Meta-Analysis on Coronary Artery Bypass Grafting With Single Versus Bilateral Internal Mammary Artery Grafts in Patients With End-Stage Renal Disease
Abstract
Patients with end-stage renal disease (ESRD) and concomitant coronary artery disease (CAD) present unique challenges for coronary revascularization. While coronary artery bypass grafting (CABG) is recommended over percutaneous coronary intervention in this population, the optimal surgical strategy remains controversial. This meta-analysis provides an updated comparison of outcomes for ESRD patients undergoing CABG with either bilateral internal thoracic artery (BITA) or single internal thoracic artery (SITA) grafting. A total of nine studies involving 911 patients were included. Our findings revealed no significant differences in perioperative mortality (p = 0.57), deep sternal wound infection (p = 0.41), or major adverse cardiac and cerebrovascular events (p = 0.54) between groups. Long-term survival rates were also comparable at one, three, five, and seven years postoperatively. The pooled hazard ratio for all-cause mortality was 0.82 (95% CI: 0.61–1.12; p = 0.21), indicating no explicit survival advantage for either grafting strategy. These results are consistent with existing literature and suggest that both BITA and SITA grafting are safe and effective in this high-risk group. As medical advances continue to extend the life expectancy of patients with ESRD, additional research focused on optimizing the management of ESRD-related CAD will be essential to improving perioperative and long-term outcomes for these high-risk patients.
1. Introduction
Chronic kidney disease (CKD) affects more than 10% of the global population and often progresses to end-stage renal disease (ESRD), requiring dialysis [1]. Among patients with ESRD, 30%–60% develop concomitant coronary artery disease (CAD), which represents the leading cause of death in this population [2, 3]. Despite the increasing prevalence of renal dysfunction among patients requiring coronary artery bypass grafting (CABG) and the established association between reduced glomerular filtration rate (GFR) and cardiovascular mortality [4, 5], there is a paucity of data surrounding the optimal management of CAD in the context of ESRD.
CABG remains the preferred revascularization modality for dialysis-dependent patients with severe, stable CAD, consistently demonstrating superior outcomes over medical management or percutaneous coronary intervention (PCI) with new-generation drug-eluting stents [5, 6]. This Class IIa recommendation has remained unchanged since 2014 [7–9], owing to the unique characteristics of ESRD-related CAD, which typically involves diffusely diseased vessels, multivessel involvement, and a heavy plaque and calcification burden [10]. ESRD is associated with an increased risk of early and late mortality after CABG [4, 10–12]. However, the long-term survival benefit of CABG with bilateral internal thoracic artery (BITA) compared with single internal thoracic artery (SITA) grafting in this setting remains unclear [6, 13]. The present meta-analysis sought to address this knowledge gap.
2. Methods
The present study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [14] (Supporting Table 1). This pooled analysis is registered in the international prospective register of systematic reviews PROSPERO (CD 583697).
2.1. Systematic Review
MEDLINE and EMBASE were queried from 1990 to 2024, using the following terms: “dialysis” or “end-stage renal disease” or “bilateral internal mammary” or “bilateral internal thoracic.” Inclusion criteria included prospective or retrospective observational studies including ESRD patients who underwent isolated CABG with BITA compared with SITA and reported on at least one outcome of interest. Patients undergoing on- or off-pump CABG with skeletonized or pedicled internal mammary artery harvesting techniques were included in the present analysis. The exclusion criteria were reviews, case reports, commentaries, editorials, conference proceedings, noncomparative studies, and non-English language articles. Two investigators (G.M and D.F.) screened and reviewed the titles and abstracts independently. Full articles adhering to the above inclusion criteria were retrieved, with any disagreements between investigators resolved by third-party consensus (FB) (Supporting Figure 1).
