Physicochemical Qualities and Health Risk Assessment of Heavy Metals in Drinking Water From Selected Areas of the Gofa Zone, Ethiopia
Abstract
Spring and well waters are among the most important sources of drinking water in Gofa Zone rural areas; therefore, it is vital to evaluate the quality of these water sources. Hence, this study aims to determine the physicochemical parameters and metallic mineral concentration with its associated health risks of drinking water in selected rural areas of the Gofa zone, Ethiopia. The purposive sampling method was used for water sample collection. Standard analytical procedures were used to determine the physicochemical parameters and mineral contents. The average values of all physicochemical parameters of spring and well-drinking water of the selected areas were obtained as temperature (20.73°C and 20.30°C), pH (7.2 and 6.07), electrical conductivity (481.13 µS/cm and 584.24 µS/cm), dissolved oxygen (4.09 and 6.07 mg/L), turbidity (1.60 NTU and 6.55 NTU), total dissolved solids (330.83 and 338.3 mg/L), total suspended solids (84.76 and 89.24 mg/L), chloride concentrations (58.15 and 60.47 mg/L), SO42− ion (68.50 mg/L and 5.58 mg/L), NO3+ ion (3.25 and 2.75 mg/L), and fluoride ion concentration (0.49 mg/L and 0.37 mg/L). Health risk assessment indicates that Co metal showed high hazard indices compared with Fe, Cu, and Zn. Additionally, the values of the heavy metal pollution index (MPI) and heavy metal evaluation index (HEI) indicate that the Sawla Kusti spring water (SSW5), Geze Gofa Mhirzho Wola spring water (GGSW7), and Geze Gofa Bulki Guya well water (BGWW8) sources are considered highly polluted and not recommended for drinking purposes.
1. Introduction
Water is an essential substance that supports all forms of life in plants and animals [1]. Access to clean drinking water is a fundamental right of human beings. According to the report of UN-water [2], 2.3 billion people live in water-stressed countries, of which 733 million live in extremely high water-stressed countries. Another report indicated that 3.2 billion people live in agricultural areas with high to very high water scarcity, of which 1.2 billion people, roughly one-sixth of the global population, live in severely water-constrained agricultural areas [3]. Mekonnen and Hoekstra [4] also indicated that four billion people face severe water scarcity. More than 700 million people do not have access to clean and safe water, and around 2.5 billion people do not have proper sanitation. As a result, around 6-8 million people die each year due to water-related diseases and disasters [5, 6]. Therefore, water quality control is a top-priority policy agenda in many parts of the world [7].
Water quality and fitness for use are determined by its taste, odor, color, and concentration of organic and inorganic matter [8]. Contaminants in the water can affect the water quality and, consequently, human health. The potential sources of water contamination are natural sources, industrial and agricultural activities, and water treatment plants. These contaminants are further categorized as microorganisms, inorganics, organics, radionuclides, and disinfectants [9–12]. Inorganics hold a greater portion of drinking water contaminants than organics [13]. According to Marcoveccio [14], minerals and metals are the major pollutant sources of water points. Many man-made activities such as farming, mining, overgrazing, municipal wastes, and disposal of treated and untreated waste effluents containing minerals and chelates from cities and towns affect the quality of water sources [10].
Minerals, whether by nature or human activities have various sources of chemical compositions such as bicarbonates, chlorides, and Ca2+ and Mg2+ ions, which are formed from the dissolution of dolomite and pure calcite, respectively, are among the major analytes found in spring and well water. Sulfate (SO42−) determination of well and spring water quality based on health and safety regulation specifications before domestic use is highly imperative. Mineral contaminations in both well and spring water are major determinants of water quality [15]. Heavy metals tend to accumulate in human organs and the nervous system and interfere with their normal functions. In recent years, heavy metals such as lead (Pb), arsenic (As), magnesium (Mg), nickel (Ni), copper (Cu), and zinc (Zn) have received significant attention because they cause health problems [7, 16, 17]. Moreover, cardiovascular diseases, kidney-related problems, neurocognitive diseases, and cancer are related to the traces of metals such as cadmium (Cd) and chromium (Cr) as reported in epidemiological studies [10, 18, 19].
