, Table A1).
Ecology: D. orobica is a cryophilic firn-dwelling species (sensu Eisenbeis & Meyer [11]), also able to colonise supraglacial habitat. In summer, it lives at the borders of snow patches, in wet and mineral soils: performing flotation methods along a linear transect getting farther from the snow, the abundance decreased and after a distance of 1 m, on average, D. orobica disappeared (unpublished data; Figures 1D,H).
Etymology: the epithet refers to the name of the mountains — Orobian Alps — where this species was discovered.
Remarks: Desoria orobica belongs to the D. nivalis-complex sharing some important characteristics with other species of the complex (D. nivalis [Carl, 1910], D. duodecimoculata [Denis, 1927]): ‘the low number of sens on Ant. I and body tergites, the shape of mucro, oligochaetotic VT and dens, and long apical seta near mucro’ [43]. D. orobica shares also the peculiar pigmentation with D. nivalis (even if usually D. nivalis has also the distal part of its legs yellow, while D. orobica does not), but differs from this species for the different number of setae on VT (D. orobica has 1 + 1 anterior, 3 + 3 latero-distal, D. nivalis 4 + 4 anterior, 4–5 + 4–5 latero-distal), Claw without inner tooth (small but present in D. nivalis), the number of sensilla Ant. I (3–4 short and 3–7 thin sensilla in D. orobica, only 4–5 short and thin sensilla in D. nivalis), the smooth Mac (that are slightly serrate in D. nivalis).
The only record of a cryophilic ice-dwelling species for this area was by Facchini Pajetta et al. [67] who reported ‘Isotoma saltans’, but this record does not seem reliable because of its habitat (on the grass below 1900 ma.s.l.) and the absence of taxonomic notes; since the work referenced to Gisin [65], it is probably not associated with D. saltans sensu Potapov [43] (see notes for D. saltans).
9. Notes on Vertagopus alpinus Haybach, 1972 and V. helveticus Haybach, 1980
V. helveticus probably is not a valid species, as sampled population on Morteratsch (the type locality of V. helveticus) is genetically identical to V. alpinus and, following the descriptions reported in Potapov [43], morphologically they are not clearly different. We only have cox1 and 16s sequences of one specimen from the type locality of V. helveticus to strongly support this finding, and further investigation is suggested.
9.1. Vertagopus alpinus Haybach, 1972. Figure 6E
Syn: Isotoma (Vertagopus) alpina Haybach, 1972.
Isotoma (Vertagopus) helvetica Haybach, 1980.
Material examined: ITALY • 6 ♂ and 6 ♀; Valle d’Aosta, Courmayeur (Aosta province), European Alps, Mont Blanc PP465302 Miage Glacier (‘MIA/MIAC/MIAS’); in supraglacial stony debris; 45°46′52.6"N, 6°52′02.3"E, 2080 ma.s.l.; 8 Sep. 2020, B. Valle leg.; B. Valle det., collected with flotation method; Genbank (NCBI) PP441780−86; PP441788−90 (cox1), PP465302−08; PP465310−12 (16S); (SMB). FRANCE • 5 ♂ and 9 ♀; Auvergne Rhône-Alps, Chamonix-Mont-Blanc, European Alps, Mont Blanc massif, Mer de Glace glacier (‘MDG’); in supraglacial stony debris; 45°54′44.5"N, 6°56′10.4"E, 2040 ma.s.l.; 12 Aug. 2021, B. Valle leg.; B. Valle det., collected with flotation method; Genbank (NCBI) PP441770−79 (cox1), PP465292−301 (16S); (SMNS). AUSTRIA • 2 ♂ and 7 ♀; Carinthia, Heilgenblut, European Alps, Großglockner, Pasterze glacier (type locality; ‘PAS’); in supraglacial stony debris; 47°05′13.9"N, 12°43′24.9"E, 2340 ma.s.l.; 1 Aug. 2021, B. Valle leg.; B. Valle det., collected with flotation method; Genbank (NCBI) PP441826−31 (cox1), XPP465348−53 (16S); (SMNS).
Chaetotaxy: Sensory chaetotaxy is very constant and is constituted by ms-setae, accp-, al- and as-setae. Only Th. II and Abd. III have ms-setae (formula 10/001). Dorsal s-setae constituted by single al-seta on Th. II and Th. III, single as-seta on Abd. V and by accp-setae normally set within p-row. The number of accp-setae can be expressed as 3, 3/3, 3, 3, 3–4, 3. The number of anterior setae on Man is very variable, from 20 to 32.
Head: Ant. I has 9–13 s-setae in ventro-lateral position, 1–2(3) of which are shorter in the distal part; 2(3) microsetae in ventro-proximal position. Ant. II has about 5 s-setae in distal position. Ant. III has a sensory field that includes 2 inner s-setae of AO III, one lateral ms-seta and 8–10 s-setae. Ant. IV with one simple subapical, peg-shaped organite and a bifurcate pin-like seta. Absence of a seta among ommatidia A, B and C, D.
For body measurements see Table A2.
Notes on distribution: V. alpinus was known to have an Alpine distribution [43]. We could confirm an Alpine distribution with new records from Western to Eastern Alps: Graian Alps (Mont Blanc), Bernina (Morteratsch glacier) Bernese (Lang glacier) and Pennine Alps (Piéce glacier).
Notes on ecology: V. alpinus seems to be a cold-adapted, high-mountain, not strictly ice-dwelling species able to colonise both the glacier forelands [68] and the supraglacial debris (our data) (Figure 1A). On Pasterze (Grossglockner), Mer de Glace and Miage (Mont Blanc) glaciers, it has been only found in thick layer of fine supraglacial debris but not on bare ice. On Morteratsch (Bernina), Pèlerins (Mont Blanc) glaciers, it was sampled on bare ice, under stones. According to our data, V. alpinus seems more linked to thicker supraglacial debris than other ice-dwelling species.
10. Notes on Desoria saltans Nicolet, 1841 sensu Potapov (2001). Figure 6D
Syn: Desoria glacialis Nicolet, 1841.
D. saltans was described for the Bernese Alps (Lauteraar and Finsteraar according to Desor [36]) by Nicolet [69] but with an incomplete description that does not include the diagnostic morphological characteristics of the species; therefore, considering the high diversity of ice-dwelling springtails as presented in this work, it is not possible to state which ice-dwelling species Nicolet referred to. In the following decades, several authors based the reports for this species only on its peculiar ecology, including some identification keys (for example, Gisin [65]). In particular, Handschin [70] and Gisin [65] probably considered a species of the ice-dwelling Vertagopus group (as suggested in particular by the mucro shape). However, from their description, it is not possible to know which species they refer to, so these works cannot be used as valid descriptions. The same applies to many other records of D. saltans (see notes on distribution and ecology). Only Potapov [43] for the first time provided a complete and unequivocal description of D. saltans based on specimens collected by Kopeszki and Zettel; for this reason, we referred to Potapov’s [43] description for the identification of D. saltans.
Material examined: ITALY • 5 ♂ and 4 ♀; Valle d’Aosta, Courmayeur (Aosta provice), European Alps, Mont Blanc massif, Pré de Bar Glacier (‘PDB’); under stones on bare ice, 45°54′08.9"N, 7°03′04.2"E, 2700 ma.s.l.; 25 Jul. 2021, R. Ambrosini, F. Pittino leg, B. Valle det., collected by hand; Genbank (NCBI) PP441832−41 (cox1), PP465354−63 (16S); (SMB). FRANCE • 8 ♂ and 10 ♀; Provence-Alpes-Côte d’Azur, Vallouise-Pelvoux, European Alps, Écrins massif, Glacier Blanc (‘GLB’); on bare ice; 44°56′46.6"N, 6°23′48.8"E, 2970 ma.s.l.; 8 Aug. 2021, B. Valle leg.; B. Valle det., collected by hand; Genbank (NCBI) PP441751−58 (cox1), PP465273−80 (16S); (SMNS). Head Ant. I has 2–5 s-setae in ventro-lateral position, and 5–7 shorter and thicker s-setae in the distal part; 2/3 microsetae in ventro-proximal position. Ant. II has about 8–11 s-setae in distal position. Ant. III has a sensory field that includes 2 inner s-setae of AO III, one lateral ms-seta and 7–11 s-setae. Ant. IV with one simple subapical, slightly bifurcated rod-shaped organite and a bifurcate pin-like seta (basal process very small).
