Design, Synthesis, Biological Evaluation, DFT Calculations, and Molecular Docking Investigations of 99mTc-Rosuvastatin as a Promising Radiopharmaceutical for Liver Targeting
Abstract
Rosuvastatin is a lipid-lowering medication belonging to the statin group that acts as a selective inhibitor of 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase, a rate-limiting step of cholesterol biosynthesis in the liver. 99mTc-rosuvastatin was synthesized aiming to develop a potential radiopharmaceutical for targeting liver. The in vitro stability and radiochemical yield were investigated, and the yield of the 99mTc-rosuvastatin complex was 89.5 ± 1.85%. Biodistribution study revealed a good preferential localization of 99mTc-rosuvastatin in liver tissues suggesting using the labeled compound as a promising liver imaging candidate. The favorable sites of Tc labeling were determined by the analysis of natural atomic charges using the DFT method at the B3LYP/LanL2DZ level of theory. Binding modes between the rosuvastatin (and its stable technetium complex) and the human HMG-CoA reductase were determined using molecular docking, and the obtained results revealed the effectiveness of technetium in increasing the binding affinity to the active residues of HMG-CoA reductase.
1. Introduction
Rosuvastatin belongs to a well-known group of drugs called “statins” and is chemically described as a 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitor [1–3]. It showed more effective affinity for HMG-CoA enzyme active site than other statins [4–6]. It works specifically in the liver to prevent the production of cholesterol and HMG-CoA reductase [7–9]. Rosuvastatin works in the liver as a selective HMG-CoA reductase inhibitor and cholesterol biosynthesis [10, 11]. Lipophilicity/hydrophilicity is possibly the most important physicochemical property of a potential drug [12–15]. Rosuvastatin is a relatively hydrophilic statin (logD = −033) at pH 7.4 [16–19] due to the presence of polar methyl sulfonamide moiety [20] and not significantly metabolized by cytochrome P450 (CYP) enzyme [21]. The greater hydrophilicity of rosuvastatin compared to other statins makes it more hepatoselective, and the high rate of uptake is observed only in the major parenchymal cells of the liver called hepatocytes either by passive diffusion and/or active transport at low concentrations compared with nonhepatic cells [22]. Selective hepatic uptake and distribution of rosuvastatin via organic anion transporters contributed to its high pharmacological activity [23, 24]. Radiolabeling of aromatic compounds is a common technique used to produce potential bioactive molecules and radiopharmaceuticals [25–29]. Technetium-99m radiopharmaceuticals are used in morphological and dynamic imaging of many body organs, for the diagnosis of various diseases. Many different technetium imaging agents have been developed and introduced into clinical practice for the diagnosis of human body organs related to the liver, kidney, heart, brain, thyroid, lung, and tumor diseases [30–33].
The pharmacokinetics of rosuvastatin, labeled with carbon-14 (14C), have been studied in both animal and human models. In mice, the hepatic uptake rate of 14C-rosuvastatin was determined to be 0.885 mL/min/g tissue, indicating a comparatively high selective uptake within the liver relative to other statin compounds. Furthermore, the drug exhibited a diffusion rate of 3.3 × 105 particles/mm2 into hepatic tissue, suggesting a pronounced propensity for hepatic distribution [34]. In human studies involving healthy adult male volunteers, approximately 50% of the administered 14C-rosuvastatin dose was absorbed. Analysis of radiolabeled material recovered within 72 h postadministration revealed that 70% of the dose was accounted for. The primary route of elimination was hepatobiliary excretion, with 90% of the recovered dose found in feces. Renal excretion accounted for the remaining 10%, as evidenced by urinary recovery. These findings suggest that metabolism plays a minor role in the clearance of rosuvastatin, with direct excretion via the hepatobiliary system being the dominant elimination pathway [35–37].
This research focused on the development and preclinical characterization of a technetium-99m (99mTc)-labeled rosuvastatin as a potential hepatotropic radiopharmaceutical. The objectives included the synthesis of 99mTc-rosuvastatin to establish and optimize a robust radiolabeling protocol with comprehensive characterization of the resulting radiocomplex. Characterization studies included radiochemical purity, in vitro stability, and pharmacokinetic and biodistribution to evaluate the in vivo biological behavior of 99mTc-rosuvastatin. The comprehensive goal is to determine the suitability of 99mTc-rosuvastatin as a diagnostic imaging agent for hepatic pathologies based on its selective accumulation within liver tissue.