Approximately 75% of the world’s population gets a drinking water supply from unprotected ponds, wells, springs, rivers, rains, and other water sources. However, these water sources are exposed to different toxic heavy metal pollutants which are poison to human beings. In Ethiopia, the major source of water supply for both urban and rural communities is underground water, accounting for 70% of the total water supply [20]. Southern nation nationalities and peoples region (SNNPR) is among the scarcest in terms of availability of drinking water, with far less access than the national coverage. Various studies indicated that the majority of the communities use unprotected sources and rivers to obtain water for domestic use [15, 20–22].
Gofa zone and its woreda district communities obtain a water supply for drinking and domestic consumption from surface water and well water. Naturally, woredas in the Gofa zone are hot spotted and water-scarce areas; as a result, people suffer to get safe and pure water which may cause toxicity to all animals and plants and are exposed to water-borne diseases. Hence, the present study aimed (i) to determine detailed physicochemical parameters such as pH, temperature, turbidity, electrical conductivity (EC), total suspended solids (TSS), total dissolved solids (TDS), dissolved oxygen (DO), fluoride, chloride, sulfate, and nitrate) and minerals (Na, K, Mg, Ca, Fe, Cr, Co, Cu, and Zn), (ii) to evaluate the drinking water quality, and (iii) to assess associated human health risks by taking water samples from spring and well-drinking water spots in the districts of four selected woreda administrations of the Gofa zone.
2. Materials and Methods
2.1. Study Area Description
This study was conducted in selected areas of the Gofa zone, located in South Ethiopia. The area shares a boundary with the Gamo Zone on the south, the Debub Omo Zone on the southwest, the Besketo special Woreda on the west, and the Dawro Zone on the north (Figure 1). The administrative center of the zone Sawla is 275 km from Hawassa, the former capital of SNNPR. The plan commission office of the Zone reported that the Gofa Zone is situated at a latitude and longitude of 6°18′N 36°53′E and an elevation of 1395 and 4577 ft above sea level [23].
2.2. Sample Collection and Selection of Sampling Points
The criteria for selecting sampling points were based on population density, drinking water scarcity, and sanitation. The purposive sampling technique was used to collect water samples from four springs and four shallow wells, respectively (Table 1). A total of 24 water samples were collected from eight spots. The water samples were collected in the morning hours between 8:00 to 12:00 a.m. in October and November 2022.
| Numbers | Name of sample locations | Water source | Specific features |
|---|---|---|---|
| 1 | Zala Galma (ZG) | Gelta spring water (SW1) | Surface water, not protected |
| Galma well water (WW2) | Groundwater, not protected | ||
| 2 | Demba Gofa (DG) | Borda spring water (SW3) | Surface water, not protected |
| Suka well water (WW4) | Groundwater, not protected | ||
| 3 | Sawla Town (ST) | Kusti spring water (SSW5) | Surface water, not protected |
| Yala well water (WW6) | Groundwater, not protected | ||
| 4 | Geze Gofa (GG) | Mirzho Wola spring water (SW7) | Surface water, not protected |
| Bulki Guya well water (BGWW8) | Groundwater, not protected | ||
2.3. Chemicals and Reagents
The chemicals and reagents used were all analytical grades. 30% of hydrogen peroxide (H2O2), deionized water, tap water, 65% of sulfuric acid (H2SO4), 65% of hydrochloric acid (HCl), 37% of nitric acid (HNO3), and stock solutions of metals Na, K, Ca, Mg, Cr, Fe, Co, Cu, and Zn were used [15].
2.4. Sample Size and Sample Preparation
Spring and well-drinking water samples were collected from different rural kebele districts of Geze Gofa, Demba Gofa, Zala woreda, and Sawula town administration, respectively. Before the collection of water samples, polyethylene bottles were thoroughly washed and rinsed with 20% HNO3 and distilled water, respectively, to avoid contamination. Then the water samples were transported to Wolaita Sodo University and Addis Ababa University Analytical Chemistry Research laboratories and stored in a refrigerator below 4°C until analysis was carried out.