For body measurements see Table A2.
Note on pigmentation: Desoria saltans is clearly distinguished from other ice-dwelling species by the purple reflections (Figure 6D), especially in juveniles, visible at the stereomicroscope.
Notes on taxonomy, past records and distribution: D. saltans is reported in Potapov [43] as an Alpine species (Austria, Switzerland, Italy); beyond the type locality of the original description—Lauteraar and Finsteraar glaciers, Switzerland (Desor [36], Nicolet [69])—it was recorded also in other localities. However, we hypothesise that several past records must be referred to other species. This is probably true for ‘Desoria glacialis’ found by Stoppani [64] on Forni Glacier—without providing morphological evidence—where we found V. glacialis sp. nov. In Orobian Alps (Italy), close to Scais glacier where D. saltans was found (SCA), Facchini Pajetta et al. [67] reported ‘Isotoma saltans’, but this report seems not reliable (see D. orobica notes).
Even some taxonomic texts report for D. saltans characters that are more similar to those of ice-dwelling Vertagopus group: for example Handschin [70]—material examined from different sites—and Gisin [65]—material examined from Gornergletscher (Monte Rosa)—affirm that D. saltans has the mucro similar to D. nivalis and Gisin [65] states that Abd. V-VI are not well distinguished.
In contrast, other reports could be probably attributed to D. saltans, like ‘Isotoma sp. G’ [11, 71] from Rotmoos and Gaisenberg glaciers (Ötzal Alps, Austria), according to what is deducible from SEM photos reported in Eisenbeis and Meyer [71] (for example, the big apical tooth on mucro); however, Eisenbeis and Meyer [11, 71] wrote that their species did not fit with D. saltans in the taxonomic key reported in Gisin [65]: this was due to the fact that Gisin [65] reported ice-dwelling Vertagopus in place of D. saltans. Isotoma saltans reported by Kopeszki [21] for Mittelberg glacier (Ötzal Alps, Austria) is confirmed as D. saltans according to the morphological information reported.
Therefore, D. saltans’ Alpine distribution is confirmed, altough fragmented (Figure 2), adding new records collected with this work from Western to Eastern Alps: from Dauphiné Alps (Glacier Blanc), Graian Alps (Mont Blanc, Prè de Bar), Bernina group (Fellaria Ovest) and Orobian Alps (Scais Glacier).
The population on Peirabroc Glacier (Maritime Alps, Italy), according to the genetic data of the present work, could be attributed to a still unknown species close to D. saltans, but this point must be explored further.
Notes on ecology: D. saltans is a strictly ice-dwelling species [43]. Several studies were performed on D. saltans, but only Kopeszki’s [21] record was taxonomically verified as D. saltans. This species is pluriannual and spends its entire life within the upper layers of the glaciers (Figure 1E), where it inhabits ice pores [72]: in winter, it remains in the layer between the snow and ice and only in summer, where the snow melting rate is high, it moves on the snow [21, 27, 28]. The ablation period is a critical phase of its life, since many individuals perish after being transported by turbulent melting water out of their microhabitat; the daily cycle of these organisms is defined by melting water [21]. D. saltans could be found also under stones on the bare ice. The diet consists primarily of inblown organic material, like pollen granules [28]. The reproduction period probably corresponds to winter, when the proper conditions for spermatophore dispersion occur (see Kopeszki [21] for detailed information on reproduction and embryonal development). The author also provided some physiological data on this species. Some other studies on physiology (for example: the measurement of respiration rate by Block and Tillbrook [73] and the finding of large amounts of long polyunsaturated fatty acids by Zinckler and Schaller [74] should be taxonomically verified.
11. Notes on Gnathisotoma bicolor Cassagnau, 1957 From European Alps. Figure 6H, J
Material examined: FRANCE • 1 ♂ and 1 ♀; Auvergne Rhône-Alps, Chamonix-Mont-Blanc, European Alps, Mont Blanc massif, Tour glacier (‘TOU’); under stones on bare ice, 45°59′44.8"N, 6°59′07.4"E, 2660 ma.s.l.; August 24, 2022, A. Zimmer leg.; B. Valle det., collected by hand; Genbank (NCBI) PP441904 (cox1), PP465426 (16S). (SMNS).
The population of Gnathisotoma bicolor from Mont Blanc (Figure 6J) is the first report of this genus for the Alps. From the morphological identification based only on two specimens, it shows a characteristic maxilla with all lamellae elongated and a mucro with an apical dens only slightly smaller than the subapical one. Characteristic of G. bicolor from Mont Blanc is the presence on the ventral tube of 4 + 4 lateral, 3 posterior and 1 + 1 anterior setae, unlike G. bicolor from Pyrenées (Figure 6H), which lacks anterior setae on VT and has 5 + 5 laterodistal setae. Also genetically, G. bicolor from Mont Blanc shows a certain difference from Pyrenean population (4.48%). Gnathisotoma bicolor, with the characteristic yellow appendages, is a typical firn-dwelling species [11], like Desoria orobica and D. nivalis.
12. Distribution Pattern of Ice-Dwelling Species
Ice-dwelling springtails show contrasting distribution patterns in the European Alps. Vertagopus alpinus and Desoria saltans can be observed throughout the whole chain but with relatively low frequency and a very fragmented distribution. Two of the newly described species, Vertagopus glacialis and V. psychrophilus, exhibit a wide and almost continuous range distribution; the remaining species are confined to local endemic distribution on single glaciers often occurring the peripheral Alpine sectors. (Figure 2). Desoria orobica sp. nov. was found only on the Trobio Glaciers in the Orobian Alps (Southern Alps), V. glacieinigrae sp. nov. only on Glacier Noir in Western Alps and V. fradustaensis sp. nov. only on Fradusta Glaciers in Dolomites (Eastern Alps). Gnathisotoma bicolor record from Mont Blanc is the first record of the genus Gnathisotoma for the European Alps. Some important glacial areas, particularly Mont Blanc, host several species, often co-occurring on the same glacier, while elsewhere each glacier typically hosts a single species. On the Apennines, the only ice-dwelling species — D. calderonis (Figure 6G) — occurs on the single glacier still present in this mountain chain (Figure 2).
13. Discussion
13.1. Unveiling the Biodiversity of Ice-Dwelling Springtails
This study emphasises the previously unexplored biodiversity of ice-dwelling springtails on the European Alps, introducing five new species beyond the known Desoria saltans and Vertagopus alpinus. Such diversity is also supported by morphological, phylogenetic and species delimitation analyses. We provide evidence suggesting the potential existence of two additional new species on Peirabroc and Miage glaciers and clarify relationships among the taxa described. Our findings indicate that Vertagopus helveticus does not constitute a valid species, as the specimens collected from its type location are genetically and morphologically indistinguishable from V. alpinus. This study also provides the first report for the genus Gnathisotoma on the European Alps. Intra-specific genetic variability within D. saltans, G. bicolor and V. alpinus as morphologically defined is observed. Comparing our findings with the historical data, we also suggest an updated attribution of the past records (see notes for each species).
Phylogenetic analysis shows that the five new species belong to a group that includes both Vertagopus and Desoria. These genera thus need to be revised, especially Desoria which is clearly a paraphyletic genus, as observed in previous works [18, 75–77]. The extended paraphyly observed in all the three observed genera suggests the presence of weak morphological characteristics for their discrimination: the only morphological trait that differentiates Vertagopus from Desoria (the presence of clavate tenent hairs) probably does not have a strong phylogenetic significance since this structure has an ecological implication concerning locomotion on wet surfaces [19, 78] highlighting that also the morphological features that distinguish Gnathisotoma from Desoria — the reduced number of sensilla on the tergites, the modified maxilla and the absence of frontal setae on the ventral tube — are not unique to the genus and show a gradual transition between the two genera.
Our preliminary phylogenetic analysis suggests that all the ice-dwelling Vertagopus species, D. calderonis and D. orobica represent a monophyletic group that could result from a unique adaptation event to glacial environment. Conversely, D. saltans and G. bicolor appear to have adapted independently; further investigation is needed to confirm these hypotheses.