2. Materials and Methods
2.1. Chemicals and Instruments
Rosuvastatin (C22H28FN3O6S) (M.wt. = 481.5 g/mol) was a generous contribution from EIPICO, Egypt. Chromatography papers (Whatman No. 1) were obtained from Whatman International Ltd, UK. Technetium-99m was supplied from a 99Mo/99mTc generator (Gentech, Turkey) and eluted as 99mTcO4−. We obtained Sodium borohydride (NaBH4; 37.83 g/mol) from Sigma-Aldrich. In all experiments, deionized (DI) water was utilized to prepare solutions. All reagents and solvents were of high purity and consumed without further purification. The radioactivity was measured in a well-type NaI (Tl) scintillation detector coupled to a digital scaler ratemeter SR7 model, UK.
2.2. Synthesis of 99mTc-Rosuvastatin
The effect of different reaction parameters on the radiolabeling efficiency of rosuvastatin, such as the amount of rosuvastatin (100–2000 μg), the amount of reducing agent (NaBH4; 10–150 mg), the reaction pH (2–11), and reaction time (5–60 min), was examined to optimize the reaction conditions to increase the radiolabeling yield. An amount of 60 mg NaBH4 (15.8 mM) and 500 μg rosuvastatin dissolved in deoxygenated water (10 mM) was carried to a reaction vial and mixed with 100 μL of a recently eluted 99mTcO4− (400 MBq). The reaction was stirred regularly for 30 min at room temperature. The pH of the reaction medium was adjusted to 9 with phosphate buffer.
2.3. Radiochemical and Analytical Methods of Analysis
The labeling efficiency of 99mTc-rosuvastatin compound was carried out by ascending paper chromatography to estimate the percentage of 99mTc-rosuvastatin, free pertechnetate, and reduced hydrolyzed 99mTc colloidal species. Samples are positioned 2 cm over the bottom margin of the chromatography strip (13 cm length, 1 cm width). The paper strip was developed with a mixture of ammonia:water:ethanol (1:5:2), while the other strip was developed with acetone. After complete development, the two strips were dried, cut into 1-cm pieces, and then individually counted using a NaI (Tl) scintillation detector coupled to gamma-ray counter to determine the ratio of free 99mTcO4, 99mTc-rosuvastatin complex, and the hydrolyzed 99mTc.
The radiochemical yield was illustrated as the ratio of 99mTc-rosuvastatin radioactivity to the entire radioactivity multiplied by 100. The yield was the average of three experiments. The complex was analyzed chromatographically using a sodium iodide radiometric detector connected to an Agilent HPLC system. An aliquot of 10 μL 99mTc-rosuvastatin was injected under optimal conditions into a reversed phase C18 Waters column (4.6 × 150 mm, 5 μm) kept at 25°C with a mobile phase containing 0.1% triethylamine in water modified to pH 4.5 using acetonitrile/methanol and glacial acetic acid (55:45; v/v). The mobile phase was filtered, degassed, and then pumped at a flow rate of 1 mL/min. Rosuvastatin was determined by UV absorbance at 242 nm.
2.4. In Vitro Stability of 99mTc-Rosuvastatin
The complex’s in vitro stability was performed by incubating the reaction mixture with 1 mL of normal serum at 37°C for 24 h. Aliquots of exactly 2–3 μL were extracted and tested at different time periods using paper chromatography to find out the percentage of 99mTc-rosuvastatin and 99mTcO4−. Therefore, the stability of the radiolabeled compound will define its convenience for in vivo application.
2.5. Lipophilicity/Hydrophilicity
The lipophilicity/hydrophilicity (partition coefficient logD) of 99mTc-rosuvastatin was measured experimentally at pH 7.4. Simply add an aliquot of 1 mL of the complex along with 5 mL of octanol and water to a centrifuge tube. After shaking the mixture for 5 min, it was centrifuged at 4500 rpm for 10 min. After separating the two phases, 500 μL aliquots of each phase were withdrawn and counted in a gamma counter. Extraction was carried out in triplicate and the partition coefficient (logD) was estimated as the ratio of the radioactive complex in both octanol and water phase at equilibrium.
