Extraction, Characterization, and Optimization of Protein From Food Waste and Waste-Activated Sludge
Abstract
There is a growing interest in the recovery of valuable biomaterials from waste in line with reaping the benefits of a circular economy. Organic wastes, such as waste-activated sludge (WAS) and food waste (FW), contain a substantial amount of protein that can be recovered for various applications. This study compared thermal alkaline and acid hydrolysis methods for their efficiencies in extracting protein from FW and WAS. The possibility of enhancing protein yield and quality through co-extraction of WAS and FW was also investigated. Response surface methodology was used to optimize the extraction process. Before extraction, WAS was purified by removing heavy metals using acid pretreatment. It was established that FW had 21.5 g/100 g protein, while WAS had 19.9 g/100 g protein. The two extraction methods had superior extraction for WAS as compared to FW. In addition to this, there was no significant increase in protein yield in the co-extraction of protein from the FW and WAS mixture. Furthermore, optimization using RSM showed that the optimal yield of 15.8 g/100 g was obtained at a pH of 13 and a temperature of 120°C, close to the experimental yield of 16.6 g/100 g WAS. Moreover, LC–MS analysis of the extracted protein showed that WAS had a good essential amino acid profile with threonine, lysine, leucine, methionine and valine in concentrations of 3.3, 2.7, 1.8, 1.1, and 2.7 g/100 g, respectively. Significantly, the level of threonine revealed the potential of beneficiation of WAS as an animal food supplement because threonine plays an important role in the synthesis of mucosal protein that lines and protects the intestinal tract as well as modulation of nutritional metabolism and macromolecular biosynthesis in animals. In addition to that, the ratio of the first limiting amino acids (lysine and methionine) met the standards for animal feed supplementation.
1. Introduction
Rapid urbanization has resulted in the establishment of a large number of sewage treatment plants that handle high volumes of wastewater generated by the increasing urban population [1]. Most of these plants use the activated sludge (AS) process due to its ability to handle high organic loads [2, 3]. Despite the suitability of the AS process in handling the urban wastewater, its main shortcoming is the excessive generation of waste-AS (WAS) [4]. The handling and disposal of WAS increases the cost of the AS process by nearly 50% [5, 6]. To reduce the WAS impact on the environment, different disposal and valorization approaches have been used. The valorization options have become better management strategies, for they are in line with the circular economy concept. Some of the valorization methods that have been applied for sludge management are used in agriculture as fertilizer, as biofuels, for carbon, for the production of building materials, and for the generation of electricity [7].
The WAS is rich in protein, having a concentration of 20–60 g protein/100 g WAS, contributed by dead microbial cells [6, 8, 9]. Subsequently, the protein in WAS can be recovered and potentially used to supplement other protein requirements, such as in animal feed [10, 11]. Extracting protein from WAS for potential use as an animal feed supplement maximizes resource utilization and provides a sustainable and economical protein source for animal nutrition. This is because the animal feed industry faces an unprecedented challenge due to the high demand for animal feed brought about by a broad mismatch between the supply of protein and the rising animal population [12]. Furthermore, regular rises in the cost of essential commodities for the animal feed industry, such as fish meal and soybeans, are complicating the global protein market [13]. In addition to this, using human-edible cereals, soybeans, and other oilseeds as animal feed is viewed as direct competition against human food security [13].
Extraction of protein from WAS is usually achieved through various methods, including chemical treatment, physical methods, and biological [14, 15]. During the extraction process, hydrolysis of WAS is usually the rate-determining step due to the complexity of the bacterial cell wall and the rigid floc structure of WAS, particularly the extracellular polymeric substance (EPS) matrix [16]. Therefore, the key to sludge protein recovery is to select a suitable method to break the sludge cell wall and disintegrate the EPS [17]. The application of thermal alkaline hydrolysis or thermal acid hydrolysis has good performance on sludge disintegration for protein release [18].
There is limited knowledge of the types of amino acids in the protein extracted from WAS. It is therefore significant to determine the types of amino acids in the extracted protein to inform its targeted application. To improve the extracted protein quality by having a wide variety of amino acids, co-extraction of WAS and other wastes, such as food waste (FW), could be beneficial. This is because these types of wastes could increase protein yield or contribute different amino acid building blocks to the final extracted protein matrix, thus improving its diversity, value, and application [19]. The primary application of amino acids is animal feed, which accounts for more than half of the market share in the amino acid industry [20].
The main protein sources for animal nutrition in the market are fishmeal, meat and bone meal, soybean meal, wheat, corn, and barley [21]. Most of these feed ingredients, even their mixtures, do not provide a balanced protein for animal nutrition; thus, feed supplementation using the FW–WAS mixture should be explored. This study, therefore, aimed at improving the extraction of protein from WAS through the optimization of extraction conditions, including co-extraction with FW, as well as determining the amino acid composition of the extracted protein.