13.2. Distribution Pattern
The currently known distribution patterns of the Alpine ice-dwelling springtails stand out for their striking diversity. Vertogopus glacialis and V. psychrophilus exhibit a wide, nearly continuous distribution range paralleled by a general genetic uniformity within species, with V. psychrophilus more linked to the Southern Alps and almost absent from the Northern side of the chain (except for two occurrences on the French slope of the Mont Blanc massif). The outlined distribution pattern supports the hypothesis of a relatively recent differentiation of these species. In contrast, V. alpinus and D. saltans present a fragmented distribution and a marked genetic variability, corroborating the hypothesis, arising from the phylogenetic evidence, of an independent and more ancient adaptation to the ice-dweller condition for D. saltans (while V. alpinus is not a truly ice-dwelling species). The low occurrence of D. saltans in our samplings is in contrast with many historical reports for the whole Alpine chain: while the locus typicus of Lauteraar and Finsteraar in Switzerland [69] cannot be unequivocally referred to any of the known species, many old reports actually refer to other ice-dwelling species: for instance, Stoppani [64] likely refers to V. glacialis (see notes on D. saltans).
The remaining species apparently have point-like distribution on single glaciers; this evidence could be related to the limited knowledge of their actual distribution ranges. On the other hand, these species are mainly located on glaciers in peripheral/southern subchains of the Alps like Maritime Alps, Orobian Alps, Écrins and Dolomites, with the significant addition of Calderone glacier on the Apennines. Such areas are well-known biodiversity hotspots due to glaciation history and existence of refugia [79–81]. The glaciation in these areas is generally reduced and often isolated from the main Alpine glaciers. Although the dispersal potential of ice-dwelling springtails is still not known, this isolation could be a driver for speciation; at the same time, it could be a reason for concern for the survival of the exclusive biodiversity of these areas under the present climate warming [2, 14]. On the other hand, inner Alpine areas with extensive glacial cover, like Mont Blanc, show a marked species richness, with the co-occurrence of many taxa on the same site; these areas could have acted as refugia for this cryophilic biodiversity during past warm periods [13, 14, 82, 83].
The compresence of different species on the same glacier is an important phenomenon that emerges from our data. Probably this could be favoured by the niche separation of species (for example, D. saltans, more linked to bare ice than Vertagopus species — see next paragraph). However, our qualitative sampling does not allow a quantification of this phenomenon and further studies are needed, focusing separately on different microhabitats.
13.3. Ice-, Firn- and Debris-Dwelling Species and Other Ecological Considerations
From the ecological point of view, the true ice-dwelling springtails seem to include a well-defined cryophilic group of springtails, including in the Alps the new Vertagopus species and Desoria saltans and, on the Apennines. D. calderonis. As suggested by Eisenbeis and Meyer [11], ice-dwelling species live only where ice is present, and may occur on snow or stony debris only where underlying ice occurs. Such species are differentiated from firn- and debris-dwelling cryophilic springtails that, in the Alps, include Gnathisotoma bicolor, Desoria orobica and Vertagopus alpinus. Eisenbeis and Meyer [11] hypothesised that the intense body pigmentation (Figures 6A–F) of ice-dwelling springtails is a functional adaptation to protect the animal against the high UV radiation on the glacier surface, while firn- and ground-dwelling springtails are less pigmented, especially on appendages. Our data confirm this observation, with all ice-dwelling species completely dark, while G. bicolor and D. orobica show lighter appendages (Figures 6H–J). In addition, D. calderonis, which permanently lives on a glacier totally covered by stony debris [14] presents paler appendages (Figure 6G). This pigmentation pattern can thus be a good indicator of the ecology of these species.
Within true ice-dwelling species, a slight ecological difference could occur between D. saltans and Vertagopus species (including D. calderonis), as the former seems to prefer clean ice avoiding thick debris, while the latter could be regularly observed in supraglacial debris. If confirmed, this phenomenon could imply an underrepresentation of D. saltans in our sampling, as the species could be linked to the less accessible accumulation areas of the glaciers, where almost no debris occurs.
Ice-dwelling springtails were not found on rock glaciers: this is probably due to the depth at which ice is located inside the stony debris. The probability of rock glaciers as habitats for these organisms is high, but their detection must overcome the difficulty in sampling at the rock–ice interface that lays at a significant depth in the soil. Underground ice associated with voids between stones like that of rock glaciers could represent an important refugium for ice-dwelling organisms during warm climatic periods [84].
14. Conclusions
This work highlights the need for a deep understanding of the still poorly known biodiversity of glacial habitats. The knowledge of the ecology and distribution of ice-dwelling organisms, many of which are likely to become extinct before their discovery, represents a fundamental key for understanding the biogeography of mountain systems of the Earth. Being intimately linked to the occurrence of glaciers, the distribution of these organisms may improve our knowledge of the role of past and present climatic fluctuations in shaping the biodiversity of the Alpine regions.
We observed many species endemic of small glaciers of the Alps that are deemed to extinction in the next decades. Understanding how these unique components of biodiversity can be preserved under ongoing climate change should be a conservation priority in all glacier areas of the Earth. To this purpose, a deep taxonomic knowledge, combining morphological and genetic approaches, remains an indispensable tool.
14.1. Identification Key of Cryophilic, Ice-Dwelling Species Studied in This Work
- 1.
Anterior setae on VT present, Tita tenent hairs pointed (Figure 11E), apical tooth on mucro small or big, apical folds on labrum present or not ….…..………………………………………………………………………………………2
-
– Anterior setae on VT absent, Tita tenent hairs at least weakly clavate, apical tooth on mucro very small (Figures 10G, 8C), apical folds on labrum present …………………………………………………………………………….5
- 2.
Body colour violet-black, with purple reflections (Figure 6D), few maxillary lamellae slightly but visibly elongated, maxillary palp simple, apical tooth on mucro equal or slightly smaller to the subapical one, apical folds on labrum absent ………………………………………………………………………………………………. D. saltans
-
– Body colour violet-black but with paler appendages, apical folds on labrum present..……………………..3
- 3.
Appendages brownish-yellow (Figure 6G). High number of accp sens (5–6,6/5–6,5,7,6–7,4). Known distribution range limited to Calderone Glacier (Apennines) …………………………………………….D. calderonis
-
– Appendages yellow, body strongly dark …………………………………………………………………………………………4
- 4.
Maxillary lamellae not elongated, Antennae (Ant. II–IV) yellow (Figure 6I), apical tooth on mucro very small …………………………………………………………………………………………………………………………………..…D. orobica
-
– Maxillary lamellae strongly elongated, legs and Antennae (Ant. I–IV) yellow (Figures 6H,L), apical tooth on mucro equal or slightly smaller to the subapical one ……………………………………………………….…..G. bicolor
- 5.
Tenent hairs strongly clavated (2–3–3). Seta e7 on labium present..……………………………………..…V. alpinus
-
– Tenent hairs very weakly clavated (2–3–3) (Figures 7E, 9E) ……………………………………………………….…..6
- 6.
Abd V-VI not clearly separated by a narrowing (Figure 9D). Anterior setae on Man 36. Known distribution range limited to Glacier Noir (Ècrins, European Alps) ….……………………………..V. glacieinigrae
-
– Abd V-VI clearly separated by a narrowing ……………………………………………………………………………………7
- 7.
Seta among ommatidia A, B and C, D absent ∗ (Figure 8C). Seta e7 on labium could lack. Anterior setae on Man 18–22 (rarely till 28). Usually 2 accp-setae on Th. II-III and 1 on Abd. I–III. ……………V. glacialis
-
– Seta among ommatidia A, B and C, D present ∗ (Figure 7C). Seta e7 on labium present. Higher number of anterior setae on Man. Usually 3 accp-setae from Th. II to Abd. III ………………………………………………8
- 8.
Max number of anterior setae on Man 26–30. Known distribution range limited to Fradusta Glacier (Dolomites)………………………………………………………………………………………………………………….….V. fradustaensis
-
– Max number of anterior setae on Man 38–40. ………………………………………………………………..V. psychrophilus
∗ For this character it is important to compare several specimens, since it could present variation, even within different sides of the same specimens
Nomenclature
-
- Abd:
-
- abdominal segment
-
- accp-setae:
-
- accessory p-row s-setae
-
- al-setae:
-
- antero-lateral s-setae
-
- Ant:
-
- antennal segment
-
- AOIII:
-
- antennal organ III
-
- as-setae:
-
- anterosubmedial s-setae
-
- bl:
-
- basolateral field of labium (mentum)
-
- bm:
-
- basomedian field of labium (submentum)
-
- ms-setae:
-
- micro s-setae
-
- PAO:
-
- postantennal organ
-
- Px:
-
- proximal field
-
- Th:
-
- thoracic segment
-
- Tita:
-
- tibiotarsus
-
- VT:
-
- ventral tube.