2.6. Animal Studies
Biodistribution studies were performed in accordance with the Egyptian Atomic Energy Authority guidelines and were licensed by the Animal Care Committee of the Labeled Compounds Department. Albino mice (26–35 g) were housed in groups of five (n = 5). Mice were administered with 10 μL of the pure labeled compound intravenously via the tail vein (0.3 MBq/g). Mice under anesthesia were sacrificed at 0.25, 0.5, 1, 2, and 4 h after administration of 99mTc-rosuvastatin. After sacrifice, each mouse was weighed and dissected, and blood was extracted from the heart. Saline was used to wash organs and tissues, which were then collected and weighed in plastic containers. Each sample’s radioactivity and background were calculated using a NaI (Tl) detector-based well counter. In a group of five mice per time point, biodistribution data were reported as a percentage of the injected dose per organ (%ID/organ ± SD). Predosing the mice with nonradioactive rosuvastatin 0.5 h before the injection of 99mTc-rosuvastatin was expressed as average percentage of hepatic uptake at 0.5 h postinjection (n = 5). The ANOVA test was applied to determine statistical significance in tissue uptake, and all data are presented as mean ± SD [38].
2.7. DFT Calculations
Calculations of technetium complexes with rosuvastatin have been performed using density functional calculation (DFT) method at the B3LYP/LANL2DZ level of theory for geometry prediction and frequency as implemented in the Gaussian 16 software [39]. Optimized geometries were sitting at true minima. Favorable coordinates sites of rosuvastatin with technetium were determined by the analysis of natural atomic charge (NPA) of the former obtained at the same level of theory.
2.8. Molecular Docking
To determine how the rosuvastatin and its technetium complexes bind to the human HMG-CoA reductase, AutoDock package was used to study the molecular docking [40]. The initial geometries of HMG-CoA reductase and the originally docked ligand, rosuvastatin (PDB code: 1HWL), were retrieved from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank website [41]. Favorable active sites were recognized based on co-crystallized HMG-CoA reductase-rosuvastatin complex structure of target protein. The re-docking of the original rosuvastatin ligands into the active sites was relatively reproduced with root mean square deviation (RMSD) values of 0.492, 1.252, 1.199, and 0.982 Å. The technetium rosuvastatin complexes were docked into the active site of the lowest RMSD value. The molecular docking step was described in preceding work [42]. Docking calculations were performed using a workstation computer equipped with an Intel Xeon Gold 6130 CPU Processor 16 Core 2.10 GHz.
3. Results and Discussion
3.1. Coupling of Rosuvastatin and Technetium-99m
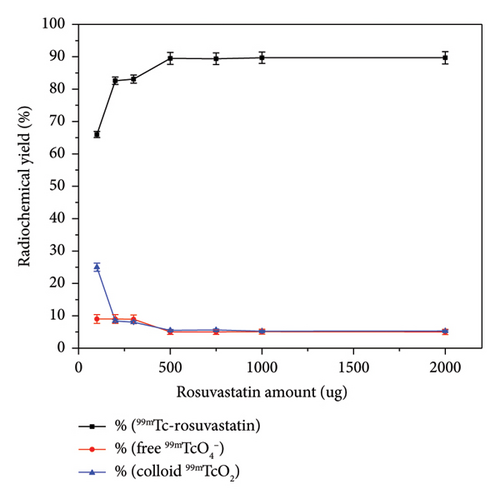
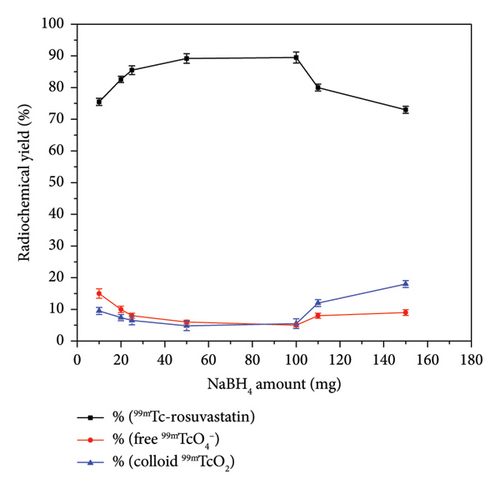
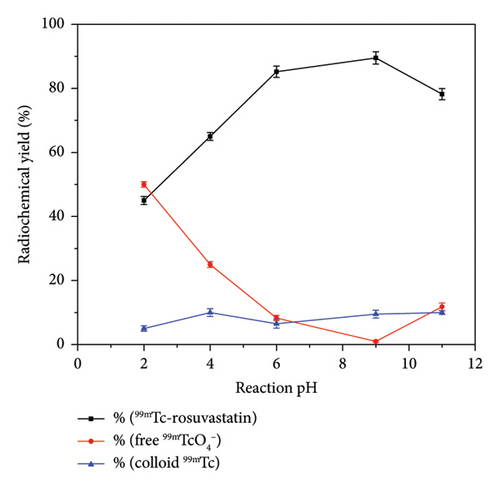
