Study of the Effect of Temperature and Microwave Action on the Structure of Polyvinyl Alcohol (PVA)
Abstract
Polyvinyl alcohol (PVA) is a widely utilized molecular polymer characterized by its strong water solubility, chemical resistance, and mechanical properties. Notably, it possesses the capability for biodegradation and finds extensive applications across various fields; thus, analyzing the fundamental properties of PVA is of paramount importance. Materials scientists have shown considerable interest in modifying polymers such as PVA. Among modification techniques, microwave irradiation has garnered significant attention due to its rapidity, efficiency, and cost-effectiveness. This study investigates the effects of temperature and microwave irradiation on PVA binary solutions using Raman spectroscopy while also analyzing intermolecular hydrogen bonding within these solutions. The results indicate that increasing temperature disrupts the tetrahedral structure formed between PVA and water, leading to a transition toward a chain-like configuration; however, an enhancement in partial hydrogen bonding occurs at 313.15 K. Furthermore, analysis of the PVA binary solution post-microwave irradiation reveals that exposure to microwaves enhances hydrogen bonding within PVA while inducing a depletion reaction after 180 s of irradiation. Atomic force microscopy further demonstrates that this duration leads to pronounced molecular chain stacking phenomena. This paper substantiates that microwave irradiation serves as an effective method for modifying PVA, thereby offering a robust strategy for polymer modification applicable in materials science, chemistry, and physics.
1. Introduction
Currently, materials science is focused on the development of biodegradable, biocompatible, and processable polymers that exhibit a diverse array of properties pertinent to their advanced applications as multifunctional materials. A prominent example is polyvinyl alcohol (PVA) [1]. This polymer finds extensive use in various sectors including adhesives, cosmetics, textiles, pharmaceuticals, and paints and even serves as a colloidal stabilizer in emulsion polymerization [2–4]. PVA possesses numerous advantageous characteristics such as chemical resistance, favorable physical properties, and complete biodegradability. Furthermore, its solubility contributes to its broad range of industrial applications owing to its high water absorption capacity. The hydroxyl groups present in PVA facilitate the formation of hydrogen bonds with both itself and water molecules [5]; this interaction results in distinctive physical and chemical properties—most notably achieving similar surface tension to that of water at specific concentrations [6–8].
For an extended period, researchers have shown considerable interest in the modification of polymers such as PVA [9–16]. In recent years, microwave irradiation has garnered significant attention and is increasingly utilized as a rapid, efficient, and cost-effective method for polymer modification [7, 17, 18]. Nechifor, Aflori, and Dorohoi [19] subjected a 10 wt.% aqueous PVA solution to microwave radiation for varying durations to fabricate films. They discovered that microwave irradiation altered the optical properties of the films; infrared spectroscopy revealed that this process significantly influenced molecular migration and facilitated intermolecular hydrogen bonding when the irradiation time was less than 68 s, resulting in more ordered polymer chains. To explore the physical and chemical characteristics associated with the thawing process of migrating PVA macromolecular chain segments, it is noted that PVA exhibits thixotropic behavior [20]. Lomovskoy et al. [21] investigated PVA samples both with and without microwave treatment across temperatures ranging from −150°C to 120°C. Their relaxation spectra indicated that microwave irradiation broadened the relaxation time spectrum. Raman spectroscopy further demonstrated a reduction in O-H group vibrations within the range of 3500–3250 cm−1 for irradiated samples; additionally, IR spectral analysis showed diminished intensity at an absorption band around 2160 cm−1. This led them to conclude that interactions between water’s O-H groups and PVA could weaken C-O bonds. Nguyen et al. [22] employed FTIR techniques revealing decreased absorbance for O-H stretching, C-H stretching, and CH2/CH3 bending correlating with increased duration of microwave exposure—concluding this phenomenon represented an extinction reaction. Concurrently, it is acknowledged that pure water’s Raman spectrum varies with temperature [23]. Therefore, it could be inferred that microwave irradiation can significantly affect the structure of PVA aqueous solution. However, there is dearth of detailed studies on the effect of microwave irradiation on the structure of PVA aqueous solution.
Raman spectroscopy is extensively employed in polymer research, encompassing six primary aspects: molecular chain conformation, molecular chain orientation, crystallinity, molecular aggregation, structural transformation, and chemical and physical composition. This study specifically utilizes Raman spectroscopy to explore hydrogen bonding kinetics in PVA aqueous solutions and examines the influence of temperature and microwave irradiation on the nature of hydrogen bonding within these solutions. We introduce a novel characterization approach that integrates Raman spectroscopy with atomic force microscopy (AFM) to investigate the structural changes in PVA induced by thermal and microwave treatment. Furthermore, we anticipate valuable insights from employing microwave irradiation techniques for modifying PVA properties. By contrasting the effects of temperature versus microwave irradiation on PVA structure, our primary objective is to elucidate the presence of distinct microwave effects. This paper lays a foundation for further exploration of PVA across materials science, chemistry, physics, and related disciplines.