2.5. Analysis of Physicochemical Parameters
On-site analyses of pH, DO, temperature, conductivity, and turbidity were carried out at the site of sample collection following the standard protocols and methods of the American Public Health Organization [24, 25]. The pH of the water samples was determined with a pH meter (HANNA Instruments, HI2020 multiparameter pH/DO/EC/TDS). The pH meter was calibrated in three standard pH buffer solutions (pH 4, pH 7, and pH 10) before analytical measurements [26]. The stable reading value of each sample was taken after submerging the pH electrode in the water samples. DO was measured by using a DO sensor. The sensor was calibrated with standard solutions and then immersed directly into water samples, and the result was recorded [27]. Turbidity was determined by the nephelometric turbidimeter method (Wag-WT3020, Halma PLC). The principle of this method was based on a comparison of the high scattered intensity by a standard reference suspension under the same conditions [21]. Temperature measurement was taken by the immersion of the temperature sensor in water samples within a beaker, and the temperature of the samples was recorded [20]. EC of water samples was determined with the help of an EC sensor, and readings are taken in μS/cm. The instrument was standardized with 12.8, 84, and 144 µS/cm standard conductivity solutions before reading samples [15].
The laboratory analysis of the TSS was carried out by filtering some amount of water sample with a preweighed Whatman filter paper. Then, the filtered paper was weighed, which was used as filter preweight, then after it was dried at 80°C temperature in the oven for 30 min and weighed again, which was used as filter postweight. Eventually, TSS was determined by the gravimetric method, as indicated by [21]. TDS was analyzed by using a portable conductivity sensor in the same manner as EC [27]. Determination of fluoride content was obtained by using a potentiometric ion-selective electrode (ISE) (Crison GLP22 model, Crison Instruments, Spain). The ISE was calibrated by two standard fluoride solutions of 1 mg/L and 10 mg/L before sample measurement. A 1000 ppm stock solution of fluoride was prepared by adding a gram of NaF in distilled water, and a series of standard solutions were prepared from the stock solution [21].
The argentometric method was used to determine chloride ion concentration in the drinking water sample. A minimum amount of each water sample was taken into an Erlenmeyer flask, and the pH of the water was measured and adjusted between 7 and 9. Subsequently, some amount of (0.1 M K2CrO4) indicator was added to it and was titrated with the previously standardized 0.1 M AgNO3 solution with a burette that changed it into the brown–red color precipitate, which indicates the endpoint. Then, the blank titration was done by taking distilled water instead of samples of water and was preceded by the same procedure as the above water samples. Then, the concentration of chloride ions was calculated as indicated by [21].
The nitrate (NO3−) ion concentration was determined using the ultraviolet–visible spectrometer (UV/Vis) (Spectra Max Pus-385, UK model). A stock solution of NO3− ion was prepared by adding 0.55 g of NaNO3 into chloroform solution that was used for stabilization and standard NaOH solution to prevent dissociation.
Sulfate (SO42−) ion was determined by a flame photometer (ZEEnit 700P #150Z7P1025 Tech: Flame Photometer, Germany). The stock solution (1000 ppm) of SO42− ion was prepared by adding a gram of Na2SO4 into a volumetric flask, and the flask was filled to the mark with distilled water from which a series of standard solutions were prepared, and the results were recorded from the instrument [28].
2.6. Digestion of Water Samples for Determination of Minerals
For the determination of nine metallic minerals such as Na, K, Mg, Ca, Fe, Cr, Co, Cu, and Zn, some ml of the triplicate water samples was taken into separate beakers, then an acid solution was added into the water in the ratio of 5 mL HNO3:3 mL HCl, and then, the solution was boiled with Kjeldahl (GP:-150 Clad-250 model) on a hot plate at 350°C for an hour, besides the mixture of HCl, HNO3, and H2O2, respectively, was added into 100 mL beakers in the ratio of 6:3:0.25 (HNO3, HCl, and H2O2) and digested to prepare blank solutions. The standard solution for each mineral element was prepared from the stock solution to plot a calibration curve before analyzing drinking water samples [29].