Data Availability Statement
Specimens collected for this work and preserved in alcohol are available in Museum collections (see Material and Methods). Genetic sequences are available on GenBank (see Material and Methods and Appendices).
Conflicts of Interest
The authors have no conflicts of interest to declare.
Author Contributions
Barbara Valle conceived of the idea, directed the project, collected samples, performed morphological and molecular analysis and wrote the draft. Marco Caccianiga and Mauro Gobbi conceived of the idea, collected samples and wrote the draft. Claudio Cucini, Francesco Nardi, Sara Boschi and Giovanni Barbon performed molecular and genetic analysis. Roberto Ambrosini, Jakub Buda, Silvio Marta, Virginia Toscano Rivalta, Riccardo Scotti and Anaïs Zimmer collected samples. Ľubomír Kováč supervise the morphological analysis. Roberto Ambrosini, Marco Caccianiga, Gentile Francesco Ficetola, Francesco Frati and Ľubomír Kováč gave financial support to the project. All authors discussed the results and contributed to the final manuscript.
Funding
This work was partially funded by European Research Council under the European Community’s Horizon 2020 Programme, Grant Agreement no. 772284 (IceCommunities) (G.F. Ficetola), by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of the Italian Ministry of University and Research funded by the European Union—NextGenerationEU (Project code CN_00000033, Concession Decree No. 1,034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP B63C22000650007, Project title ‘National Biodiversity Future Center - NBFC’) to B. Valle, F. Nardi, F. Frati, by EU Biodiversa+ (PrioritIce), by PRIN 2022 ‘ColdCase’ (Project code 2022TT8XHN, CUP G53D23002730006) to R. Ambrosini, M. Caccianiga and M. Gobbi.
Acknowledgments
The authors thank Marco Valle (Museo Civico di Scienze Naturali di Bergamo, Italy), Matteo Oreggioni, Andrea and Luca Ambrosini for the support given on the field, Davide Badano, Pietro Paolo Fanciulli for the support given in the laboratory and BMR genomics (Padua, Italy) for the consultancy relating to genetic analysis. Authors thank the Parks for the collaborations, in particular Paneveggio-Pale di San Martino, Parc National des Écrins, Parco Naturale Alpi Marittime (project ALCOTRA n. 1711 CClimaTT), Parco Nazionale dello Stelvio, Parco Regionale delle Orobie Bergamasche and Parc National du Pyrenées Francaise.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Appendix
| Site names | Geographic area | Land form | Code | Historical reports for the same glaciers | Species name | Country | Sampling date | Microhabitat | Latitude | Longitude | Sampling altitude [m asl] |
Morphology | Specimens sequenced | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Sforzellina | Ortles-Cevedale, Southern Rhaetian Alps | G | SFO |
|
IT | August 5, 2020 | On supraglacial snow and in supraglacial debris (FM) | 46°20′58.7"N | 10°30′44.1"E | 2,860 | Morph. Descr. | 8 | |
| Dosdè | Ortles-Cevedale, Southern Rhaetian Alps | G | DOS |
|
IT | August 5, 2020 | Under stones on bare ice | 46°23′34.8"N | 10°12′58.9"E | 2,760 | Morph. Descr. | 10 | ||
| Agola | Brenta Dolomites, Southern Rhaetian Alps Eastern Alps | G | AGO |
|
IT | August 10, 2020 | On supraglacial snow and ice | 46°08′57.9"N | 10°51′27.0"E | 2,580 | Morph. Descr. | 9 | ||
| Predarossa | Valtellina, Southern Rhaetian Alps | G | PRE |
|
IT | August 19, 2021 | Under stones on bare ice | 46°15′37.4"N | 9°44′21.8"E | 2,850 | Morph. Descr. | 6 | ||
| Fellaria Ovest | Bernina, Wester Rhaetian Alps | G | FEL ∗ |
|
IT | September 14, 2022 | Under stones on bare ice | 46° 20′ 51” N | 9°56′09” E | 2,840 | — | 1 | ||
| Rutor | Rutor-Léchaud chain, Graian Alps | G | RUT |
|
IT | June 30, 2023 | In supraglacial debris (FM) | 45°39′57.7"N | 7°00′09.3"E | 2,670 | — | 5 | ||
| Valtournanche | Breuil basin, Pennine Alps | G | BRE ∗ |
|
IT | July 23, 2023 | Under stones on bare ice | 45°55′33.0"N | 7°41′58.8"E | 3,100 | — | 1 | ||
| Tour ∗ | Mont Blanc, Graian Alps | G | TOU ∗ |
|
FR | Augut 24, 2022 | Under stones on bare ice | 45°59′44.8"N | 6°59′07.4"E | 2,660 | — | 5 | ||
| Orny | Mont Blanc, Graian Alps | G | ORN ∗ |
|
CH | August 17, 2022 | Under stones on bare ice | 45°59′59.8"N | 7°04′03.7"E | 2700 | — | 6 | ||
| Forni | Ortles-Cevedale, Rhaetian Alps | G | GFO | Isotomidae sp (Buda et al. 2020), Desoria glacialis” (Stoppani, 1876) | Vertagopus glacialis sp. nov. | IT | August 3, 2020 | Under stones on bare ice | 46°23′42.95"N | 10°35′24.79"E | 2630 | Morph. Descr. | 7 | |
| Vedretta d’Amola | Adamello, Southern Rhaetian Alps | G | AMO | Vertagopus glacialis sp. nov. | IT | July 21, 2020 | On supraglacial snow and in supraglacial debris (FM) | 46°13′8.95"N | 10°41′12.25"E | 2700 | Morph. Descr. | 10 | ||
| Giogo alto | Val Senales, Southern Rhaetian Alps | G | GIO | Vertagopus glacialis sp. nov. | IT | August 21, 2020 | Under stones on bare ice | 46°46′52.1"N | 10°48′20.7"E | 2840 | Morph. Descr. | 9 | ||
| Pers | Bernina, Wester Rhaetian Alps | G | PER | Vertagopus glacialis sp. nov. | CH | July 30, 2021 | Under stones on bare ice | 46°24′25.9"N | 9°57′32.4"E | 2670–2700 | Morph. Descr. | 8 | ||
| Morteratsch ∗ | Bernina, Western Rhaetian Alps | G | MOR ∗ | Vertagopus glacialis sp. nov. | CH | July 10, 2021 and August 25, 2021 | Under stones on bare ice | 46°24′55.5"N | 9°56′04.5"E | 2200–2300 | Morph. Descr. | 4 | ||
| Mittelberg | Ötztal Alps, Southern Rhaetian Alps | G | MIT | Vertagopus glacialis sp. nov. | AT | August 20, 2021 | Under stones on bare ice | 46°55′09.5"N | 10°53′43.7"E | 2680–2700 | Morph. Descr. | 6 | ||
| Lang | Bernese Alps | G | VAL ∗ | Vertagopus glacialis sp. nov. | CH | August 10, 2021 | Under stones on bare ice | 46°27′28.3"N | 7°55′55.8"E | 2450–2550 | Morph. Descr. | 6 | ||
| Oberaar ∗ | Bernese Alps | G | OBE | Isotoma ‘sp. G’ (Zettel 1984). Desoria saltans (Nicolet, 1841) | Vertagopus glacialis sp. nov. | CH | August 14, 2021 | Under stones on bare ice | 46°32′08.6"N | 8°13′12.4"E | 2380–2440 | Morph. Descr. | 6 | |
| Moiry | Pennine Alps | G | MOI | Vertagopus glacialis sp. nov. | CH | August 11, 2021 | Under stones on bare ice | 46°05′15.5"N | 7°35′25.3"E | 2620–2660 | Morph. Descr. | 10 | ||
| de Pièce | Pennine Alps | G | PIE ∗ | Vertagopus glacialis sp. nov. | CH | August 17, 2021 | Under stones on bare ice | 45°59′59.7"N | 7°28′12.2"E | 2750–2800 | Morph. Descr. | 10 | ||
| Valtournanche | Breuil basin, Pennine Alps | G | BRE ∗ | Vertagopus glacialis sp. nov. | IT | July 23, 2023 | Under stones on bare ice | 45°55′33.0"N | 7°41′58.8"E | 3100 | — | 3 | ||
| Lauson | Valnontery, Gran Paradiso Graian Alps,Western Alps | G | LAU | Vertagopus glacialis sp. nov. | IT | August 11, 2023 | In supraglacial debris (FM) | 45°33′58.6"N | 7°17′20.6"E | 3070 | — | 5 | ||