2. Experimental
PVA (molecular weight: 205,000, 99% hydrolyzed) was obtained from Aladdin. PVA was mixed with deionized water to prepare a solution with a mass fraction of 7%.
The Raman spectra were all acquired on a Renishaw inVia-Qontor Raman spectrometer (UK). The instrument is equipped with a 532 nm semiconductor laser, an 1800 lines/mm−1 grating, a 50x Olympus telephoto lens, and a resolution of ±1 cm−1. Raman spectra were acquired in the range of 100∼4000 cm−1, the exposure time was 10 s, and the cumulative number of exposures was 1. The atomic force was measured using a Bruker Icon atomic force microscope in smart mode with a SCANASYST-AIR probe with a measurement range of 5 × 5 μm and a resolution of 256 × 256.
We used WiRE 5.1 for integrated and parametric analysis of Raman spectral data. PeakFit software was used to fit the spectra in the following steps: (1) in the Raman spectra, a range of 3000–3800 cm−1 ware selected; (2) the baselines ware automatically corrected using a linear equation; (3) the “Residuals” method was used to fit the spectral peaks with a “Gauss Amp” method; (4) Make multiple iterations. Tap water is deionized in an ultrapure water machine (ULUPURE Ultrapure Technology Co., Ltd., UPD-I-20T HYJD-D, Sichuan) with a resistivity of 18.25 MΩ·cm. Microwave heating equipment (Galanz, G70F23CN2P-BM1 (S0), Guangdong), rated output power 700 W, rated microwave frequency 2450 MHz. Ding Xin Yi (DXY-5H) Digital display thermostatic oil bath equipment (Ding Xin Yi, DXY-5H, China), power 600 W, temperature accuracy ± 0.5°C. The error of the temperature measuring instrument is ±0.1°C.
In this work, the quantum chemical calculations were performed with the BDF software [24–28] by using the hybrid density functional B3LYP [29, 30] method in conjunction with the 6-311 G (d, p) basis set.
3. Results and Discussion
Generally, hydroxyl groups (-OH) present on the polymer chains of PVA can engage in three types of hydrogen bonding with water molecules: intermolecular hydrogen bonds with water, intramolecular chain hydrogen bonds, and intermolecular chain hydrogen bonds. Notably, the presence of intermolecular chain hydrogen bonding contributes significantly to the increased viscosity of PVA in aqueous solutions. In this study, a PVA sample with a molecular weight of 205,000 was utilized to prepare an aqueous solution with a mass fraction of 7%.
Figure 1 presents the Raman spectra of a 7% PVA-H2O solution, which exhibits characteristic groups including C-C, C-H, C-O, and O-H bonds. Notably, the O-H stretching vibration serves as critical information for elucidating hydrogen bonding interactions. These functional groups within the PVA-H2O matrix are essential for understanding its internal structure (see Figure 2 and Table 1). Raman spectroscopy facilitates the recording of vibrational modes and interaction details associated with these groups. The observed red shift and blue shift of characteristic peaks reflect molecular interactions and microstructural changes in the mixed solution. The assignment of these characteristic peaks in the Raman spectra was established through a comprehensive literature review, as detailed in Table 2.