2.7. Statistical Analysis
The data were analyzed using OriginPro 9.0 and Microsoft Excel. The findings of physicochemical quality and average metal contents of the investigated drinking water samples were compared with the World Health Organization (WHO), Ethiopian Standards Agency (ESA), and Kenyan Bureau of Standards (KEBS) guidelines for drinking water quality. Statistical significant differences of all physicochemical properties and minerals concentration in the spring and well-drinking water samples were analyzed by using the one-way ANOVA at p < 0.05, and significant differences within samples were determined based on calculated relative standard deviation (RSD).
2.8. Determination of Drinking Water Quality
2.9. Assessment of Human Health Risk
The total potential noncarcinogenic health impacts caused by exposure to a mixture of heavy metals in drinking water were calculated using the HI and were computed as stated by the US Environmental Protection Agency (EPA) guidelines for health risk assessment using Equation (5). The sum of all HQs of the different heavy metals in drinking water gives an estimate of total potential health risks or chronic health hazards. HI should not exceed one; a value exceeding 1.0 implies significant noncancer risks, which increase with increasing value of HI and if it is below 1.0, shows health benefits for the consumption of drinking water and that the consumers are safe [36, 41].
3. Results and Discussions
3.1. Analysis of Physicochemical Parameters in Spring and Well Water During the dry Season
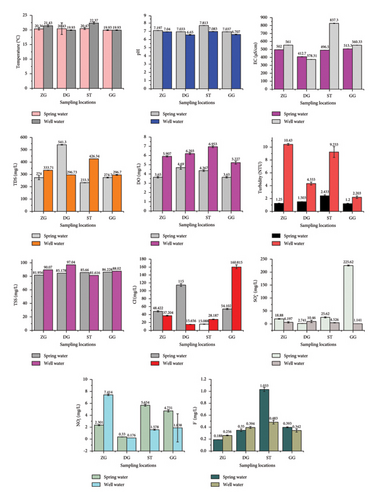
In this study, the physiochemical parameters of drinking water (spring and well water) in the Gofa zone community, south Ethiopia, were assessed, and the results are presented in Figure 2. All water samples were collected during the dry season of the year. Physicochemical parameters, such as pH, temperature, EC, DO, TDS, TSS, turbidity, chloride, NO3−, SO42−, and fluoride, were evaluated.
3.1.1. Temperature
Water temperature is one of the physicochemical parameters used to evaluate the quality of drinking water. Temperature affects the rate of chemical reactions in the water body, reduction in solubility of gases, and changes in tastes and colors of water [42]. The temperature obtained from both spring and well water varies from 22.37 ± 0.33°C to 19.93 ± 0.12°C (in well water) and 20.47 ± 0.32 to 19.93 ± 0.12°C (in spring water) is considered below the WHO maximum permissible limit (< 30°C) [7, 43]. The highest (22.37 ± 0.33°C) and lowest (19.93 ± 0.11°C) temperatures were recorded from Sawula Town Yala well water and Demba Gofa Sukka well water (DGWW4), respectively (Figure 2). Thus, the water temperature obtained in this study is likely suitable for drinking purposes. The findings of this study are comparable to other similar studies reported within a range of 19.5°C–21°C [44], 10.18°C–19.73°C [45], 20.5°C–22°C [46], 19.01°C–23.93°C [47], 21.6 to 24.9 [48], 21.0°C–23.91°C [49], and 19.0 to 19.2 [50].