| Pelerins | Mont Blanc, Graian Alps | G | PEL ∗ | Vertagopus glacialis sp. nov. | FR | August 23, 2022 | Under stones on bare ice | 45°53′43.9"N | 6°53′04.9"E | 2320 | — | 4 | ||
| Orny | Mont Blanc, Graian Alps | G | ORN ∗ | Vertagopus glacialis sp. nov. | CH | August 17, 2022 | Under stones on bare ice | 45°59′59.8"N | 7°04′03.7"E | 2700 | — | 1 | ||
| Miage ∗ | Mont Blanc, Graian Alps | G | MIA/MIAS | Vertagopus alpinus Haybach, 1972 | IT | September 08, 2020 | In supraglacial debris (FM) | 45°46′52.6"N | 6°52′02.3"E | 2080 | Morph. Ident. and notes | 10 | ||
| Mer de Glace | Mont Blanc, Graian Alps | G | MDG | Vertagopus alpinus Haybach, 1972 | FR | August 12, 2021 | In supraglacial debris (FM) | 45°54′44.5"N | 6°56′10.4"E | 2040 | Morph. Ident. and notes | 10 | ||
| Pasterze | Grossglockner, Western Tauern Alps | G | PAS | Vertagopus alpinus Haybach, 1972 | Vertagopus alpinus Haybach, 1972 | AT | August 01, 2021 | In supraglacial debris (FM) | 47°05′13.9"N | 12°43′24.9"E | 2340 | Morph. Ident. and notes | 6 | |
| Morteratsch | Bernina, Wester Rhaetian Alps | G | MOR ∗ | Vertagopus helveticus (Haybach, 1980) | Vertagopus alpinus Haybach, 1972 | CH | August 25, 2021 | Under stones on bare ice | 46°24′55.5"N | 9°56′04.5"E | 2200–2300 | Morph. Ident. and notes | 1 | |
| Pelerins | Mont Blanc, Graian Alps | G | PEL ∗ | Vertagopus alpinus Haybach, 1972 | FR | August 23, 2022 | Under stones on bare ice | 45°53′43.9"N | 6°53′04.9"E | 2,320 | 4 | |||
| Lang gletscher | Bernese Alps | G | VAL20 ∗ | Vertagopus alpinus Haybach, 1972 | CH | August 10, 2021 | Under stones on bare ice | 46°27′28.3"N | 7°55′55.8"E | 2450–2550 | — | 1 | ||
| Pièce | Pennine Alps | G | PIE20 ∗ | Vertagopus alpinus Haybach, 1972 | CH | August 17, 2021 | Under stones on bare ice | 45°59′59.7"N | 7°28′12.2"E | 2750–2800 | — | 1 | ||
| Glacier Noir | Ecrins, Dauphiné Alps | G | GLN | Vertagopus glacieinigrae sp. nov. | FR | August 9, 2021 | In supraglacial debris (FM) | 44°55′11.3"N | 6°23′37.8"E | 2240 | Morph. Descr. | 6 | ||
| Vedretta della Fradusta | Pale San Martino Dolomites, Southern-Eastern Alps | G | FRA | Vertagopus fradustaensis sp. nov. | IT | August 8, 2023 | Under stones on bare ice and in ice fractures | 46°15′06.8"N | 11°52′21.4"E | 2845 | Morph. Descr. | 3 | ||
| Pré de Bar |
|
G | PDB | Desoria saltans Nicolet, 1841 | IT | July 25, 2021 | Under stones on bare ice | 45°54′08.9"N | 7°03′04.2"E | 2700 | Morph. Ident. and notes | 10 | ||
| Glacier Blanc | Ecrins, Dauphiné Alps | G | GLB | Desoria saltans Nicolet, 1841 | FR | August 08, 2021 | In ice fractures | 44°56′46.6"N | 6°23′48.8"E | 2970 | Morph. Ident. and notes | 8 | ||
| Scais | Orobian Alps | G | SCA | Desoria saltans Nicolet, 1841 | IT | September 18, 2020 | In supraglacial debris (FM) | 46°03′52.8"N | 9°58′56.1"E | 2820 | — | 5 | ||
| Fellaria Ovest | Bernina, Wester Rhaetian Alps | G | FEL ∗ | Desoria saltans Nicolet, 1841 | IT | September 14, 2022 | Under stones on bare ice | 46° 20′ 51” N | 9°56′09” E | 2840 | — | 1 | ||
| Peirabroc | Maritime Alps | G | PEI | Desoria cf. saltans | IT | September 14, 2020 | In supraglacial debris (FM) | 44°07′18.7"N | 7°24′51.1"E | 2540 | — | 1 | ||
| Trobio Ovest | Orobian Alps | G | TRO | Desoria orobica sp. nov. | IT | August 10, 2023 | In supraglacial debris (FM) and under stone on bare ice | 46°03′16"N | 10°05′11"E | 2550 | Morph. Descr. | — | ||
| Trobio Est | Orobian Alps | G | TRO | Desoria orobica sp. nov. | IT | July 18, 2020 | In debris at the snow patch border (FM) | 46°03′25.5"N | 10°05′30.0"E | 2700 | Morph. Descr. | 5 | ||
| Tour | Mont Blanc, Graian Alps | G | TOU ∗ | Gnathisotoma bicolor Cassagnau, 1957 | FR | August 24, 2022 | Under stones on bare ice | 45°59′44.8"N | 6°59′07.4"E | 2660 | Morph. Ident. and notes | 1 | ||
| Miage | Mont Blanc, Graian Alps | G | MIA | Isotomidae sp. | IT | September 8, 2020 | In supraglacial debris (FM) | 45°46′52.6"N | 6°52′02.3"E | 2080 | — | 2 | ||
| Aletsch | Bernese Alps | G | NF | CH | August 1, 2021 | Under stones on bare ice | 46°26′36.2"N | 8°04′41.5"E | 2340 | — | — | |||
| Passo Valsecca | Orobian Alps | N | NF | IT | September 19, 2020 | On snow and in the stony debris on the border of the snow (FM) | 46°02′22.8"N | 9°54′22.2"E | 2390 | — | — | |||
| Lago della Malgina | Orobian Alps | N | NF | IT | July 17, 2020 | On snow and in the stony debris on the border of the snow (FM) | 46°04′49.5"N | 10°03′01.8"E | 2570 | — | — | |||
| Secreti | Orobian Alps | N | NF | IT | September 18, 2020 | On snow and in the stony debris on the border of the snow (FM) | 46°03′28.3"N | 9°58′54.8"E | 2700 | — | — | |||
| Mandrone | Adamello, Southern Rhaetian Alps | G | NF | IT | August 25, 2020 and August 2023 | Under stones on bare ice | 46°11′5.80′’N | 10°33′34.67′’E | 2606 | — | — | |||
| Marmolada | Marmolada Dolomites, Southern-Eastern Alps | G | NF | IT | September 3, 2020 | Under stones on bare ice | 46°26′21.3"N | 11°51′49.4"E | 2900 | — | — | |||
| Vedretta Piana | Ortles-Cevedale, Southern Rhaetian Alps | G | NF | IT | August 19, 2020 | Under stones on bare ice | 46°30′29.6"N | 10°28′06.7"E | 3200 | — | — | |||
| Ventina | Valmalenco, Southern Rhaetian Alps | G | NF | IT | September 5, 2020 | Under stones on bare ice and in supraglacial debris (FM) | 46°17′08.9"N | 9°46′52.2"E | 2100 | — | — | |||
| Belvedere | Monte Rosa, Pennine Alps | G | NF | IT | August 10, 2020 | In supraglacial debris (FM) | 45°57′33.5"N | 7°54′47.0"E | 2040 | — | — | |||
| Cima Uomo | Marmolada Dolomites, Southern Rhaetian Alps | G | NF | IT | August 25, 2020 | In supraglacial debris (FM) | 46°24′33.5"N | 11°48′21.1"E | 2550 | — | — | |||
| Lazaunkar | Val Senales, Southern Rhaetian Alps | RG | NF | IT | August 6, 2020 | Ice not found, too deep | 46°44′50.3"N | 10°45′23.1"E | 2540 | — | — | |||
| Rock glacier Amola | Adamello, Southern Rhaetian Alps | RG | NF | IT | August 22, 2020 | Ice not found, too deep | 46°12′11.5"N | 10°42′11.0"E | 2460 | — | — | |||
| Ap | Calderone | Gran Sasso, Apennines | G | CAL | Desoria calderonis Valle, 2021 | Desoria calderonis Valle, 2021 | IT | July 8, 2020 | In supraglacial debris (FM) | 42°28ʹ16.2″ N | 13°34ʹ05.8″ E | 2700 | (Valle et al. 2021, [18]) | 8 |
| Py | Troumouse | Gavarnie-Gédre, Pyrenées | N | TGN | Gnathisotoma bicolor Cassagnau, 1957 | Gnathisotoma bicolor Cassagnau, 1957 | FR | July 8, 2021 | Stony debris at the border of a snowpatch, along the glacial stream (FM) | 42°43′14.3"N | 0°07′32.8"E | 2500 | Morph. Ident. and notes | 9 |
| Tourettes | Gavarnie-Gédre, Pyrenées | G | TRS | — | Gnathisotoma bicolor Cassagnau, 1957 | FR | August 2023 | Supraglacial debris (FM) | 42°41′59.1"N | 0°02′41.4"W | 2470 | Morph. Ident. | — | |
- Abbreviations: Al, European Alps; Ap, Apennines; Descr., description; FM, sampled with flotation method; G, glacier; Ident., Identification; Morph., morphological; N, snow-patch; Py, Pyrenées; RG, rock glacier.