2. Materials and Methods
2.1. Materials and Reagents
The materials used include WAS obtained from sludge drying beds of the Kariobangi Sewerage Treatment Plant, a local wastewater treatment facility in Nairobi, Kenya. The plant uses an AS system to treat wastewater, and its treatment capacity is 96,000 m3 per day. FW material included kitchen waste and peels obtained from various eateries. Reagent-grade alkali (sodium hydroxide) and acid (sulfuric acid) were used for protein extraction from the wastes. A heating mantle was used for the extraction process. H2SO4, Cu catalyst, K2SO4, NaOH, H3BO3, and HCl were used in protein quantification.
2.2. Sample Collection and Pretreatment
The AS samples were collected and stored at 4°C until use. The sludge was pretreated with 1N HCl solution to remove heavy metals. The solution was then filtered and rinsed with distilled water to recover the pretreated sludge as supernatant and heavy metals as filtrate. Heavy metal concentrations were determined in the filtrate. Similarly, FW was collected from various eateries and stored in a refrigerator at 4°C. To prepare the sample for analysis, FW was dried in an oven to reduce the moisture content. The dried sample was then ground into small particles. Ethanol was added to FW in a ratio of 1:1 [22] to remove fats and oils, and the mixture was centrifuged to separate the solvent from the FW.
2.3. Sludge and FW Characterization
The characterization of WAS and FW was performed following the American Public Health Association (APHA) prescribed standard methods [23]. Protein content was determined by the Kjeldahl method through direct measurement of nitrogen and subsequent multiplication by a conversion factor, usually 6.25 [24]. The method involves three steps: digestion, distillation, and titration. The sample is digested in sulfuric acid to convert the protein nitrogen to ammonium sulfate at a boiling point elevated by the addition of potassium sulfate with a copper catalyst. Ammonia is released by alkaline steam distillation and quantified titrimetrically with a standard acid. Elemental analysis was also performed to establish the presence of heavy metals. Finally, the amino acid analysis of the protein supernatant was done to determine the nutritional properties of the sludge.
2.4. Alkaline Thermal Hydrolysis Experiments
2.5. Co-Extraction of Protein
To study the extraction of protein from WAS and FW, FW and sludge were combined in the ratios of 1:0, 3:1, 1:1, 1:3, and 0:1. In this study, alkaline and acid methods of hydrolysis were used under similar conditions as explained above. The performance of the two hydrolysis methods on protein recovery was compared.
2.6. Optimization of Protein Extraction Using Response Surface Methodology (RSM)
RSM was used to optimize the process of protein extraction. RSM is a set of statistical and mathematical methods effective in constructing models and analyzing the problems in which several independent variables influence dependent variables or responses [26]. To analyze the process parameters, Design-Expert software Version 13.0 was used to study the interactive effect of pH, temperature, and extraction time on protein yield and extraction efficiency.
2.7. Sample Analysis
Fourier transform infrared spectroscopy (FTIR) analysis to identify the functional groups in the raw waste and extracted protein was conducted using the Shimadzu IRSpirit spectrometer. Heavy metals were analyzed using an inductively coupled plasma (ICP) spectrometer, model ICPE-9000, after wet digestion using a nitric acid–hydrochloric acid mixture. Amino acid analysis was conducted using Agilent 1290 high-performance liquid chromatography (HPLC) paired with a 6120 series single quad mass spectrometer (MS) (Agilent Technologies Inc., Santa Clara, CA, USA) outlined as follows.
The recovered protein supernatant was analyzed for its amino acid composition using the HPLC–MS technique described by Kibet et al. [27]. The crude protein supernatant was hydrolyzed into amino acids with 1.5 mL of 6N HCl at 110°C for 24 h in a stream of nitrogen. Hydrolysis products were subsequently dried by evaporation at 40°C under nitrogen, and the residues were dissolved in 1 mL of 0.01% formic acid/acetonitrile (95:5). The mixture was vortexed for 30 s before sonication for 30 min and subsequently centrifuged at 14,000 rpm. The resulting solution was filtered through a 0.45 μm syringe filter and analyzed on HPLC–MS. Zorbax RX-C18, 4.6 × 250 mm, 5 μm column, operated at 40°C, was used to achieve chromatographic separation. An authentic standard of amino acids (Sigma-Aldrich, St. Louis, MO, USA) was analyzed by LC–MS and used to externally quantify the amino acids. All the analyses were performed in triplicates [27].