2.2. Data Retrieval and Study Quality Assessment
Data extraction was performed by two independent investigators, capturing the following variables: authors, study period, year of publication, number of participating centers, surgical technique, left ventricular ejection fraction, dialysis duration, EuroSCORE II, internal thoracic artery (ITA) harvesting technique, early and late major adverse cardiac and cerebrovascular events (MACCE), repeat myocardial revascularization, deep sternal wound infection (DSWI), and intensive care unit stay > 48 h. The risk of bias of observational studies was assessed using the to the National Heart Lung Blood Institution criteria (Supporting Table 2) [15].
2.3. Study Outcomes
The primary outcomes of interest were short- and long-term all-cause mortality. Secondary outcomes included DSWI and MACCE, defined as a composite of death, cardiac or cerebrovascular events, and/or repeat coronary revascularization.
2.4. Statistical Analysis
Risk ratio (RR) estimates for each study were computed for early mortality and MACCE, as well as for binary variables (e.g., sex, hypertension, and diabetes), while mean differences (MD) were applied for continuous variables (e.g., age, body mass index, hemodialysis duration, and the EuroSCORE II) (Table 1). Data from the Kaplan–Meier (KM) plots of the included studies were digitized using the Plotdigitizer software (available online at: https://plotdigitizer.com). Individual patient survival data were reconstructed with the “KM_reconstruct” function from the R package “reconstructKM,” assuming constant censoring over time. The reconstructed survival data were used to estimate the pooled survival curve for long-term mortality and the hazard ratios (HRs) of each study using the Cox proportional hazards method. HRs were estimated to compare long-term all-cause mortality between the BITA and SITA groups. The random-effects model and the inverse variance methods were used to calculate the weight of each study and pool the RRs and MDs. Heterogeneity between studies was classified as low (0 < I2 ≤ 25%), moderate (26 < I2 ≤ 50%), or high (I2 > 50%), with the Cochran’s Q test applied to assess the presence of heterogeneity among study results. Potential publication bias was assessed by funnel plots and Egger’s Test. Individual and pooled results were reported with their 95% and confidence intervals (95% CI). Statistical analyses were performed using the “meta” package in R software (Version 4.3.1).
| Variable | N studies | BITA | SITA | Estimate [95% CI] | Method | p value |
|---|---|---|---|---|---|---|
| Age (years) | 8 | 65.2 | 65.4 | −0.19 [−1.45, 1.07] | MD | 0.77 |
| Male (%) | 8 | 79.8 | 78.0 | 1.03 [0.96, 1.10] | RR | 0.44 |
| BMI (kg/m2) | 4 | 22.9 | 22.7 | 0.20 [−0.70, 1.10] | MD | 0.67 |
| Diabetes (%) | 8 | 59.7 | 63.2 | 0.95 [0.78, 1.16] | RR | 0.61 |
| Hypertension (%) | 7 | 81.9 | 79.6 | 1.03 [0.96, 1.11] | RR | 0.39 |
| HD duration (days) | 7 | 6.2 | 5.6 | 0.59 [−0.66, 1.83] | MD | 0.36 |
| EuroSCORE II (%) | 4 | 4.4 | 5.5 | −1.16 [−3.76, 1.45] | MD | 0.38 |
- Note: Data are presented as means or percentages. BITA, bilateral internal thoracic graft; SITA, single internal thoracic graft.
- Abbreviations: BMI, body mass index; CI, confidence interval; HD, hemodialysis; MD, mean difference; RR, relative risk.
3. Results
3.1. Included Studies and Their Quality Assessment
Nine studies published between 2001 and 2021 were included in this meta-analysis, with a mean follow-up of 3.6 ± 3.0 years and a follow-up completion rate above 94% on average [16–20]. Among the 911 included patients with severe CAD and concomitant ESRD requiring dialysis, 51.0% (n = 465) underwent CABG with BITA grafting and 49.0% (n = 446) with SITA grafting. Six patients out of 911 (0.7%) were on peritoneal dialysis.