- ‘ ∗’ is associated with glaciers where more than one species occurs. Nomenclature of Alpine sectors follows SOIUSA [85].
| Head (dorsal) | Head and body | Ant I |
Ant II |
Ant III |
Ant IV |
Antenna | Head, body and antenna | Cephalic diagonal | Furca | Man | Dens | Mucro | Mac (Abd. V) |
Abd. V |
Inner edge of Claw3 | Mandible | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vertagopus psychrophilus sp. nov. | Mean value | 314 | 1490 | 64 | 97 | 89 | 176 | 427 | 1922 | 381 | 447 | 168 | 267 | 11 | 53 | 92 | 39 | 85 |
| St.dev | 46 | 291 | 7 | 9 | 9 | 16 | 36 | 308 | 20 | 30 | 19 | 30 | 1 | 5 | 12 | 2 | 8 | |
| N° of measurements | 12 | 11 | 13 | 13 | 13 | 13 | 13 | 11 | 3 | 11 | 12 | 12 | 11 | 13 | 13 | 13 | 7 | |
| Vertagopus glacialis sp. nov. | Mean value | 341 | 1729 | 64 | 102 | 94 | 190 | 451 | 2175 | 416 | 447 | 172 | 267 | 11 | 51 | 96 | 42 | 88 |
| St.dev | 31 | 188 | 10 | 11 | 11 | 16 | 42 | 210 | 36 | 38 | 22 | 24 | 1 | 6 | 11 | 4 | 9 | |
| N° of measurements | 29 | 29 | 33 | 33 | 33 | 33 | 33 | 29 | 5 | 29 | 31 | 29 | 31 | 30 | 28 | 29 | 14 | |
| Vertagopus glacieinigrae sp. nov. | Mean value | 284 | 1296 | 53 | 90 | 85 | 153 | 380 | 1676 | 335 | 461 | 151 | 297 | 12 | 62 | 63 | 29 | 69 |
| St.dev | 22 | 100 | 5 | 12 | 11 | 14 | 37 | 118 | 18 | 42 | 15 | 28 | 1 | 7 | 9 | 4 | 8 | |
| N° of measurements | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 3 | 10 | 10 | 10 | 10 | 9 | 9 | 8 | 7 | |
| Desoria saltans | Mean value | 403 | 1864 | 68 | 119 | 109 | 233 | 529 | 2393 | 484 | 665 | 230 | 421 | 15 | 59 | 102 | 50 | 123 |
| St.dev | 38 | 178 | 3 | 7 | 5 | 18 | 21 | 183 | 52 | 59 | 25 | 40 | 1 | 5 | 6 | 3 | 6 | |
| N° of measurements | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 4 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 6 | |
| Vertagopus alpinus | Mean value | 288 | 1420 | 53 | 76 | 71 | 142 | 342 | 1757 | 330 | 473 | 154 | 304 | 11 | 36 | 73 | 33 | 77 |
| St.dev | 24 | 157 | 5 | 3 | 3 | 12 | 17 | 173 | 17 | 62 | 28 | 37 | 1 | 5 | 14 | 5 | 9 | |
| N° of measurements | 12 | 11 | 9 | 9 | 9 | 9 | 9 | 11 | 3 | 12 | 9 | 9 | 9 | 9 | 9 | 9 | 4 | |
| Vertagopus fradustaensis sp. nov. | Mean value | 293 | 1584 | 55 | 92 | 89 | 171 | 407 | 1992 | 323 | 503 | 182 | 311 | 10 | 55 | 88 | 32 | 85 |
| St.dev | 16 | 96 | 5 | 8 | 6 | 19 | 34 | 132 | 18 | 26 | 18 | 17 | — | 4 | 7 | 2 | 8 | |
| N° of measurements | 9 | 9 | 7 | 7 | 7 | 7 | 7 | 7 | 9 | 9 | 9 | 9 | 1 | 6 | 5 | 8 | 5 | |
| Desoria orobica sp. nov. | Mean value | 275 | 1463 | 50 | 75 | 72 | 152 | 341 | 1812 | — | 415 | 140 | 265 | 9 | 58 | 98 | 28 | 77 |
| St.dev | 17 | 136 | 7 | 9 | 6 | 11 | 20 | 161 | — | 27 | 20 | 13 | 1 | 9 | 12 | 2 | 2 | |
| N° of measurements | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 6 | — | 7 | 7 | 7 | 7 | 6 | 7 | 7 | 2 | |
| Specimen | Species | cox1 | 16S | Specimen | Species | cox1 | 16S | |
|---|---|---|---|---|---|---|---|---|
| AGO_1 | Vertagopus psychrophilus | PP441687 | PP465209 | MOI_6 | Vertagopus glacialis | PP441804 | PP465326 | |
| AGO_2 | Vertagopus psychrophilus | PP441688 | PP465210 | MOI_7 | Vertagopus glacialis | PP441805 | PP465327 | |
| AGO_3 | Vertagopus psychrophilus | PP441689 | PP465211 | MOI_8 | Vertagopus glacialis | PP441806 | PP465328 | |
| AGO_4 | Vertagopus psychrophilus | PP441690 | PP465212 | MOI_9 | Vertagopus glacialis | PP441807 | PP465329 | |
| AGO_5 | Vertagopus psychrophilus | PP441691 | PP465213 | MOR_11 | Vertagopus glacialis | PP441808 | PP465330 | |
| AGO_6 | Vertagopus psychrophilus | PP441692 | PP465214 | MOR_16 | Vertagopus glacialis | PP441809 | PP465331 | |
| AGO_7 | Vertagopus psychrophilus | PP441693 | PP465215 | MOR_18 | Vertagopus alpinus | PP441810 | PP465332 | |
| AGO_8 | Vertagopus psychrophilus | PP441694 | PP465216 | MOR_19 | Vertagopus glacialis | PP441811 | PP465333 | |
| AGO_9 | Vertagopus psychrophilus | PP441695 | PP465217 | MOR_20 | Vertagopus glacialis | PP441812 | PP465334 | |
| AMO_1 | Vertagopus glacialis | PP441696 | PP465218 | OBE_1 | Vertagopus glacialis | PP441813 | PP465335 | |
| AMO_10 | Vertagopus glacialis | PP441697 | PP465219 | OBE_2 | Vertagopus glacialis | PP441814 | PP465336 | |
| AMO_2 | Vertagopus glacialis | PP441698 | PP465220 | OBE_3 | Vertagopus glacialis | PP441815 | PP465337 | |
| AMO_3 | Vertagopus glacialis | PP441699 | PP465221 | OBE_4 | Vertagopus glacialis | PP441816 | PP465338 | |
| AMO_4 | Vertagopus glacialis | PP441700 | PP465222 | OBE_5 | Vertagopus glacialis | PP441817 | PP465339 | |
| AMO_5 | Vertagopus glacialis | PP441701 | PP465223 | OBE_6 | Vertagopus glacialis | PP441818 | PP465340 | |
| AMO_6 | Vertagopus glacialis | PP441702 | PP465224 | ORN_21 | Vertagopus psychrophilus | PP441819 | PP465341 | |
| AMO_7 | Vertagopus glacialis | PP441703 | PP465225 | ORN_22 | Vertagopus psychrophilus | PP441820 | PP465342 | |
| AMO_8 | Vertagopus glacialis | PP441704 | PP465226 | ORN_23 | Vertagopus psychrophilus | PP441821 | PP465343 | |
| AMO_9 | Vertagopus glacialis | PP441705 | PP465227 | ORN_24 | Vertagopus psychrophilus | PP441822 | PP465344 | |
| BRE_1 | Vertagopus glacialis | PP441706 | PP465228 | ORN_26 | Vertagopus psychrophilus | PP441823 | PP465345 | |
| BRE_2 | Vertagopus glacialis | PP441707 | PP465229 | ORN_27 | Vertagopus glacialis | PP441824 | PP465346 | |