3. Results and Discussion
3.1. Characteristics of WAS and FW
The characterization of FW and WAS included the determination of the following parameters: pH, moisture content, TSS, VSS, heavy metals, and protein content, as shown in Table 1. A moisture content of 69% in WAS implied that the sludge used in this study had been partially dewatered but still retained a substantial amount of water. The VSS of approximately 66% of the TSS indicated that a significant portion of the sludge is organic.
| Parameter | Units | WAS | FW |
|---|---|---|---|
| Moisture content | % | 69.22 | 75 |
| Protein | % | 19.92 | 21.45 |
| pH | 6.5 | 5.5 | |
| TSS | Mg/L | 4673 | 10,350 |
| VSS | Mg/L | 3080 | 7580 |
| VSS/TSS | Mg/L | 0.66 | 0.73 |
| Cd | Mg/L | 7.41 | — |
| Fe | Mg/L | 18,705 | 569 |
| Ni | Mg/L | — | — |
| Mn | Mg/L | 6705 | 1200 |
| Pb | Mg/L | 42.35 | — |
| Cr | Mg/L | 53.50 | — |
| Zn | Mg/L | 867.65 | 291 |
| Cu | Mg/L | 155 | 6.95 |
The VSS/TSS ratio of 73% suggests that a large portion of the total suspended solids in FW is volatile. In addition, the results of elemental analysis in FW demonstrate high levels of contamination against the safety standards set by the World Health Organization (WHO) and Food Agricultural Organization (FAO). The concentrations of Cu, Zn, Mn, and Fe were above WHO and FAO limits [28]. According to Scutaraşu and Trincă [29], the high levels of these elements are primarily due to their natural abundance in food, and their levels may have been elevated by industrial growth and the excessive use of chemicals in agriculture. However, Cr, Ni, Cd, and Pb were present in low concentrations below detectable levels.
3.2. Pretreatment for Heavy Metals Removal
The metals detected in WAS included Ni, Cr, Cd, Fe, Mn, Pb, Zn, and Cu, which were above the permissible levels for animal feeds. However, after a two-stage removal process involving pretreatment using dilute acid followed by removal during protein extraction, substantial amounts of heavy metals were reduced to allowable limits, as shown in Table 2. A removal efficiency of more than 90% was recorded for lead, manganese, cadmium, and zinc. Conversely, the pretreatment step had the least removal efficiency for copper, 22%. The low removal efficiency of Cu during pretreatment may be attributed to its binding affinity to organic matter in the sludge to form stable complexes, which can make it resistant to leaching by acid [32]. Despite this observation, the extraction step after pretreatment led to further purification due to its high selectivity towards protein. This led to a further reduction in the amount of the heavy metals in the extracted protein, with concentrations of Cd, Mn, and Pb below the limits of detection.
| Element | Raw sludge (Mg/L) | Pretreated sludge (Mg/L) | Extracted protein (Mg/L) | Removal efficiency (%) | WHO/FAO/EU limits (Mg/L) [30, 31] |
|---|---|---|---|---|---|
| Cr | 53.50 | 24.80 | 0.37 | 53.64 | 1 |
| Ni | 13.9 | 7.80 | 0.16 | 43.80 | 2 |
| Cd | 7.41 | ND | ND | 100 | 0.5 |
| Mn | 6705 | 37.70 | ND | 99.44 | 150 |
| Pb | 42.35 | 2.50 | ND | 94.10 | 10 |
| Zn | 867.65 | 38.60 | 1.59 | 95.55 | 150 |
| Cu | 155 | 121 | 0.68 | 21.94 | 170 |
- Abbreviation: ND, Not detectable.
3.3. Effects of Temperature and pH on Protein Extraction
3.3.1. Effect of Temperature on Protein Extraction
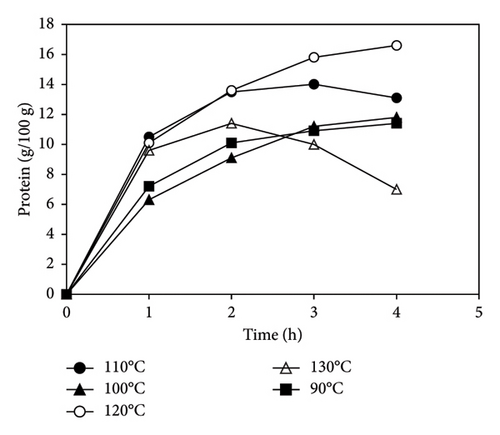
It was observed that the amount of protein extracted increased from 11 g/100 g to 16 g/100 g when the temperature was increased from 90°C to 120°C, after which there was a remarkable decline to 7 g/100 g when the temperature was increased stepwise to 130°C (Figure 1). A similar trend was reported by Gao et al. [6]. As the temperature increases from 90 to 120, the structure of the sludge is destroyed and microbial cells lysed, releasing organic material into the liquid phase and thus increasing the protein content [33]. However, a decline in protein concentration after 120°C could be due to favorable conditions that promote the Maillard reaction (MR) between amino acids and carbohydrates, thus lowering the protein yield [34].