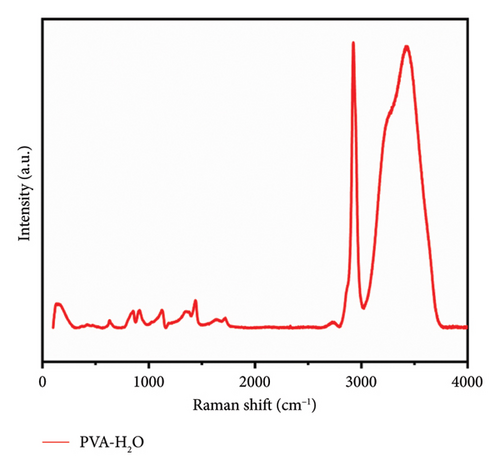
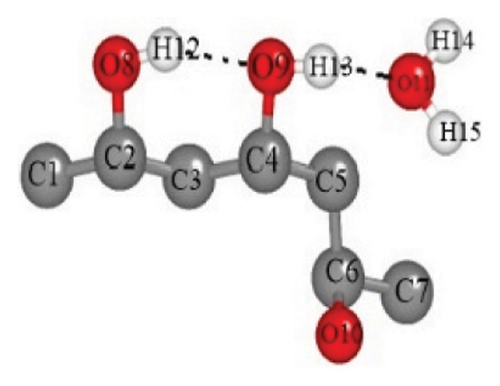
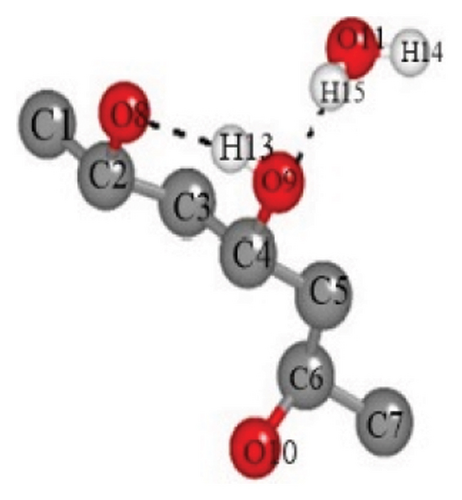
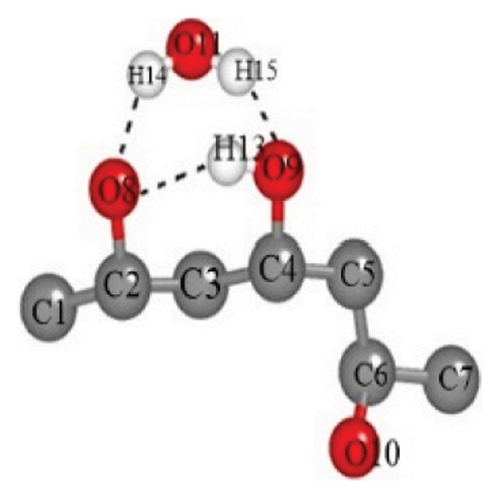

| O9-H12 | O11-H13 | O8-H13 | O9-H15 | O8-H14 | O8-H15 | |
|---|---|---|---|---|---|---|
| (a) | 1.83687 | 1.89755 | ||||
| (b) | 1.87448 | 1.87095 | ||||
| (c) | 1.94783 | 2.06452 | 2.18162 | |||
| (d) | 1.92408 | 1.93578 | 1.90475 |
3.1. Effect of Temperature on the Microstructure of PVA Solution
The solubility of PVA in water at ambient temperature is notably low, typically necessitating the application of heat and agitation for effective dissolution. To investigate the structural alterations occurring within the solution during the heating process, Raman spectroscopy was employed to analyze the internal structural modifications of PVA binary solutions. Figure 3 illustrates the Raman spectra of a 7% mass fraction PVA aqueous solution as a function of temperature. The C-H stretching vibrations observed in the range of 2900–3000 cm−1 and OH stretching vibration modes between 3000 and 3800 cm−1 represent the most prominent Raman peaks in these aqueous solutions. As temperature varies, notable frequency shifts are observed in the characteristic Raman peaks corresponding to OH stretching vibrations; this phenomenon can be attributed to differing forms and extents of intermolecular interactions present within the aqueous PVA system at varying temperatures.
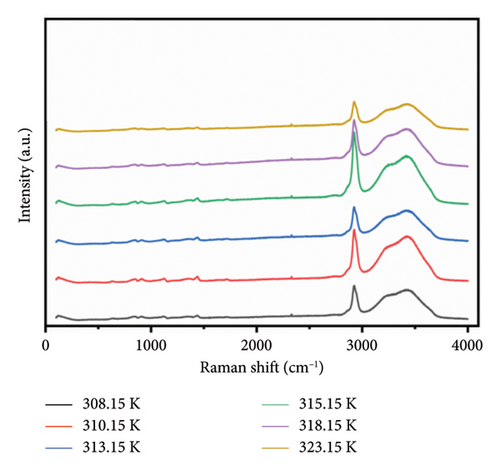
During heating, both intramolecular and intermolecular hydrogen bonds can form between PVA and pure water. The analysis of the hydrogen bonding characteristics in PVA aqueous solutions can be conducted by examining the frequency shifts of characteristic peaks within the 3000–3800 cm−1 range. The spectra show that the OH stretching band can be resolved into three distinct components, corresponding to three types to OH stretching vibrations [35, 36]. Figure 4(a) illustrates the structures associated with symmetric OH stretching vibrations (Figure 4(b)), asymmetric OH stretching vibrations (Figure 4(c)), and free OH groups (Figure 4(d)) observed in this spectral region as depicted in Figure 3. The symmetric OH stretching vibration is referred to as a fully hydrogen-bonded structure, while the asymmetric OH stretching vibration corresponds to a partially hydrogen-bonded structure.