In situ right ITA was anastomosed to the LAD and in situ left ITA was anastomosed to the circumflex artery in eight out of nine studies. The right ITA, when not long enough to reach the LAD, was grafted to the circumflex artery through the transverse sinus or used as a composite Y graft from the left ITA, while the left ITA was grafted to the LAD. Only in one study, sixty-five patients over one-hundred and five in the BITA group included the right ITA composite graft based on in situ left ITA (i.e., single inflow bypass grafting). None of the SITA patients had a composite bypass grafting.
Three studies employed propensity score matching or similar methods to minimize selection bias [16, 17, 21], one study used adjusted regression methods [18], and the remaining five studies did not apply any risk adjustment method [19, 20, 22–24]. The present analysis demonstrated a comparable risk profile of the study groups (Table 1).
3.2. Early Outcomes
No statistically significant associations were observed between BITA and SITA grafting strategies for the early outcomes studied. Specifically, no differences were seen in perioperative mortality (RR: 0.84; 95% CI: 0.47–1.52; and p = 0.57), myocardial infarction (RR: 0.81; 95% CI: 0.23–2.82; and p = 0.74), stroke (RR: 0.84; 95% CI: 0.30–2.37; and p = 0.74), MACCE (RR: 0.78; 95% CI: 0.34–1.76; and p = 0.54), or DSWI (RR: 1.34; 95% CI: 0.67–2.66; and p = 0.41) (Figures 1, 2, 3, 4, and 5). Heterogeneity for these early endpoints was low (I2 = 0%-14%) across studies. Funnel plots for perioperative mortality, myocardial infarction, stroke, and MACCE (Supporting Information Figures 2, 3, 4, and 5) showed a symmetrical distribution of studies, suggesting no substantial publication bias; these findings were further supported by the results Egger’s test (p value > 0.05). For DSWI (Supporting Information Figure 6), a slight asymmetry in the distribution of studies was observed; however, Egger’s test did not indicate any significant publication bias (p value = 0.617). Five studies [16–18, 21, 22] included patients who underwent surgery employing the skeletonized harvesting technique in all cases. The use of this technique resulted in comparable risk of DSWI in the study groups (RR: 1.52; 95% CI: 0.64–3.62, and p = 0.35; I2: 0%).
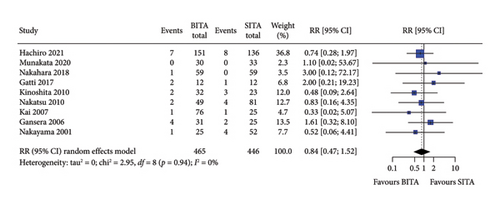
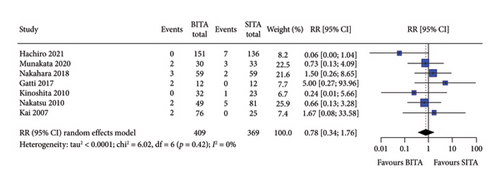
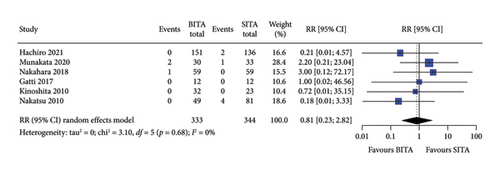
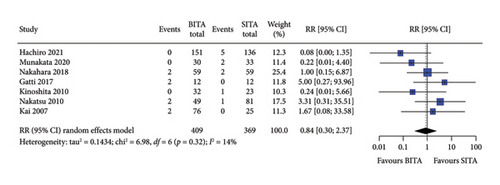
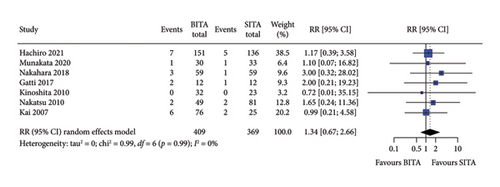
3.3. Long-Term Survival