3.3.2. Effect of pH on Protein Extraction
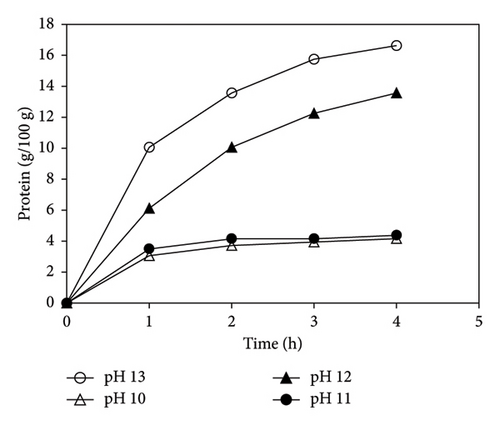
The effect of pH on protein concentration was investigated at a constant temperature of 120°C. Figure 2 shows that as the pH was increased from 10 to 13, the amount of extracted protein increased from 4 g/100 g to 16 g/100 g. Compared to pH 12 and 13, the rates of extraction at pH 10 and 11 were lower. This can be attributed to the low alkali concentration that was insufficient to break or lyse the cell membrane of cells in the sludge to release protein [35]. Conversely, at pH 13, the alkali concentration was adequate, resulting in an effective release of protein into the aqueous phase since a high alkaline concentration facilitates the solubility of sludge proteins [36]. As shown in Figure 2, compared to the effect of temperature, pH had more influence on the extraction of protein from sludge. This is due to the direct influence of pH on the solubility of WAS proteins [37].
3.3.3. Combined Effect of Temperature and pH on Protein Extraction
In Figure 3, the protein extraction increased gradually with an increase in both temperature and pH. When the pH was increased from 10 to 13, with a subsequent increase in temperature from 90°C to 120°C, the protein yield increased four-fold, peaking at 16.6 g/100 g. The increase in protein concentration as a result of an increase in both alkali concentration and temperature can be attributed to the destruction of sludge structure as a result of high temperatures, resulting in ease of permeation of alkali into the sludge flocs, leading to solubilization of the membrane proteins, saponification of the membrane lipids, and destruction of microbial cells, resulting in a higher release of protein into the liquid phase [38].
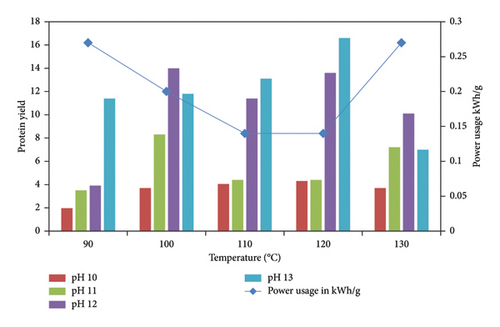
There was a notable decline in protein yield at 130°C and a high pH of 13. At this temperature and pH, it was observed that the extracted solution was dark brown, which could be attributed to the MR, where protein degrades into amino acids and reacts with simple sugars to form dark brown–colored melanoidins [39]. Therefore, the availability of amino acids and proteins is significantly reduced by the MR compounds formed under high temperatures and pH [40]. Under these conditions, the MR can affect the quality of the recovered protein [41].
At lower temperatures of 90°C and 100°C, there was a higher protein yield at high pH values of 12 and 13 than those at pH of 10 and 11. This could be because at high pH, proteins are negatively charged, which increases their solubility [42]. Moreover, at low temperatures, the stability of proteins is high, and therefore this contributed to high protein concentrations at low temperatures and high alkaline concentrations.
3.4. Energy Efficiency of Thermal Alkaline Hydrolysis
3.5. Co-Extraction of Protein From Sludge and FW by Alkaline Hydrolysis
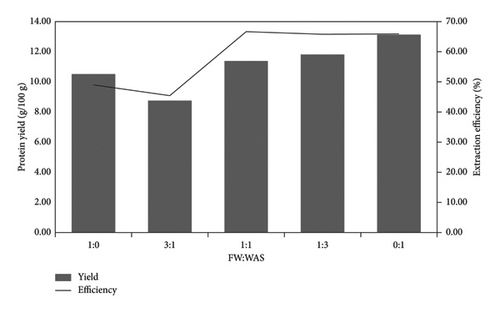
Co-extraction of protein from FW and WAS was studied to establish if there could be any synergy that would improve the protein yield. The ratios of FW to WAS studied were 1:0, 3:1, 1:1, 1:3, and 0:1 at a pH of 13. The protein concentration in raw sludge was 19.9 g/100 g, while FW had a protein concentration of 21.5 g/100 g, as shown in Table 3. Even though FW had a higher protein content, thermal alkaline hydrolysis had lower protein extraction efficiency from FW than WAS. Subsequently, when the FW: WAS was lower, the protein yield was higher. This may be due to the difference in composition between FW and sludge, as the FW protein often co-exists with compounds such as starch, cellulose, pectin, and lipids in the cells, which may lower the protein extraction efficiency [43]. Conversely, alkaline thermal hydrolysis has a good sludge lysis ability, thus releasing protein into the aqueous phase.