| BRE_3 | Vertagopus psychrophilus | PP441708 | PP465230 | ORN_31 | Vertagopus psychrophilus | PP441825 | PP465347 | |
| BRE_4 | Vertagopus glacialis | PP441709 | PP465231 | PAS_17 | Vertagopus alpinus | PP441826 | PP465348 | |
| CAL_1 | Desoria calderonis | PP441710 | PP465232 | PAS_18 | Vertagopus alpinus | PP441827 | PP465349 | |
| CAL_10 | Desoria calderonis | PP441711 | PP465233 | PAS_19 | Vertagopus alpinus | PP441828 | PP465350 | |
| CAL_2 | Desoria calderonis | PP441712 | PP465234 | PAS_20 | Vertagopus alpinus | PP441829 | PP465351 | |
| CAL_3 | Desoria calderonis | PP441713 | PP465235 | PAS_21 | Vertagopus alpinus | PP441830 | PP465352 | |
| CAL_4 | Desoria calderonis | PP441714 | PP465236 | PAS_22 | Vertagopus alpinus | PP441831 | PP465353 | |
| CAL_5 | Desoria calderonis | PP441715 | PP465237 | PDB_1 | Desoria saltans | PP441832 | PP465354 | |
| CAL_6 | Desoria calderonis | PP441716 | PP465238 | PDB_10 | Desoria saltans | PP441833 | PP465355 | |
| CAL_8 | Desoria calderonis | PP441717 | PP465239 | PDB_11 | Desoria saltans | PP441834 | PP465356 | |
| CAL_9 | Desoria calderonis | PP441718 | PP465240 | PDB_12 | Desoria saltans | PP441835 | PP465357 | |
| DOS_1 | Vertagopus psychrophilus | PP441719 | PP465241 | PDB_13 | Desoria saltans | PP441836 | PP465358 | |
| DOS_10 | Vertagopus psychrophilus | PP441720 | PP465242 | PDB_14 | Desoria saltans | PP441837 | PP465359 | |
| DOS_2 | Vertagopus psychrophilus | PP441721 | PP465243 | PDB_2 | Desoria saltans | PP441838 | PP465360 | |
| DOS_3 | Vertagopus psychrophilus | PP441722 | PP465244 | PDB_3 | Desoria saltans | PP441839 | PP465361 | |
| DOS_4 | Vertagopus psychrophilus | PP441723 | PP465245 | PDB_4 | Desoria saltans | PP441840 | PP465362 | |
| DOS_5 | Vertagopus psychrophilus | PP441724 | PP465246 | PDB_5 | Desoria saltans | PP441841 | PP465363 | |
| DOS_6 | Vertagopus psychrophilus | PP441725 | PP465247 | PEI_4 | Desoria cf saltans | PP441842 | PP465364 | |
| DOS_7 | Vertagopus psychrophilus | PP441726 | PP465248 | PEL_11 | Vertagopus alpinus | PP441843 | PP465365 | |
| DOS_8 | Vertagopus psychrophilus | PP441727 | PP465249 | PEL_12 | Vertagopus alpinus | PP441844 | PP465366 | |
| DOS_9 | Vertagopus psychrophilus | PP441728 | PP465250 | PEL_13 | Vertagopus alpinus | PP441845 | PP465367 | |
| FEL_1 | Vertagopus psychrophilus | PP441729 | PP465251 | PEL_14 | Vertagopus alpinus | PP441846 | PP465368 | |
| FEL_2 | Desoria saltans | PP441730 | PP465252 | PEL_30 | Vertagopus glacialis | PP441847 | PP465369 | |
| FEL_3 | Vertagopus psychrophilus | PP441731 | PP465253 | PEL_31 | Vertagopus glacialis | PP441848 | PP465370 | |
| FRA_2 | Vertagopus fradustaensis | PP441732 | PP465254 | PEL_34 | Vertagopus glacialis | PP441849 | PP465371 | |
| FRA_3 | Vertagopus fradustaensis | PP441733 | PP465255 | PEL_36 | Vertagopus glacialis | PP441850 | PP465372 | |
| FRA_4 | Vertagopus fradustaensis | PP441734 | PP465256 | PER_1 | Vertagopus glacialis | PP441851 | PP465373 | |
| GFO_1 | Vertagopus glacialis | PP441735 | PP465257 | PER_2 | Vertagopus glacialis | PP441852 | PP465374 | |
| GFO_10 | Vertagopus glacialis | PP441736 | PP465258 | PER_3 | Vertagopus glacialis | PP441853 | PP465375 | |
| GFO_2 | Vertagopus glacialis | PP441737 | PP465259 | PER_4 | Vertagopus glacialis | PP441854 | PP465376 | |
| GFO_3 | Vertagopus glacialis | PP441738 | PP465260 | PER_5 | Vertagopus glacialis | PP441855 | PP465377 | |
| GFO_6 | Vertagopus glacialis | PP441739 | PP465261 | PER_6 | Vertagopus glacialis | PP441856 | PP465378 | |
| GFO_7 | Vertagopus glacialis | PP441740 | PP465262 | PER_7 | Vertagopus glacialis | PP441857 | PP465379 | |
| GFO_8 | Vertagopus glacialis | PP441741 | PP465263 | PER_8 | Vertagopus glacialis | PP441858 | PP465380 | |
| GIO_10 | Vertagopus glacialis | PP441742 | PP465264 | PIE_1 | Vertagopus glacialis | PP441859 | PP465381 | |
| GIO_11 | Vertagopus glacialis | PP441743 | PP465265 | PIE_10 | Vertagopus glacialis | PP441860 | PP465382 | |
| GIO_12 | Vertagopus glacialis | PP441744 | PP465266 | PIE_2 | Vertagopus glacialis | PP441861 | PP465383 | |
| GIO_13 | Vertagopus glacialis | PP441745 | PP465267 | PIE_20 | Vertagopus alpinus | PP441862 | PP465384 | |
| GIO_14 | Vertagopus glacialis | PP441746 | PP465268 | PIE_3 | Vertagopus glacialis | PP441863 | PP465385 | |
| GIO_15 | Vertagopus glacialis | PP441747 | PP465269 | PIE_4 | Vertagopus glacialis | PP441864 | PP465386 | |
| GIO_16 | Vertagopus glacialis | PP441748 | PP465270 | PIE_5 | Vertagopus glacialis | PP441865 | PP465387 | |
| GIO_4 | Vertagopus glacialis | PP441749 | PP465271 | PIE_6 | Vertagopus glacialis | PP441866 | PP465388 | |
| GIO_5 | Vertagopus glacialis | PP441750 | PP465272 | PIE_7 | Vertagopus glacialis | PP441867 | PP465389 | |
| GLB_13 | Desoria saltans | PP441751 | PP465273 | PIE_8 | Vertagopus glacialis | PP441868 | PP465390 | |
| GLB_14 | Desoria saltans | PP441752 | PP465274 | PIE_9 | Vertagopus glacialis | PP441869 | PP465391 | |
| GLB_15 | Desoria saltans | PP441753 | PP465275 | PRE_12 | Vertagopus psychrophilus | PP441870 | PP465392 | |
| GLB_16 | Desoria saltans | PP441754 | PP465276 | PRE_13 | Vertagopus psychrophilus | PP441871 | PP465393 | |
| GLB_17 | Desoria saltans | PP441755 | PP465277 | PRE_14 | Vertagopus psychrophilus | PP441872 | PP465394 | |