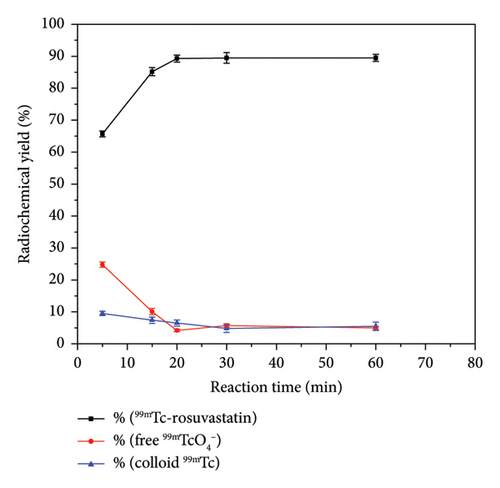
Coupling of rosuvastatin and technetium-99m (t1/2 = 6 h) was performed via direct radiolabeling process. The radiochemical synthesis of 99mTc-rosuvastatin was optimized by studying different reaction parameters (Figure 1) such as the effect of amount, reducing agent, pH, and time. The optimum amount (Figure 1(a)) of ligand required to obtain maximum radiochemical yield (89.5%) was exactly 500 μg. Below this value, the rosuvastatin amount was insufficient to react with all the reduced 99mTc. At higher amount, the labeling yield was stable up to 1000 μg. The radiochemical yield was dependent on the amount of reducing agent (NaBH4) present in the reaction mixture. Sodium borohydride (NaBH4) was selected as a good reducing agent to prevent the interference of colloidal tin (IV) oxide (SnO2) in the tin (II) chloride (SnCl2) procedure. According to Figure 1(b), an amount of 100 μg was sufficient to reduce the present technetium-99m to the desirable pertechnetate (99mTcO4−). At lower amounts (10–25 μg), the yield decreased due to the large percentage of free 99mTcO4− while at higher amounts (110–150 μg) of reducing agent, the yield decreased dramatically to 73%. This decline may be due to the formation of undesirable colloids, which can compete with rosuvastatin for reduced technetium-99m. The effect of pH (Figure (1c)) on the radiochemical yield was studied to determine the optimum pH for maximum radiolabeling yield. At pH 9, the highest radiochemical yield was attained due to the strong reducing power of NaBH4 in alkaline medium [43, 44] reducing all pertechnetates to lower oxidation state technetium favoring the formation of 99mTc-rosuvastatin. The labeling percentage decreased to 78% at low pH (2–4) due to the destructive ability of the reducing agent. At pH above 9, the yield decreased to 78.2% due to the formation of the reduced hydrolyzed technetium-99m as the main impurity in the process, which did not bind to the rosuvastatin molecule. The labeling yield increased when increasing the reaction time from 5 to 30 min (Figure 1(d)), which was enough to complete the reaction.
In paper chromatography developed with acetone as the solvent, free 99mTcO4− (Rf = 1) migrated with the solvent front, whereas the radiolabeled rosuvastatin and reduced hydrolyzed technetium stayed at the base. The second paper strip was developed using a combination of ammonia:water:ethanol (1:5:2), where 99mTc-rosuvastatin and free 99mTcO4− species moved with different solvent fronts while hydrolyzed reduced technetium-99m colloid (TcO2) remained at the origin (Rf = 0). Radiochemical purity was estimated by subtracting the total of free pertechnetate and colloid percentages from 100%. Furthermore, it was confirmed through chromatographic analysis using HPLC, where the retention times of free technetium and 99mTc-rosuvastatin were 1.25 and 4.5 min, respectively, as indicated in the chromatogram (Figure 2).