| Mixing ratio (FW: WAS) | Protein (g/100 g) | Extracted protein (g/100 g) | Efficiency (%) |
|---|---|---|---|
| 1:0 | 21.45 | 11.38 | 53 |
| 3:1 | 19.26 | 5.69 | 30 |
| 1:1 | 17.07 | 9.63 | 56 |
| 1:3 | 17.95 | 14.01 | 78 |
| 0:1 | 19.92 | 16.63 | 83 |
3.6. Co-Extraction of Protein From Sludge and FW by Acid Hydrolysis
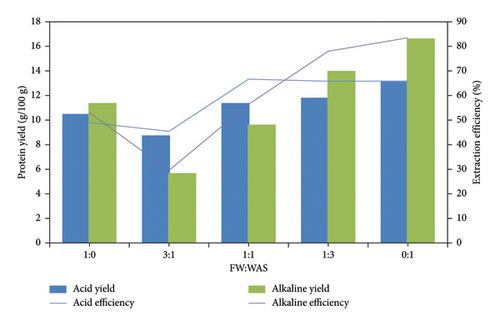
The acid method of hydrolysis was used to investigate the effect of acid on protein concentration in FW and WAS. The extracted protein in FW and sludge was 10.5 g/100 and 13.1 g/100 g, respectively. A FW: WAS ratio of 3:1 had a protein extraction of 8.8 g/100 g, while at a FW: WAS ratio of 1:1, 11.4 g/100 g protein was recovered, as shown in Figure 4. Subsequently, extraction of 11.8 g/100 g was achieved when the FW: WAS ratio was 1:3. Generally, the amount of protein recovered increased as the ratio of FW to WAS decreased. This shows that acid thermal hydrolysis is more efficient in extracting WAS protein than the FW protein. This efficiency may be due to the composition of WAS, which contains a higher proportion of microbial cells and EPSs that are more readily broken down by acid thermal hydrolysis [44]. In contrast, FW often contains a variety of complex organic materials, including fats, oils, and carbohydrates, which can make protein extraction more challenging [45].
3.7. Comparative Analysis of the Use of Alkaline and Acid Methods for Protein Extraction
A comparative study on the performance of alkali and acid hydrolysis of mixed FW and WAS was carried out to determine the best extraction method. As shown in Figure 5, there was no significant increase in protein yield in co-extraction. Generally, it was observed that thermal alkaline hydrolysis was superior to the thermal acid hydrolysis method at all ratios tested except at FW: WAS ratios of 3:1 and 1:1. The highest protein extraction of 16.3 g/100 g was attained at a FW: WAS ratio of 0:1. This observation can be linked to the acid solubilization, which has limited floc disruption compared to alkaline hydrolysis, which, apart from breaking sludge floc, damages the cell membrane, hence robust protein release [46].
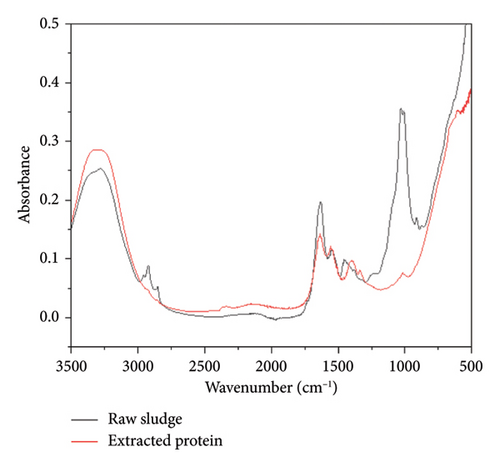
3.8. Characterization Analysis of Hydrolysis Products
FTIR spectra of sludge and the extracted protein are displayed in Figure 6. The broad, mid-intense band between 3400 and 3200 cm−1 represents O–H stretching vibrations of hydroxyl groups. An intense peak at around 1640 cm−1 was assigned to the stretching vibrations of the –C=O group of Amide I, while the peaks at 1560–1520 cm−1 are attributed to the N–H bending vibrations of the Amide II band [47]. The absorption bands in the region from 3000 to 2800 cm−1 can be linked to the presence of hydrocarbon chains on the organic matter of the sludge [48]. These bands are associated with the asymmetric stretching of the C–H methyl bonds (2955 cm−1) and methylene (2920 cm−1) groups and are also related to the symmetric C–H stretching of methylene (2850 cm−1) groups. Double peaks at 1030 and 1009 cm−1 suggest specific functional groups, which can be linked to C–O–C and C–O stretching vibrations of carbohydrates and polysaccharides in the biomass [49, 50]. The appearance of peaks in the treated sludge at 1400 cm−1 represents symmetric stretching vibrations of the carboxylate groups (COO–) commonly found in proteins and other organic compounds broken down during the hydrolysis process [51]. Similarly, the appearance of a small peak at 1340 cm−1 can be assigned to the bending vibrations of CH3 groups or the C–N stretching vibrations in amides, which are also the components of proteins.