3.1.2. pH
pH of water is an important parameter in evaluating the acid-base balance. pH measurements are related to the acidity or alkalinity of the water. A sample is considered to be acidic if the pH is below 7.0; it is alkaline if the pH is higher than 7.0. Acidic water can lead to the corrosion of metal pipes and plumping systems, while alkaline water shows disinfection in water. The normal drinking water pH range mentioned in WHO guidelines is between 6.5 and 8.5. The average pH values in both spring and well-drinking water samples are found to be in the range between 7.81 ± 0.01 and 6.65 ± 0.05 (Figure 2), where the lowest and highest values are from samples DGWW4 (6.65 ± 0.05) and Sawla Town Kusti Spring water (SSW5) (7.81 ± 0.01), respectively. The present investigation was in line with the studies reported by Amanial [21] and Aremu et al. [51] conducted in Arbaminch town, Ethiopia, and Eggon, Nasarawa state, Nigeria, respectively.
3.1.3. EC
EC measures the ionic process of a solution that enables it to transmit current. Conductivity is determined for several purposes, such as the determination of the existence of minerals such as potassium, calcium, and sodium, and estimating the amount of chemical reagents is used to treat the water sample [25, 52–55]. The presence of dissolved solids such as calcium, chloride, and magnesium in water samples carries the electric current through the water. The measured EC values of all the drinking water samples (spring and well) are presented in Figure 2. According to WHO [7] standards, the EC value should not exceed 400 µS/cm (Table 2), but the results of this were above the permissible limit except that of DGWW4. The lowest and highest conductivity values correspond to DGWW4 (Demba Gofa Suka well water) (378.31 ± 1.08 µS/cm) and SWW6 (Sawla Town Yala well water) (837.3 ± 3.08 µS/cm) samples, respectively. According to Dissmeyer [8], the differences in EC values are based on various factors, such as agricultural and industrial activities and land use, which affect the mineral contents and hence the EC of the drinking water. High conductivity may lead to lowering the esthetic value of the water by giving a mineral taste to the water. For industrial and agricultural activities, the EC of water is critical to monitor. Water with high EC may cause corrosion of the metal surface of equipment such as a boiler. It is also applicable to home appliances such as water heater systems and faucets [56–58].
| Parameters | Organizations | ||||
|---|---|---|---|---|---|
| WHO (2008, 2011) | KEBS (2010) | ESA (2013) | Spring water in this study | Well water in this study | |
| Temp (°C) | 30 | 28–32 | — | 20.73 | 20.30 |
| pH | 6.5–85 | 6.5–8.5 | 6.5–8.5 | 7.27 | 6.37 |
| TDS (mg/L) | 1500 | 1200 | 1000 | 330.83 | 338.37 |
| TSS(mg/L) | ≤ 30 | 250 | 250 | 84.76 | 89.24 |
| Turbidity | 5NTU | 5NTU | 5NTU | 1.60 NTU | 6.55 NTU |
| EC (μs/cm) | 400 | 1400 | 1500 | 481.13 | 584.24 |
| DO (mg/L) | 4–6 | — | — | 4.09 | 6.07 |
| SO4−2 mg/L | 250 | 250 | 250 | 68.50 | 5.58 |
| NO3− mg/L | 50 | 10 | 10 | 3.25 | 2.75 |
| Cl− mg/L | 250 | 250 | 250 | 58.15 | 60.47 |
| F− mg/L | 1.5 | 1.5 | 1.5 | 0.49 | 0.37 |
- Abbreviations: ESA, Ethiopian Standard Agency; KEBS, Kenyan Bureau of Standards; WHO, World Health Organization.
3.1.4. DO
DO is an important water quality parameter and is an indicator of water contamination. DO content of any water body depends on the mixing and aeration of water, water temperature, duration of sunlight received, and altitude of the area [59]. In this study, analysis of different spring and well water samples revealed mean DO values ranging between 3.65 ± 0.08 and 6.95 ± 0.10 mg/L. The higher mean levels of DO in spring water samples are found to be 4.69 ± 0.19 mg/L in the DGSW3 and 3.65 ± 0.08 mg/L in the Zala Galma Gelta spring water (ZSW1), respectively. The high values of DO of the well water samples were found to be 6.95 ± 0.10 mg/L in SWW6 and 5.23 ± 0.16 mg/L in Geze Gofa Bulki Guya well water (BGWW8) (Figure 2).