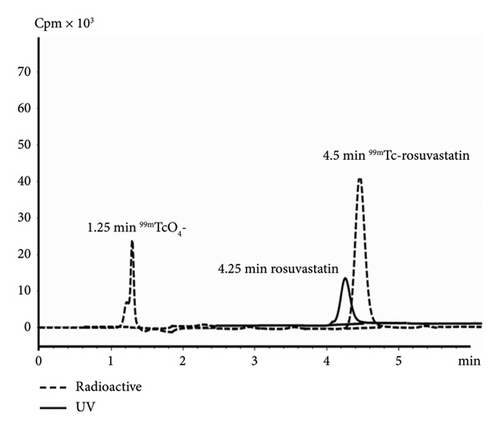
The appropriate injection time was determined based on the in vitro stability of 99mTc-rosuvastatin to prevent the formation of unwanted products due to radiolysis of the radiolabeled compound. In vitro stability results revealed that 99mTc-rosuvastatin remained stable in serum for up to 24 h.
The hydrophilicity/lipophilicity determines the binding affinity of the labeled compound and affects its hepatocyte uptake and excretion rate. The lipophilicity was measured by determining the separation of 99mTc-rosuvastatin between n-octanol and water solution in the physiologically relevant pH range (7.4). The experimental logD of the labeled compound (−0.34 ± 0.02) was found to be greater than the logD value of rosuvastatin (−0.33), which may be owing to the coupling of technetium to the structure. The negative value of logD of the labeled complex would lead to determine that 99mTc-rosuvastatin would be of lower lipophilicity and more likely to be water soluble in the body. The characteristic methane sulfonamide group in the complex enhanced hydrophilicity and led to an improved ionic binding interaction with the HMG-CoA enzyme. Therefore, the retention time of rosuvastatin was observed before 99mTc-rosuvastatin on a reversed-phase high-performance liquid chromatogram (RP-HPLC), as shown in Figure 2. The relative hydrophilic character of 99mTc-rosuvastatin is vital in predicting the uptake and excretion pathway implying attenuated access to nonhepatic cells because of low passive diffusion and considerable hepatic cell uptake via selective organic anion transport.
3.2. Biodistribution Studies
The biodistribution and retention of 99mTc-rosuvastatin were studied by tracking the compound in various organs of mice following intravenous injection. No adverse effects such as pain, redness, edema, or death were observed in the injected mice. Figure 3 shows a high and steady activity in blood after IV injection throughout the study period, with slow clearance due to the ninety percent binding of rosuvastatin to blood proteins [17, 20]. 99mTc-rosuvastatin was efficiently eliminated from the bloodstream, leaving only 1.53% ± 0.15% in the blood after 4 h postinjection compared to 15.42% ± 0.05% at 0.25 h postinjection. The liver-targeting ability of 99mTc-rosuvastatin revealed a high uptake in the liver of 50.0 ± 0.45% at 0.25 h postinjection, then increased to reach 53.15% ± 0.56% after 0.5 h, and then decreased to 45.34% ± 1.12% after 2 h. These results revealed the stability of 99mTc-rosuvastatin for several hours and demonstrated the ability to bind to the active site of HMG-CoA in vivo with high affinity and specificity as a liver imaging agent, suggesting the use of the labeled compound as a promising candidate for liver imaging. The relatively high hydrophilicity of rosuvastatin compared to other statins may explain the minor nonspecific distribution to extrahepatic tissues and may be consistent with low uptake in other organs. The effectiveness of hepatocyte uptake and the clearance rate may be dependent on the physiochemical and structural relationship between the lipophilic and hydrophilic functional groups on 99mTc-rosuvastatin. The absorption in the intestine was 11.42 ± 0.32% at 0.25 h and increased to 12.61 ± 0.49% at 0.5 h, which assumed that the clearance pathway proceeded through the hepatobiliary excretory route via the intestine. The labeled compound displayed accumulation in the kidneys (3.46% ± 0.34% at 0.5 h and 7.56% ± 0.01% at 1 h) supporting a second elimination route through the urinary renal excretory system. It is clear from the clearance model in mice that 99mTc-rosuvastatin was effectively cleared via the hepatobiliary excretory pathway (liver and intestine) and through the renal urinary system to a lesser extent. The radioactivity retained in the stomach was 0.45% ± 0.25% at 0.5 h as compared to 0.12% ± 0.02% at 4 h confirming suitable in vivo stability and negligible dissociation due to metabolism. Predosing mice with nonradioactive rosuvastatin 0.5 h before administration of 99mTc-rosuvastatin inhibited the liver uptake to 15.43 ± 0.34% ID/organ. A similar hepatocellular-targeted radiolabeled 99mTc-atorvastatin has been prepared with radiolabeling efficiency of 90.3% to target and selectively image the liver or hepatocellular carcinoma. The maximum liver uptake was 37.6% ID/g within 15 min [45], indicating a higher hepatic uptake efficiency of 99mTc-rosuvastatin compared to a compound from the same class of statins. This suggests the use of 99mTc-rosuvastatin compound as a promising selective candidate for liver imaging.