3.9. Amino acid Analysis
The high levels of protein in WAS make it a rich source of amino acids [52]. The amino acid distribution in protein obtained from WAS compared to conventional protein sources is presented in Table 4. 15 amino acids consisting of 9 essential amino acids (EAAs) and 6 non-EAAs (NEEAs) were detected. Among the EAAs, threonine was the most abundant. Threonine plays a critical role in macromolecular biosynthesis, gut homeostasis, and the modulation of nutritional metabolism [55]. It also promotes body weight gain, feed intake, and carcass weight when used as a feed supplement [56]. Furthermore, threonine works synergistically with lysine and methionine by playing a critical role in antioxidant and immune functions [57], thus complementing the growth and health benefits provided by lysine and methionine, which are considered co-limiting amino acids [58].
| Amino acid | Extracted WAS protein | Soybean meal | Fishmeal | FAO reference protein [54] |
|---|---|---|---|---|
| Valine | 2.7 | 5.2 | 4.7 | 4.2 |
| Threonine | 3.3 | 4.4 | 3.8 | 2.8 |
| Phenylalanine | 2.2 | 5.3 | 3.5 | 2.8 |
| Methionine | 1.1 | 1.3 | 2.9 | 2.2 |
| Lysine | 2.7 | 6.6 | 7.6 | 4.2 |
| Leucine | 1.8 | 7.6 | 6.5 | 4.8 |
| Isoleucine | 0.2 | 5.8 | 3.9 | 4.2 |
| Histidine | 0.5 | 2.7 | 2 | — |
| Alanine | 4.3 | — | — | — |
| Arginine | 1.6 | 7.3 | 6.8 | — |
| Glutamic acid | 5.7 | — | — | — |
| Serine | 2.1 | — | — | — |
| Glycine | 3.2 | — | — | — |
| Proline | 1.8 | — | — | — |
| Tyrosine | 1.5 | 4.1 | 3 | 2.8 |
The ratio of lysine to methionine is critical for optimal growth and development of body tissues, particularly in young animals [59]. The current study found a ratio of 2.5, which is above the 1.9 threshold recommended by FAO. This value correlates with the fishmeal’s ratio of 2.6, indicating the possibility of adding WAS protein to the fishmeal. Moreover, the presence of other EAAs such as valine, arginine, histidine, phenylalanine, and leucine in WAS protein emphasizes its potential as a feed supplement. Supplementation of animal feeds using protein derived from WAS will not only reduce the environmental burden associated with the disposal of WAS but also contribute to global food security by reducing the reliance on human-edible protein sources for animal feeds.
Vriens et al. [60] carried out feeding experiments on pigs, rats, poultry, and steers using WAS. In their report, satisfactory results were obtained with tests on poultry, as no adverse effects on the growth and health of chicks and on layers were noted. Similarly, Nkhalambayausi-Chirwa and Lebitso [61] assessed the nutritional value of single-cell protein from WAS as a protein supplement in poultry feed and noted that chicks fed with WAS gained more weight than chicks fed with conventional feed. The current study, therefore, sought to determine the potential of WAS protein as a nutritional supplement in chicken feed. However, to date, there is no existing literature about the protein content of WAS-derived protein, and therefore, the extracted protein should undergo further testing to determine if there are any antinutritional factors as well as to determine its digestibility.