As compared to the WHO guideline value for DO, both well and spring water samples are within and below the acceptable limit of drinking water (5–7 mg/L) [60]. The small amount of DO in water indicates microbial contamination or corrosion of chemical substances in the aquifer [61, 62]. Other studies also indicate that the temperature of water influences the amount of DO, with only less oxygen dissolved in warm water than in cold water [63, 64]. According to Ebenezer [65], the level of DO in natural water is highly reduced with increasing water temperature and high organic concentrations as a result of increased decomposer activities. Hence, the high temperature of the water sources could be one of the factors for the low DO values recorded in the current study. The findings are similar to a study conducted in hand-dug well water samples of Kafta Humera Tigray, Ethiopia; it varies from 5.6 ± 0.06 to 6.2 ± 0.04 mg/L [66]. Drinking water samples in Samunaber and Piazza district, Gondar Town (Ethiopia), are between 4.7 ± 0.32 and 5.2 ± 0.35 mg/L [67].
3.1.5. Turbidity
Turbidity is a measure of the cloudiness of water and is caused by a variety of solid matter present in the form of suspended solids. Turbidity is also a measure of light emitting properties of water, and its test is used to indicate the quality of waste discharge for the colloidal of the matter. It is also related to the content of diseases causing organisms in water, which may come from soil runoff. The turbidity values measured for all drinking water samples studied are presented in Figure 2. The recommended maximum turbidity limit set by the WHO for drinking water is 5 nephelometric turbidity units (NTUs) [7, 60].
The highest turbidity values of 10.43 ± 0.15 NTU were found for water samples from Zala Galma well water (ZWW2), and the lowest value of 1.2 ± 0.07 NTU was found for samples from Geze Gofa Mirzho Wola spring water (GGSW7) (Figure 2). The findings of this study indicated that the spring water had the lowest turbidity values and was all below the WHO standard limit of 5 NTU (Table 2). However, the turbidity of well water samples except for the SWW6 (9.23 ± 0.84) and ZWW2 (10.43 ± 0.15) was below the recommended standard limit of 5 NTU by the WHO. The high turbidity observed in some of the water sources (SWW6 and ZWW2) did not agree with WHO standards (5 NTU) and may be due to weathering of igneous rocks, leaching of soils, erosions, and agricultural waste discharges [62].
3.1.6. TDS
TDS is the amount of mobile charged ions, including minerals, salts, or dissolved metals in a given volume of water [62, 68, 69]. The TDS values for all drinking water samples analyzed are shown in Figure 2. The findings of this study indicated that higher TDS value was measured in the DGSW3 (541.3 ± 2.16) and low TDS levels (233.3 ± 1.08) in the SSW5, while SWW6 exhibited higher TDS values (426.34 ± 0.26) and Geze Gofa Bulki Guyo well water (BGWW8) had low TDS value (296.7 ± 3.19). However, the average TDS value of 330.82 ± 5.80 mg/L was measured in the spring water samples, and the average TDS values of 338.37 ± 1.15 mg/L correspond to well water samples from the study sites. According to the WHO, the desired limit for TDS in drinking water was 500 mg/L and the maximum limit of the water was 1000 mg/L, prescribed for drinking purposes (Table 2). The values found from the drinking water samples are all below the maximum limit of 1000 mg/L. TDS level > 1000 mg/L is not suitable for consumption, and values above 1200 mg/L significantly affect consumers [70]. Higher TDS quantity is more toxic to aquatic organisms and enhances the risks of diabetes, hypertension, and renal problems; similar findings were reported by [62, 69, 70].