3.3. DFT Results
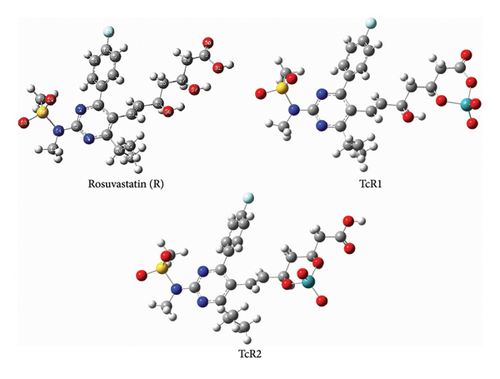
Fukui indices were calculated to elucidate the structure, which was confirmed by DFT calculation applying the hybrid functional B3LYP together with LanL2DZ basis set in gas and in a polarizable continuum model (PCM), in which IEF-PCM formalisms were evaluated. Figure 4 represents the optimized geometries of TcR1 and TcR2 complexes. NPA analysis revealed that technetium may coordinate to two main sets of rosuvastatin atomic sites. In the TcR1 complex, Tc is coordinated to O57 and O31 atomic sites (NPAs of −0.838 and −0.783) of rosuvastatin, while in the TcR2 complex, it is coordinated to O56 and O57 atomic sites (NPAs of −0.826 and −0.838). The calculated free Gibbs energy change of the two complexes revealed that TcR2 is thermodynamically more stable than TcR1 with a relative Gibbs energy of 8 kcalmol−1.
3.4. Molecular Docking
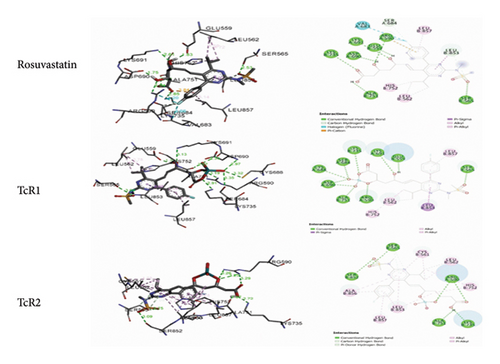
The intermolecular interactions between rosuvastatin and its technetium complexes were determined by analyzing the docking outputs into HMG-CoA reductase active site. Table 1 outlines the predicted binding free energies (kcal/mol) of the stable ligand-receptor (LR) complexes and number of conventional intermolecular hydrogen bonds (HBs) formed between rosuvastatin and its technetium complexes on one side and HMG-CoA reductase active site residues on the other side. The docking results showed that the complexes formed between rosuvastatin and its technetium complexes and the active residues exhibited negative binding energies, revealing that the inhibition of the target receptor was both thermodynamically and spontaneously favorable (Table 1). Docking analysis revealed that the coordination position of technetium with rosuvastatin may have a strong effect on its binding to the active residues of HMG-CoA reductase (Figure 5). It stabilized the TcR2-HMG-CoA reductase complex by 0.41, while destabilizing TcR1-HMG-CoA reductase by 0.81 kcalmol−1.
| Free binding energy (kcal/mol) | Conventional H-bonds (HBs) | Number of closest residues to the docked compounds | |
|---|---|---|---|
| Rosuvastatin | −9.42 | 7 | 13 |
| TcR1 | −8.61 | 10 | 13 |
| TcR2 | −9.83 | 6 | 11 |
4. Conclusions
99mTc-rosuvastatin was successfully prepared using direct labeling technique with a radiochemical yield of 89.5 ± 1.8%. Molecular docking predicted the binding affinity of 99mTc-rosuvastatin to the human HMG-CoA enzyme binding sites in the liver. Biodistribution results showed that 99mTc-rosuvastatin has a high targeting ability toward hepatocytes compared to nonhepatic tissues indicating that the new couple is a promising radiopharmaceutical for liver imaging.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was supported by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, project number 2022/01/20095.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through the project number 2022/01/20095.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