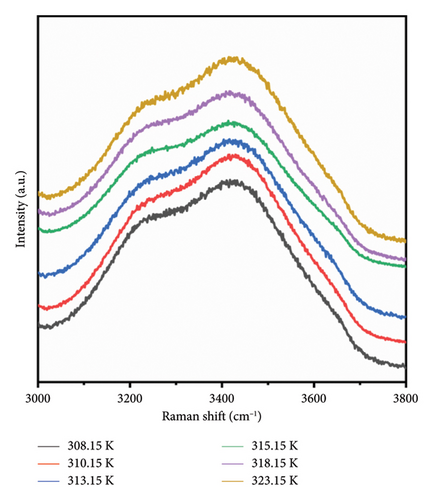
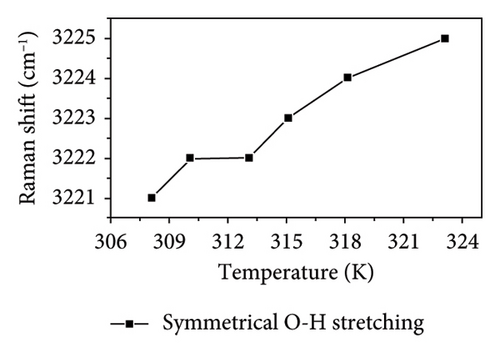
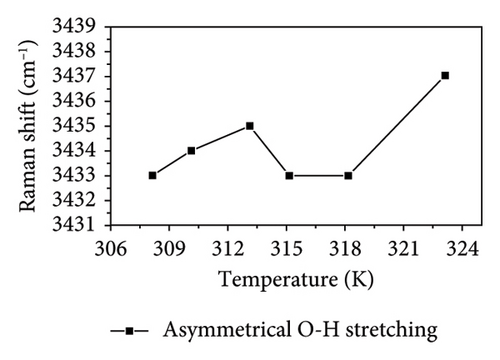
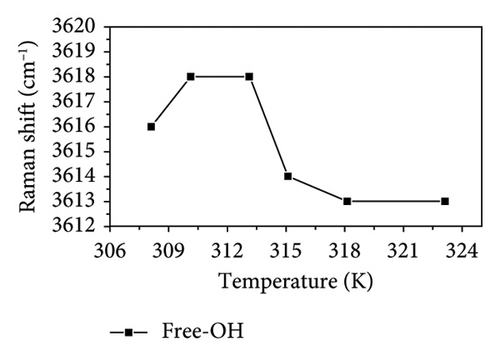
As shown in Figure 4, the symmetric OH stretching vibration demonstrates a consistent trend of shifting to higher wavenumbers as temperature increases, indicating that the hydrogen bonds associated with the symmetric O-H stretching vibration in PVA aqueous solution are being disrupted. In general, the hydrogen bonds between PVA-H2O, PVA-PVA, and H2O-H2O weaken the covalent O-H bond, causing O-H to shift toward lower vibrational frequency (i.e., red shift). Therefore, as the temperature increases, the hydrogen bonds progressively weaken and eventually break down. Notably, between 310.15 and 313.15 K, there is no significant change in either the symmetric OH stretching or free OH vibrations; however, the asymmetric OH stretching vibration continues to progress toward higher wavenumbers. This indicates that within this temperature range, the asymmetric OH stretching vibrations in the PVA aqueous solution system are more significantly affected by temperature, leading to a transformation from partial hydrogen bond structures to fully formed hydrogen bond structures and free OH groups. Although the fully formed hydrogen bond structures and free OH groups are influenced by temperature, this effect is mitigated by the transformation in hydrogen bond structure. Nevertheless, as temperature rises further, it leads to a weakening of the symmetric OH stretching vibration; thus, its wavenumber remains relatively stable as shown in Figures 4(b) and 4(c) while the asymmetric OH stretching shifts to higher frequencies. Additionally, as shown in Figures 4(c) and 4(d), it can be observed that within the temperature range of 313.15–315.15 K, the asymmetric OH stretching vibration and free OH groups exhibit a tendency to shift toward lower wavenumbers, while the symmetric OH stretching shifts to higher frequencies. This indicates that within this temperature range, the symmetric OH stretching vibrations in the PVA aqueous solution system are more significantly affected by temperature, leading to a transformation from fully formed hydrogen bond structures to partial hydrogen bond structures and free OH groups. Between temperatures of 315.15 and 318.15 K, changes in asymmetrical vibrations appear more subdued; this suggests a transformation from disrupted tetrahedral configurations back into free hydroxyl structures alongside potential generation and breakdown of extensive PVA-H2O-PVA chain networks within this interval. Finally, at temperatures spanning from 318.15 to 323.15 K, rapid movement of asymmetric OH stretches toward high wavenumbers occurs as elevated temperatures lead to substantial destruction of symmetric vibrational structures transitioning into those associated with asymmetric stretches instead. In conclusion, thermal elevation facilitates a transition from tetrahedral arrangements toward chain-like configurations within aqueous PVA solutions. However, at specific temperature thresholds where energy absorption by hydroxyl groups occurs alongside partial disruption of hydrogen bonds, new interactions may emerge leading to observable inflection points on graphical representations. At different temperatures, temperature influences distinct hydrogen bond structures in PVA aqueous solution. This intriguing finding holds significant value for the processing and preparation of PVA materials.