Similarly, no statistically significant differences in long-term survival were observed between the two groups at any of the specified time points (p = 0.66) (Figure 6). One-year survival rates were comparable, with 88.0% (95% CI: 84.8%–91.3%) for BITA and 88.1% (95% CI: 84.7%–91.5%) for the SITA group. At 3 years, survival rates were 70.5% (95% CI: 65.7%–75.3%) for patients undergoing BITA grafting and 68.7% (95% CI: 63.5%–73.9%) for SITA. By 5 years, survival was 53.3% (95% CI: 47.5%–59.1%) for BITA and 55.4% (95% CI: 49.6%–61.2%) for SITA, while 7-year survival rates were 39.3% (95% CI: 32.7%–46.0%) for BITA and 35.4% (95% CI: 28.2%–42.7%) for SITA (Table 2). The pooled HR for all-cause mortality was 0.82 (95% CI: 0.61–1.12; p = 0.21), with moderate heterogeneity between studies (I2 = 40%) (Figure 7). A subgroup analysis was conducted, dividing the studies into those that employed propensity score matching or statistical adjustments [16, 18, 21] and those that did not [17, 20, 22]. In the first subgroup, no significant long-term survival benefit was observed for BITA over SITA, with a pooled HR of 0.89 (95% CI: 0.52–1.51; p = 0.66) and moderate-to-high heterogeneity (I2 = 56%). However, BITA was associated with a significant survival benefit in the unadjusted subgroup, demonstrating a pooled HR of 0.70 (95% CI: 0.49–0.98; p = 0.04) and low between-study heterogeneity (I2 = 0%) (Figure 7). These figures were derived from pooled reconstructed KM curves from seven of the nine included studies that specifically compared survival between SITA and BITA groups (Supporting Figure 7) [16–22].
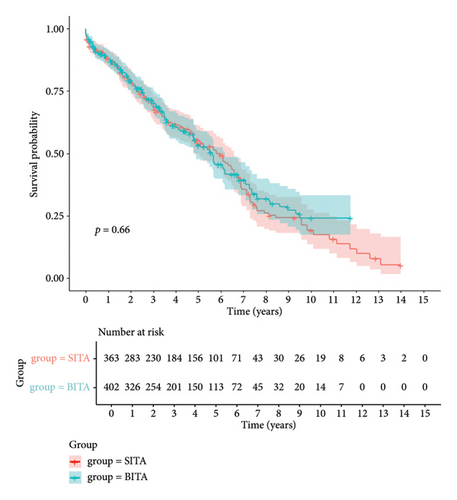
| Group | 1-year survival | 3-year survival | 5-year survival | 7-year survival |
|---|---|---|---|---|
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| BITA | 88.0 (91.3–84.8) | 70.5 (75.3–65.7) | 53.3 (59.1–47.5) | 39.3 (46.0–32.7) |
| SITA | 88.1 (91.5–84.7) | 68.7 (73.9–63.5) | 55.4 (61.2–49.6) | 35.4 (42.7–28.2) |
- Note: Data are presented as percentages with 95% confidence intervals. BITA, bilateral internal thoracic graft; SITA, single internal thoracic graft.
- Abbreviation: CI, confidence interval.
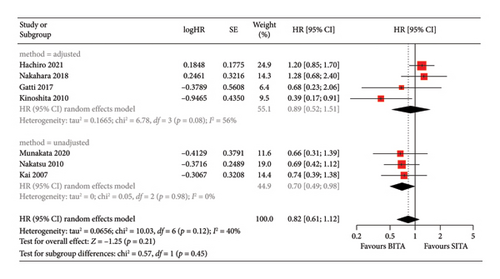
4. Discussion
Our analysis did not demonstrate any benefit of using BITA over SITA revascularization in patients with ESRDs. The present findings have a clinical relevance because the superiority of BITA grafting versus SITA grafting is controversial. Several observational studies showed that BITA grafting may be superior to SITA grafting. However, the policy of multiple arterial grafting for coronary revascularization can be biased by patient’s characteristics, comorbidities, and coronary artery anatomy. Since BITA grafting is a more complex technique which may require a specific expertise, an expeditious procedure using the SITA grafting can be preferred in patients with an increased risk profile such as those with severe renal failure.