3.1.7. TSS
The major sources of the TSSs in water are soil and silt, which directly contribute to the water turbidity. The TSS values of all the drinking water samples studied are shown in Figure 2. The highest value of 97.04 ± 0.00 mg/L was found in water samples from the DGWW4, whereas the lowest value of 81.62 ± 0.00 was found for samples from SWW6. According to WHO, the maximum allowable limit of TSS in drinking water is < 30 mg/L (Table 2). The enhanced values of the present study may be attained due to the disposal of domestic sewage and surface runoff. These high values indicate that water samples contain high concentrations of bacteria, nutrients, pesticides, and metals. High TSS content decreases the effectiveness of drinking water disinfection agents by allowing microorganisms to hide from disinfectants within solid aggregates. It suggests that these samples should be filtered before being consumed to remove the suspended solids present in them [48, 71].
3.1.8. Chloride (Cl−)
Chloride ion is one of the major inorganic anions in drinking water and wastewater. Chloride anion can originate from natural and anthropogenic activities, such as sewage and industrial effluents. Naturally, chloride level indicates sewage pollution and imparts leachate effects on soils. Generally, surface water bodies usually have low concentrations of chlorides as compared to ground well water. Chloride has significant importance for metabolic activities in the human body and other main physiological processes. A high amount of chloride in water damages metallic pipe structures and harms growing plants [62, 72]. According to ESA, the permissible standard value of chloride in drinking water is 250 mg/L (Table 2). Relatively high chloride content was detected in BGWW8 (160.82 ± 4.82) and DGSW3 (115 ± 3.24) well and spring water samples, while low concentrations of chloride were detected in DGWW4 (15.66 ± 0.44) and SSW5 (15.09 ± 0.43), respectively (Figure 2). The high amount of chloride in BGWW8 and DGSW3 compared to other sites may be due to runoff, agricultural discharges, use of inorganic fertilizers, discharge of sewage, and wastes. However, the chloride contents in all water samples of this study were below ESA and WHO maximum standard limits of 250 mg/L.
3.1.9. Sulfate (SO42−)
The sources of most SO42− are the oxidation of sulfite ores, the presence of shales, and industrial wastes. High levels of sulfate ions in drinking water may be due to oxidation of pyrite and mining drainages. The concentration of the SO42− ion in natural water ranges from a few to 100 mg/L, but it has no major negative impact on human health [62]. The mean SO42− concentration of spring water samples ranged from 225.62 ± 2.19 to 2.74 ± 0.29 mg/L, while in well water samples, the average SO42− concentration ranged from 10.46 ± 4.55 to 1.14 ± 0.13 (Figure 2). According to the WHO, the highest intended level of SO42− for human consumption in drinking water is 250 mg/L (Table 2). A high amount of SO42− was detected in GGSW7 (225.62 ± 2.19 mg/L), whereas a low level of it was detected in BGWW8 (1.14 ± 0.13 mg/L) (Figure 2). Hence, the results exhibit that the SO42− concentration of spring and well-drinking waters of the study sites were all below the allowable limits of WHO.
3.1.10. Nitrate (NO3−)
Nitrate concentrations in all the sampling sites varied between 0.33 ± 0.01 to 5. 65 ± 0.16 mg/L in spring water samples and 0.18 ± 0.00 to 7.41 ± 0.13 mg/L in well-drinking water (Figure 2). A relatively high concentration of nitrate was observed at ZWW2, while low concentrations of nitrate were observed at DGWW4. Hence, the results exhibit that the nitrate levels of spring and well-drinking waters of the study sites were all below the tolerable limits of WHO (50 mg/L) and ESA of 10 mg/L (Table 2).
3.1.11. Fluoride Ion
The mean fluoride concentration of drinking water samples was within the range of 0.19 ± 0.00 to 1.03 ± 0.03 mg/L (Figure 2). A higher value of fluoride was determined in the SSW5 (1.03 ± 0.03) and lower in the ZSW1 samples (0.19 ± 0.00), respectively. The concentrations of fluoride recorded in water samples from the study sites were below the standard set by WHO (1.5 mg/L) (Table 2).