In the PVA-H2O solution, a characteristic C-H peak is observed in the range of 2800–3000 cm−1, as illustrated in Figure 5(a). This peak can be subdivided into two components (see Figure 5(b)): CH2 stretching vibration (Peak I) and broad alkyl valence band vibration associated with C-H (Peak II). The temperature-dependent displacements of Peak I and Peak II are represented graphically in Figure 5. As shown in Figure 5(c), both peaks exhibit an inflection point at approximately 313.5 K, which closely aligns with the inflection point identified in Figure 4. This correlation suggests that the anomaly observed at 313.15 K is linked to changes in hydrogen bonding within the PVA-H2O solution. The frequency shift data from the Raman spectra’s characteristic peaks provide insights into hydrogen bonding interactions between PVA molecules and water molecules. A downward shift in Raman frequency indicates an enhancement of hydrogen bonding between the PVA chain groups and hydroxyl groups present in water, leading to a complete hydrogen-bonding network involving asymmetric OH stretching vibrations of both PVA molecular chains and H2O, thereby reinforcing the hydrogen bond linkage between PVA and H2O. Consequently, it can be concluded that at 313.15 K, there is a strengthening of hydrogen bonds between CH groups and water molecules.
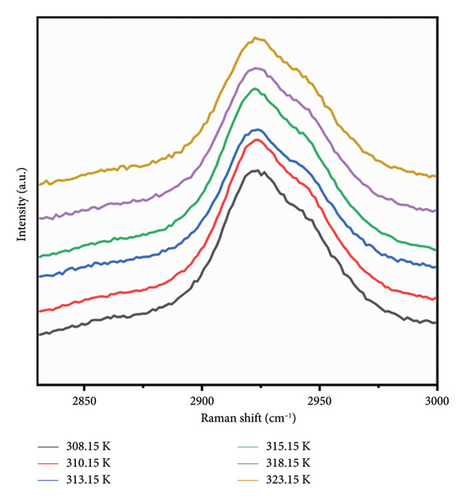
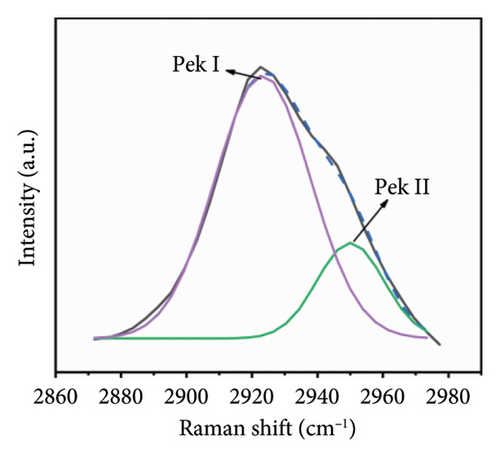
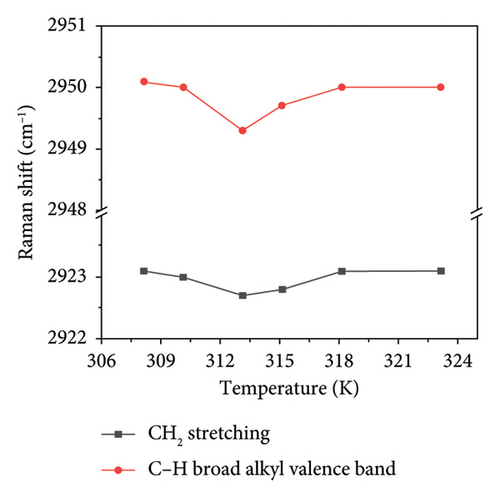
Based on the aforementioned observations, it can be inferred that variations in temperature induce alterations in the stretching vibrations of OH groups. At a temperature of 313.15 K, the middle CH groups within the PVA molecular chain exhibit a minor Raman frequency shift. The elevation in temperature disrupts the stable tetrahedral structure present in PVA aqueous solutions, resulting in a significant transformation of tetrahedral structural hydrogen bonds into chain structures. Consequently, hydroxyl groups within PVA absorb energy to establish both chain hydrogen bond structures and a limited number of tetrahedral hydrogen bond configurations with water’s OH groups. However, an increase in temperature tends to diminish hydrogen bonding interactions within the PVA-H2O solution.