ESRD is marked by a particularly high mortality rate, largely driven by the CAD that develops as a result of accelerated vascular calcification, inflammation, and the atherosclerotic effects of renal dysfunction [4, 25, 26]. Both ESRD and CAD share common etiologies and comorbidities, including diabetes and hypertension, which are independent predictors of adverse cardiovascular events following CABG [2, 27, 28]. While BITA grafting has demonstrated significant reductions in long-term mortality in the general population [11], this advantage does not appear to extend to ESRD patients, highlighting the need for more granular, disease-specific data. Given that patients with ESRD are more prone to developing complex, multivessel CAD, surgical revascularization is a common occurrence in this population, making evidence-based surgical management strategies a necessity. In addition, the successful resolution of CAD is often a prerequisite to renal transplantation candidacy.
Building on the previous meta-analysis by Tam and colleagues [9], which included five studies and 428 patients, our study more than doubles the sample size (nine studies, 911 patients), thereby increasing statistical power and providing a more robust, updated comparison of the short- and long-term outcomes of ESRD patients undergoing CABG with either BITA or SITA grafting. Consistent with previous findings [9], we observed no significant differences in early outcomes, including perioperative mortality, DSWI and MACCE, or long-term survival at one, three, five, and seven years postoperatively. These results further support the comparable safety and efficacy of both CABG grafting strategies in this high-risk population.
The reluctance to use BITA grafts in patients with ESRD is often attributed to concerns over sternal wound complications, lowered life expectancy, and coronary steal in those with an arteriovenous fistula [9]. However, ITA grafts have greater resistance to atherosclerosis and slower progression of native atherosclerotic disease than the venous grafts used in SITA CABG [10]. This benefit may offset some of the perceived risks associated with BITA grafting in ESRD patients and help explain the similar outcomes observed.
Interestingly, a recent study comparing BITA and SITA CABG in patients with CKD (estimated GFR [eGFR] < 60 mL/min/1.73 m2) reported comparable early outcomes but significantly improved long-term survival (upto 20 years) in CKD patients receiving BITA grafting [4]. These findings suggest that the protective effects of BITA revascularization observed in CKD patients may diminish in patients with more advanced renal dysfunction (ESRD, eGFR < 15 mL/min/1.73 m2), including those in this study.
5. Limitations
These findings should be considered in light of several important limitations.
First, despite the efforts to conduct a comprehensive analysis of the available literature, only nine studies met the inclusion criteria for the final analysis, which may leave this meta-analysis underpowered.
Second, the generalizability of the results is limited, as most of the included studies were conducted in Japan, where the incidence of ESRD is the third highest in the world, and cardiovascular mortality rates are among the lowest [29]. Similar studies should be accomplished in other regions and demographics to make the results generalizable. Third, while the previous meta-analysis reported a roughly 70% 4-year survival for the overall cohort [9], consistent with the literature on patients treated with PCI or CABG (75%–80% 5-year survival) [3], our survival estimates demonstrated a lower overall survival of 54% at five years. Fourth, the unadjusted results showed a benefit in favor of the BITA group in terms of late mortality and that this advantage was homogenous among all the unadjusted studies. Indeed, the heterogeneity was confirmed by I2 0%. Still, we believe that the pooled analysis of adjusted HRs is more reliable because it considers the effect of potential confounders which are behind the selection bias in choosing the surgical technique. We do recognize that adjusted studies showed a relatively high heterogeneity (I2: 50%), but the retrospective nature and fair quality of the studies justify such heterogenous results. This could be related also to the different length of follow-up between the studies. Overall, the main bias of the pooled adjusted risk estimates seems to be related to the small size of two studies [17, 20] contributing with their limited weight and possibly a type II error. Furthermore, the small number of studies with adjusted risk estimates of late mortality prevented sensitivity analysis as well as leave-one-out analysis.