| GLB_18 | Desoria saltans | PP441756 | PP465278 | PRE_15 | Vertagopus psychrophilus | PP441873 | PP465395 | |
| GLB_19 | Desoria saltans | PP441757 | PP465279 | PRE_16 | Vertagopus psychrophilus | PP441874 | PP465396 | |
| GLB_20 | Desoria saltans | PP441758 | PP465280 | PRE_17 | Vertagopus psychrophilus | PP441875 | PP465397 | |
| GLN_12 | Vertagopus glacieinigrae | PP441759 | PP465281 | RUT_1 | Vertagopus psychrophilus | PP441876 | PP465398 | |
| GLN_13 | Vertagopus glacieinigrae | PP441760 | PP465282 | RUT_2 | Vertagopus psychrophilus | PP441877 | PP465399 | |
| GLN_14 | Vertagopus glacieinigrae | PP441761 | PP465283 | RUT_3 | Vertagopus psychrophilus | PP441878 | PP465400 | |
| GLN_15 | Vertagopus glacieinigrae | PP441762 | PP465284 | RUT_4 | Vertagopus psychrophilus | PP441879 | PP465401 | |
| GLN_16 | Vertagopus glacieinigrae | PP441763 | PP465285 | RUT_5 | Vertagopus psychrophilus | PP441880 | PP465402 | |
| GLN_17 | Vertagopus glacieinigrae | PP441764 | PP465286 | SCA_1 | Desoria saltans | PP441881 | PP465403 | |
| LAU_1 | Vertagopus glacialis | PP441765 | PP465287 | SCA_2 | Desoria saltans | PP441882 | PP465404 | |
| LAU_2 | Vertagopus glacialis | PP441766 | PP465288 | SCA_3 | Desoria saltans | PP441883 | PP465405 | |
| LAU_3 | Vertagopus glacialis | PP441767 | PP465289 | SCA_4 | Desoria saltans | PP441884 | PP465406 | |
| LAU_4 | Vertagopus glacialis | PP441768 | PP465290 | SCA_5 | Desoria saltans | PP441885 | PP465407 | |
| LAU_5 | Vertagopus glacialis | PP441769 | PP465291 | SFO_10 | Vertagopus psychrophilus | PP441886 | PP465408 | |
| MDG_12 | Vertagopus alpinus | PP441770 | PP465292 | SFO_3 | Vertagopus psychrophilus | PP441887 | PP465409 | |
| MDG_13 | Vertagopus alpinus | PP441771 | PP465293 | SFO_4 | Vertagopus psychrophilus | PP441888 | PP465410 | |
| MDG_14 | Vertagopus alpinus | PP441772 | PP465294 | SFO_5 | Vertagopus psychrophilus | PP441889 | PP465411 | |
| MDG_15 | Vertagopus alpinus | PP441773 | PP465295 | SFO_6 | Vertagopus psychrophilus | PP441890 | PP465412 | |
| MDG_16 | Vertagopus alpinus | PP441774 | PP465296 | SFO_7 | Vertagopus psychrophilus | PP441891 | PP465413 | |
| MDG_17 | Vertagopus alpinus | PP441775 | PP465297 | SFO_8 | Vertagopus psychrophilus | PP441892 | PP465414 | |
| MDG_18 | Vertagopus alpinus | PP441776 | PP465298 | SFO_9 | Vertagopus psychrophilus | PP441893 | PP465415 | |
| MDG_19 | Vertagopus alpinus | PP441777 | PP465299 | TGN_12 | Gnathisotoma bicolor | PP441894 | PP465416 | |
| MDG_20 | Vertagopus alpinus | PP441778 | PP465300 | TGN_13 | Gnathisotoma bicolor | PP441895 | PP465417 | |
| MDG_21 | Vertagopus alpinus | PP441779 | PP465301 | TGN_14 | Gnathisotoma bicolor | PP441896 | PP465418 | |
| MIAC_10 | Vertagopus alpinus | PP441780 | PP465302 | TGN_15 | Gnathisotoma bicolor | PP441897 | PP465419 | |
| MIAC_11 | Vertagopus alpinus | PP441781 | PP465303 | TGN_16 | Gnathisotoma bicolor | PP441898 | PP465420 | |
| MIAC_6 | Vertagopus alpinus | PP441782 | PP465304 | TGN_17 | Gnathisotoma bicolor | PP441899 | PP465421 | |
| MIAC_9 | Vertagopus alpinus | PP441783 | PP465305 | TGN_18 | Gnathisotoma bicolor | PP441900 | PP465422 | |
| MIAS_6 | Vertagopus alpinus | PP441784 | PP465306 | TGN_19 | Gnathisotoma bicolor | PP441901 | PP465423 | |
| MIAS_8 | Vertagopus alpinus | PP441785 | PP465307 | TGN_20 | Gnathisotoma bicolor | PP441902 | PP465424 | |
| MIAS_9 | Vertagopus alpinus | PP441786 | PP465308 | TOU_1 | Vertagopus psychrophilus | PP441903 | PP465425 | |
| MIA_1 | Isotomidae sp. | PP441787 | PP465309 | TOU_14 | Gnathisotoma bicolor | PP441904 | PP465426 | |
| MIA_2 | Vertagopus alpinus | PP441788 | PP465310 | TOU_2 | Vertagopus psychrophilus | PP441905 | PP465427 | |
| MIA_3 | Vertagopus alpinus | PP441789 | PP465311 | TOU_3 | Vertagopus psychrophilus | PP441906 | PP465428 | |
| MIA_4 | Vertagopus alpinus | PP441790 | PP465312 | TOU_4 | Vertagopus psychrophilus | PP441907 | PP465429 | |
| MIA_5 | Isotomidae sp. | PP441791 | PP465313 | TOU_5 | Vertagopus psychrophilus | PP441908 | PP465430 | |
| MIT_1 | Vertagopus glacialis | PP441792 | PP465314 | TRO_1 | Desoria orobica | PP441909 | PP465431 | |
| MIT_2 | Vertagopus glacialis | PP441793 | PP465315 | TRO_18 | Desoria orobica | PP441910 | PP465432 | |
| MIT_3 | Vertagopus glacialis | PP441794 | PP465316 | TRO_3 | Desoria orobica | PP441911 | PP465433 | |
| MIT_4 | Vertagopus glacialis | PP441795 | PP465317 | TRO_4 | Desoria orobica | PP441912 | PP465434 | |
| MIT_5 | Vertagopus glacialis | PP441796 | PP465318 | TRO_5 | Desoria orobica | PP441913 | PP465435 | |
| MIT_6 | Vertagopus glacialis | PP441797 | PP465319 | VAL_11 | Vertagopus glacialis | PP441914 | PP465436 | |
| MOI_1 | Vertagopus glacialis | PP441798 | PP465320 | VAL_12 | Vertagopus glacialis | PP441915 | PP465437 | |
| MOI_10 | Vertagopus glacialis | PP441799 | PP465321 | VAL_13 | Vertagopus glacialis | PP441916 | PP465438 | |
| MOI_2 | Vertagopus glacialis | PP441800 | PP465322 | VAL_14 | Vertagopus glacialis | PP441917 | PP465439 | |
| MOI_3 | Vertagopus glacialis | PP441801 | PP465323 | VAL_15 | Vertagopus glacialis | PP441918 | PP465440 | |
| MOI_4 | Vertagopus glacialis | PP441802 | PP465324 | VAL_16 | Vertagopus glacialis | PP441919 | PP465441 | |
| MOI_5 | Vertagopus glacialis | PP441803 | PP465325 | VAL_20 | Vertagopus alpinus | PP441920 | PP465442 |