3.2. Effect of Microwave Irradiation on the Structure of PVA
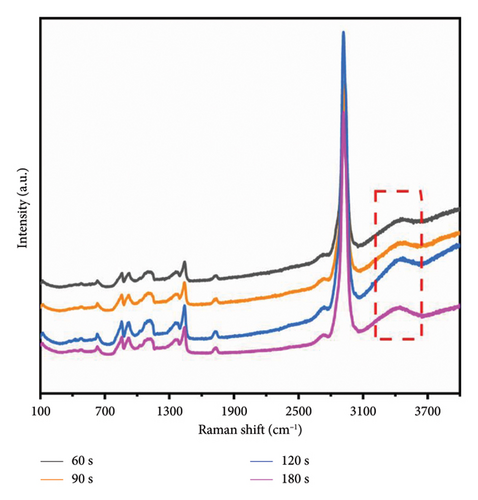
Figure 6(a) illustrates the Raman spectra obtained following microwave treatment durations of 60, 90, 120, and 180 s. The samples were subsequently deposited onto a glass substrate and allowed to dry naturally at room temperature for three days. Microwave irradiation induces structural modifications in PVA-H2O that persist for an extended period (up to several days) [21]. Thus, it is justifiable to maintain the PVA-H2O solution under ambient temperature and pressure conditions for three days during this experiment. The spectral region between 3100 and 3700 cm−1 was analyzed; this range corresponds to the vibrational modes of OH groups. As depicted in Figure 6(b), there is a reduction in the OH stretching vibration features compared to those observed in the solution, with only the primary peak remaining. This alteration can be attributed to the effects of microwave electric fields which stretch PVA macromolecules, disrupting symmetric OH stretching vibrations while leading to the disappearance of free OH structures as water molecules evaporate—resulting solely in asymmetric OH stretching vibrations within dehydrated PVA. Furthermore, as illustrated in Figure 6(c), increasing exposure time to microwave electromagnetic fields correlates with a downward shift in Raman frequency of the main peak toward lower wavenumbers; notably, shifts after exposures of 90 and 120 s exhibit pronounced slopes. This phenomenon arises from microwave-induced disruption of symmetric OH stretching vibrations within PVA solutions transitioning them into asymmetric forms, consequently resulting in molecular elongation and dehydration where free H2O structures vanish while inter- and intramolecular hydrogen bonding remains intact within PVA matrices. The observed shift toward lower wavenumbers indicates that microwaves enhance hydrogen bonding interactions among PVA molecules. According to Nagura et al. [37], such phenomena may stem from structural alterations occurring within crystalline regions of irradiated PVA films. When subjected to microwave irradiation at an energy density of approximately 0.36 kJ/mL, initial decreases are noted in XRD frequency shifts followed by increases at higher absorption energies—wherein these high-frequency shifts signify enhanced intramolecular hydrogen bonding.
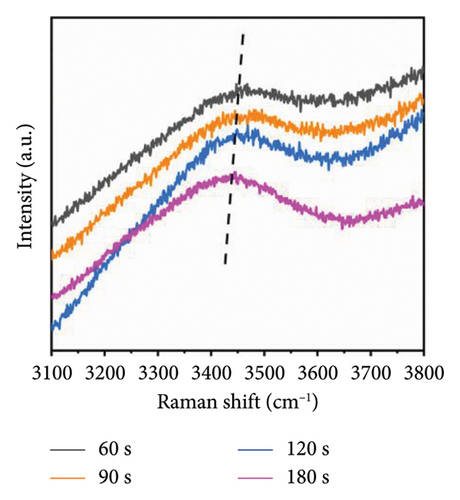
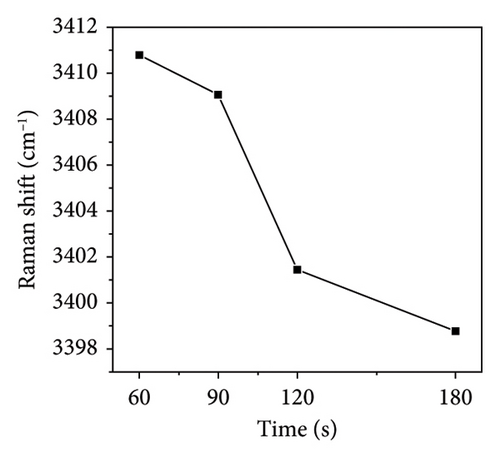
During microwave irradiation, the order of the polymer chains increases. This process facilitates the formation of both intramolecular and intermolecular hydrogen bonds with water; specifically, intermolecular hydrogen bonds are established by syndiotactic sequences within PVA molecules, while intramolecular hydrogen bonds arise from isotactic sequences [38]. As the duration of microwave irradiation extends and water evaporates, PVA chains develop intramolecular hydrogen bonds characterized by syndiotactic sequences [39]. The molecular rearrangement induced by microwave electromagnetic fields may result in denser molecular packing, with hydroxyl groups regularly distributed along one side of the main chain, thereby forming more robust hydrogen bonding structures [19].