Fifth, we recognize the significance of cardiovascular mortality for a throughout assessment of the durability of invasive cardiac procedures. However, the policy of evaluation of causes of death has changed in many countries with a reduction of rates of autopsy and this makes the estimation of mortality secondary to cardiovascular events not reliable. Indeed, most of the recent studies rely on the assessment of MACE or MACCE, which is a more comprehensive and reliable measure of long-term outcome. Sixth, the studies included in this meta-analysis did not provide outcome data stratified by the suggested variables (cause, duration, and modality of dialysis), which prevents us from exploring the comparison between the two techniques in these specific subgroups. Finally, in the general population, the survival benefit of BITA grafting becomes evident around 7 years postoperatively [30]. Given that our long-term survival analysis does not extend beyond 7 years, it is possible that the full benefits of BITA revascularization in dialysis-dependent ESRD patients have not yet emerged or been captured in the present data. Additional research with extended follow-up is warranted to explore this potential benefit and to investigate the underlying mechanisms driving these observations.
6. Conclusions
The recommendation for CABG over PCI in patients with concomitant ESRD and CAD is well supported by extensive research. However, equipoise still surrounds the optimal surgical revascularization strategy in this complex patient population. In line with previous literature, our meta-analysis of over 900 patients found no significant differences in early outcomes or long-term survival between BITA and SITA grafting, reinforcing that both are safe and effective options for CABG in ESRD patients requiring dialysis. As medical advancements continue to extend the life expectancy of patients with ESRD, who bear a disproportionately high burden of CAD, ongoing research aimed at optimizing CABG outcomes and graft patency in the context of renal dysfunction will be essential to minimizing perioperative complications and improving the long-term outcomes of CABG in this high-risk group.
Nomenclature
-
- BITA
-
- bilateral internal thoracic artery
-
- BMI
-
- body mass index
-
- CABG
-
- coronary artery bypass grafting
-
- CAD
-
- coronary artery disease
-
- CKD
-
- chronic kidney disease
-
- DSWI
-
- deep sternal wound infection
-
- eGFR
-
- estimated glomerular filtration rate
-
- ESRD
-
- end-stage renal disease
-
- GFR
-
- glomerular filtration rate
-
- HR
-
- hazard ratio
-
- ITA
-
- internal thoracic artery
-
- KM
-
- Kaplan–Meier
-
- MACCE
-
- major adverse cardiac and cerebrovascular events
-
- MD
-
- mean difference
-
- PCI
-
- percutaneous coronary intervention
-
- RR
-
- risk ratio
-
- SITA
-
- single internal thoracic artery
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Giorgio Mastroiacovo and Aliya Izumi contributed equally to this work.
Funding
This study was supported by the Italian Ministry of Health, Ricerca Corrente to Centro Cardiologico Monzino IRCCS. The funder did not have any role in the analysis and writing of this study.
Supporting Information
Supporting Figure 1: Study flowchart.
Supporting Figure 2: Funnel plot for perioperative mortality.
Supporting Figure 3: Funnel plot for myocardial infarction.
Supporting Figure 4: Funnel plot for stroke.
Supporting Figure 5: Funnel plot for MACCE.
Supporting Figure 6: Funnel plot for DSWI.
Supporting Figure 7: Funnel plot for long-term mortality.
Supporting Table 1: PRISMA statement.
Supporting Table 2: Quality assessment of the included studies according to the National Heart Lung Blood Institution criteria.
Open Research
Data Availability Statement
The data of this study are not publicly available due to privacy issues.