4. Statistical Optimization of Protein Extraction Using RSM
The ANOVA for protein yield and extraction efficiency are presented in Tables 5 and 6, respectively. The significance of the model was checked by examining the p-factor and the F-factor values. The model was significant as suggested by a p value of 0.0001 for both protein yield and efficiency and F-values of 46.94 and 45.67 for protein yield and extraction efficiency, respectively. Also, the model terms A, B, C, and A2 and the interactive effect of BC are significant in the model equation.
| Source | Sum of squares | df | Mean square | F-value | p value | |
|---|---|---|---|---|---|---|
| Model | 1192.11 | 9 | 132.46 | 46.97 | < 0.0001 | Significant |
| A-temperature | 68.63 | 1 | 68.63 | 24.34 | < 0.0001 | |
| B-pH | 893.38 | 1 | 893.38 | 316.79 | < 0.0001 | |
| C-time | 119.50 | 1 | 119.50 | 42.37 | < 0.0001 | |
| AB | 7.93 | 1 | 7.93 | 2.81 | 0.0981 | |
| AC | 2.18 | 1 | 2.18 | 0.7733 | 0.3822 | |
| BC | 23.93 | 1 | 23.93 | 8.49 | 0.0048 | |
| A2 | 58.89 | 1 | 58.89 | 20.88 | < 0.0001 | |
| B2 | 10.58 | 1 | 10.58 | 3.75 | 0.0568 | |
| C2 | 7.10 | 1 | 7.10 | 2.52 | 0.1171 | |
| Residual | 197.41 | 70 | 2.82 | |||
| Cor total | 1389.52 | 79 |
| Source | Sum of squares | df | Mean square | F-value | p value | |
|---|---|---|---|---|---|---|
| Model | 29,600.75 | 9 | 3288.97 | 45.67 | < 0.0001 | Significant |
| A-temperature | 1612.71 | 1 | 1612.71 | 22.39 | < 0.0001 | |
| B-pH | 22,392.43 | 1 | 22,392.43 | 310.95 | < 0.0001 | |
| C-time | 2869.85 | 1 | 2869.85 | 39.85 | < 0.0001 | |
| AB | 220.15 | 1 | 220.15 | 3.06 | 0.0848 | |
| AC | 86.31 | 1 | 86.31 | 1.20 | 0.2774 | |
| BC | 632.99 | 1 | 632.99 | 8.79 | 0.0041 | |
| A2 | 1398.95 | 1 | 1398.95 | 19.43 | < 0.0001 | |
| B2 | 234.89 | 1 | 234.89 | 3.26 | 0.0752 | |
| C2 | 152.46 | 1 | 152.46 | 2.12 | 0.1501 | |
| Residual | 5040.93 | 70 | 72.01 | |||
| Cor total | 34,641.68 | 79 |
In addition to the p value, other statistical parameters, including the coefficient of determination (R2), the coefficient of variation (CV%), predicted R2 (), and adjusted R2 (), shown in Table 7, were used to analyze the effectiveness of the generated model. R2 and values close to one and smaller standard deviation values indicate that the empirical model fits the experimental data [62]. The and the calculated in this study are consistent since their difference is less than 0.2, indicating a better-predicting response of the quadratic model in both responses. Adeq precision evaluates the signal-to-noise ratio, and a value greater than 4 is desirable [63]. Thus, 25.76 in this study represents an adequate signal to navigate the design space.
| Std. dev. | 1.68 |
| Mean | 7.02 |
| CV % | 23.92 |
| R2 | 0.8579 |
| Adjusted R2 | 0.8397 |
| Predicted R2 | 0.8227 |
| Adeq precision | 25.7594 |
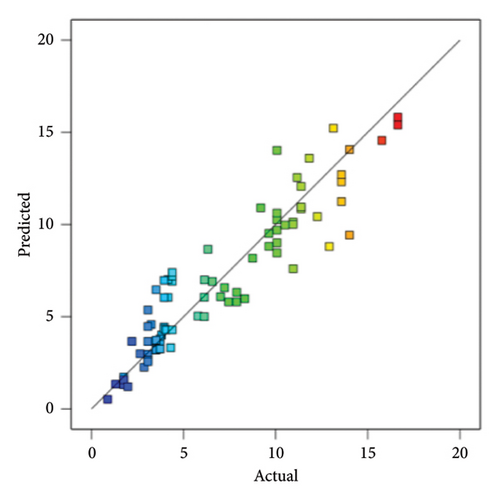
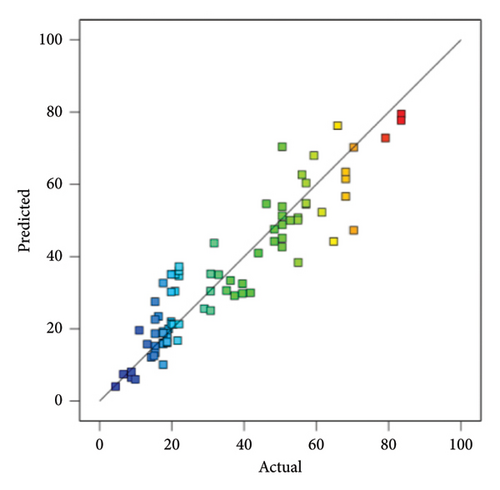
In addition, at a 95% confidence level, there exists a correlation between the predicted models and observed response values, as shown in Figure 7. This suggests that the quadratic model can accurately predict both responses.