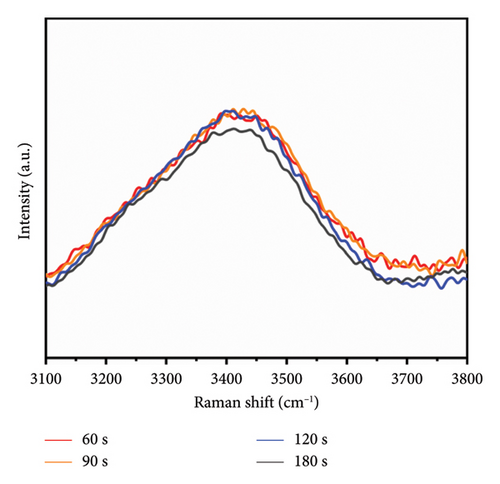
The Raman spectra presented in Figure 7 were obtained through baseline correction and FFT filtering applied to data collected via Raman spectroscopy. Notably, the intensity of the characteristic OH stretching vibration peak near 3410 cm−1 exhibited no significant reduction during microwave irradiation for durations between 60 and 120 s; however, a marked decrease in intensity was observed following 180 s of irradiation. Previous studies indicate that PVA undergoes an elimination reaction post-microwave irradiation, leading to a diminished presence of hydroxyl groups [32].
3.3. AFM Analysis
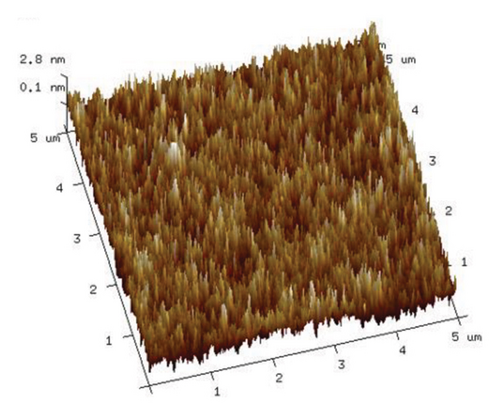
To elucidate the impact of microwave electromagnetic fields on aqueous PVA solutions, we employed AFM to examine the structural changes in PVA following natural drying. Utilizing the smart mode of AFM, we selected a sample area measuring 5 × 5 μm. The AFM images obtained from samples without microwave treatment are presented in Figure 8, revealing that PVA molecules were distributed relatively uniformly with no observable aggregation of molecular chains.

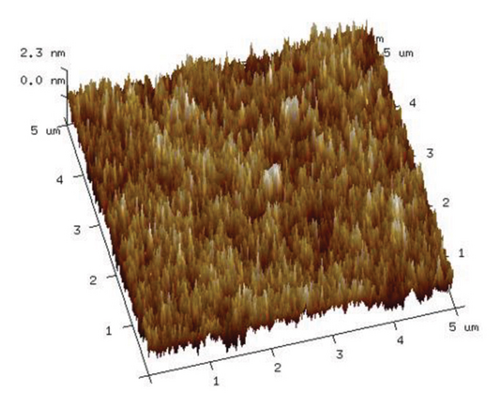
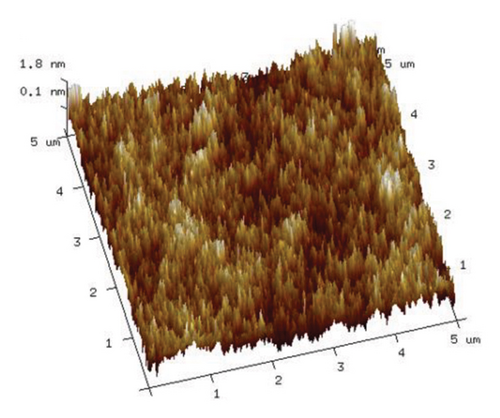
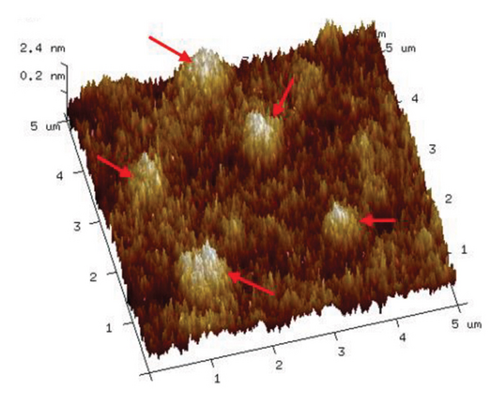
As illustrated in Figure 9, an increase in the duration of microwave irradiation correlates with a tendency for PVA molecular chains to aggregate. This phenomenon is particularly pronounced at an irradiation time of 180 s. The observed aggregation may be attributed to the prolonged exposure to the microwave electromagnetic field, which polarizes PVA molecules within the electric field, thereby enhancing polymer order. Consequently, PVA chains form stronger hydrogen bonds through cross-linking via ether/ester bridges and interactions among molecular hydroxyl groups, leading to a denser stacking of molecular chains. This observation aligns with the enhanced hydrogen bonding indicated by the Raman spectra presented in Figure 6.
Based on the aforementioned results from the analysis of microwave irradiation effects in PVA-H2O solutions, we have constructed a simplified schematic diagram to illustrate the impact of microwave action. In a prepared PVA-H2O solution, the PVA molecular chains are dispersed in an unordered state within the aqueous medium, forming hydrogen bonding interactions with water molecules. Upon application of microwave irradiation to this solution, the electromagnetic field induces polarization and alignment of dipoles, leading to disruption and subsequent recombination of hydrogen bonds. This process facilitates the formation of isotactic sequences within the PVA molecular chains present in the aqueous environment. A schematic representation depicting these effects is presented in Figure 10.
4. Conclusions
In this study, the microstructure of PVA under varying temperature and microwave conditions was investigated using Raman spectroscopy and AFM. The modes of group stretching vibrations in PVA solutions were systematically summarized. Based on the data obtained from Raman spectroscopy, we can draw several conclusions. (1) Temperature significantly influences the hydrogen bonding dynamics between PVA and water; specifically, an increase in temperature facilitates a transition from a tetrahedral hydrogen-bonded structure to a chain-like configuration within PVA–water solutions. Notably, at certain temperatures, numerous PVA-H2O-PVA chain structures are formed and disrupted, resulting in distinct inflection points observed in the Raman spectrum. (2) Analysis of the Raman spectrum for PVA solutions subjected to microwave irradiation followed by natural dehydration revealed that with prolonged microwave exposure, the primary peak corresponding to OH stretching vibrations shifted toward lower wavenumber frequencies. This indicates that microwaves enhance hydrogen bonding within the PVA molecules and that significant structural changes occurred after 180 s of irradiation. Furthermore, AFM results corroborate these findings by demonstrating molecular stacking of PVA molecules at this time point. Microwave irradiation is inferred to modify the structure of PVA aqueous solutions, consequently altering their surface tension, viscosity, and other physical properties, which ultimately influences the macroscopic mechanical characteristics of the resulting material. Thus, microwave technology provides a feasible method for the successful creation of polymer materials with excellent properties based on multiple hydrogen bonds.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Nannan Wu and Rui Ma contributed equally to this work and are co-first authors.
Funding
This work was supported by the National Natural Science Foundation of China (21864019), Inner Mongolia Major Basic Research Open Project (0406091701), Guangzhou Higher Education Teaching Quality and Teaching Reform Project (2024YBJGO52), Guangdong Provincial Special Innovative Project for Ordinary Higher Education Institutions (2024KTSCX147), and Tertiary Education Scientific Research Project of Guangzhou Municipal Education Bureau (2024312053 and 2024312054). We gratefully acknowledge HZWTECH for providing computation facilities.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21864019), Inner Mongolia Major Basic Research Open Project (0406091701), Guangzhou Higher Education Teaching Quality and Teaching Reform Project (2024YBJGO52), Guangdong Provincial Special Innovative Project for Ordinary Higher Education Institutions (2024KTSCX147), and Tertiary Education Scientific Research Project of Guangzhou Municipal Education Bureau (2024312053 and 2024312054). We gratefully acknowledge HZWTECH for providing computation facilities.
Open Research
Data Availability Statement
Data are available upon reasonable request.