4.1. Response Surface Plots
3D surface plots were used to investigate the combined effect of independent variables on the responses. Figure 8 shows the effect of increasing temperature from 90°C to 130°C and pH from 10 to 13 on protein yield. At a temperature of 120°C and pH < 12, the contour lines were sparse, which implied that the protein yield and extraction efficiency were relatively low. However, at pH above 12 and temperatures greater than 120°C, the contour lines become dense, and the shading on the response curves becomes intense. This shows that an increase in both temperature and pH leads to an increase in protein yield up to around 120°C and pH 13, where the maximum protein yield and extraction efficiency are recorded. Beyond this temperature, the conditions become unfavorable in the extraction of protein from WAS. This is depicted by a decline in yielded protein at 130°C. Also, the curves close to pH were denser, suggesting that pH had more influence than temperature during thermal alkaline hydrolysis.
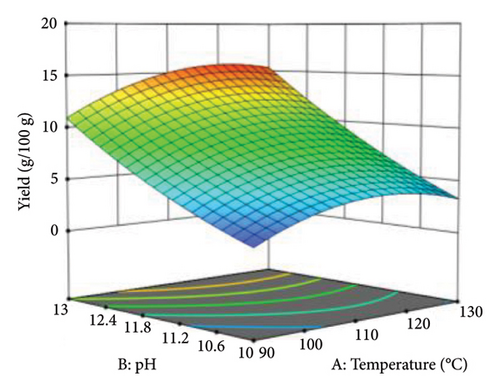
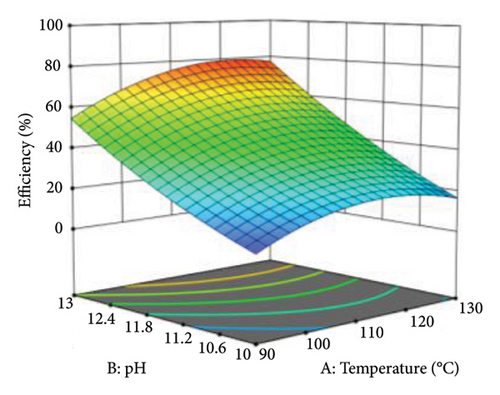
4.2. Optimization of Thermal Alkaline Hydrolysis
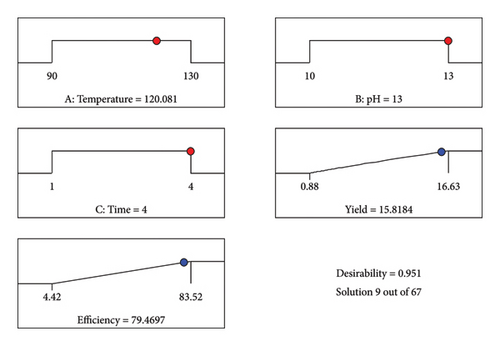
The desirability of the model was observed at optimum conditions of pH at 13 and a temperature of 120°C, as shown in Figure 9. At the optimized conditions, 15.8 g/100 g and 79.5% protein yield and extraction efficiency were obtained by the model. To validate the reliability of the model, experimental runs were conducted at the identified optimal conditions, giving a protein yield and extraction efficiency of 16.6 g/100 g and 83.4%, respectively. This is consistent with the predicted values, confirming the response surface models’ precision and accuracy.
5. Conclusions
The protein extracted from FW and WAS was characterized, and the process of extraction was successfully optimized using RSM. The pretreatment method used in the removal of heavy metals prior to extraction was highly effective and led to the reduction of heavy metals to levels acceptable for animal feeds with Cd, Mn, and Pb below the detection limit. It was found that FW had a higher protein concentration than WAS. However, due to the difference in the composition of WAS and FW, the chemical methods used in extraction had higher efficiency in accessing WAS protein than the FW protein. Furthermore, there was no significant increase in protein yield in the co-extraction of protein from the FW and WAS mixture, as a maximum of 14 g/100 g protein was recovered from the mixture compared to 16 g/100 g extracted from the WAS substrate. The optimum conditions from the RSM for the extraction of protein from wastes were identified as a pH of 13 and a temperature of 120°C. Finally, amino acid analysis demonstrated that WAS had nearly all the EAAs, with threonine being the most abundant. The abundance of threonine and the presence of the first limiting amino acids (lysine and methionine) in an appropriate ratio demonstrate the suitability of WAS protein in feed supplementation pending digestibility tests and analysis of protein content to determine possible antinutritional factors.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Open Research
Data Availability Statement
The data used to support the findings of this study are available upon request from the corresponding author.