3.2. Quality Control/Quality Assurance (QC/QA)
QC and QA were checked for all parameters investigated in this study. Calibration curves were constructed to determine the linearity with each batch sample analysis (R2 ≥ 0.998) and the unknown concentration of minerals in the sample. Analytical method validation was conducted using a limit of detection (LOD) and limit of quantification (LOQ) from triplicate analysis of five method blanks. The precision and accuracy of the results were calculated by replicating measurements of standard or sample solutions of recovery experiments. The recovery results indicated that the spiked sample analysis was successful and showed worthy recovery within the acceptable range of 80%–120%. In addition, the RSD and standard error of the mean (SEM) of triplicate measurements of each sample were used to obtain precision.
3.3. Analysis of Minerals Status in Spring and Well-Drinking Water
The presence of metallic minerals in drinking water higher than the desired level can cause harmful impacts on human well-being. Hence, the analysis of metallic minerals in drinking water is an important parameter, and most of the studies on drinking water quality involve the investigation of these minerals. In the present study, the results of the mineral contents of spring and well waters analyzed, such as Na, K, Mg, Ca, Cr, Fe, Cu, and Zn, are presented in Tables 3 and 4. One-way ANOVA indicated that there is no statistical significance difference (p < 0.05) between mean values of Na, Co, Cu, Zn, and TDS, pH, EC, NO3−, Cl−, and F− of spring and well water samples whereas there are statistical significance differences (p > 0.05) of the average levels of K, Ca, Mg, and Fe minerals and DO, TSS, turbidity, and SO42−. The results of metallic minerals content and physicochemical parameters investigated were compared with the safe limits set by WHO, KEBS, and the ESA (Tables 2 and 5).
| Metals/minerals | Sampling sites/locations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zala Galma (ZG) | Demba Gofa (DG) | Sawla Town (ST) | Geze Gofa (GG) | |||||||||
| Spring water | SEM | RSD | Spring water | SEM | RSD | Spring water | SEM | RSD | Spring water | SEM | RSD | |
| Na (mg/L) | 0.06 ± 0.02 | 0.01 | 39.65 | 2.64 ± 0.07 | 0.04 | 2.50 | 0.86 ± 0.05 | 0.03 | 6.14 | 0.46 ± 0.05 | 0.03 | 11.54 |
| K (mg/L) | 2.08 ± 0.08 | 0.04 | 3.70 | 0.003 ± 0.00 | 0.00 | 33.33 | 1.58 ± 0.15 | 0.09 | 9.44 | 0.25 ± 0.01 | 0.01 | 4.76 |
| Mg (mg/L) | 1.24 ± 0.02 | 0.01 | 1.86 | 0.37 ± 0.07 | 0.04 | 19.13 | 0.95 ± 0.02 | 0.01 | 1.67 | 1.24 ± 0.29 | 0.17 | 23.07 |
| Ca (mg/L) | 0.58 ± 0.09 | 0.05 | 14.95 | 0.35 ± 0.01 | 0.01 | 2.30 | 0.58 ± 0.22 | 0.01 | 3.95 | 0.64 ± 0.01 | 0.01 | 1.73 |
| Cr (mg/L) | BDL | BDL | BDL | BDL | ||||||||
| Fe (mg/L) | 0.42 ± 0.01 | 0.01 | 1.92 | 0.42 ± 0.01 | 0.01 | 1.88 | 1.55 ± 0.04 | 0.02 | 2.71 | 1.94 ± 0.03 | 0.02 | 1.59 |
| Co (mg/L) | 0.06 ± 0.00 | 0.00 | 0.48 | BDL | 0.11 ± 0.00 | 0.06 | 0.66 | 0.23 ± 0.00 | 0.00 | 0.21 | ||
| Cu (mg/L) | 0.06 ± 0.00 | 0.00 | 6.25 | 0.06 ± 0.00 | 0.00 | 3.27 | 0.06 ± 0.01 | 0.01 | 20.63 | 0.25 ± 0.00 | 0.00 | 1.57 |
| Zn (mg/L) | 0.06 ± 0.00 | 0.00 | 6.25 | 0.06 ± 0.00 | 0.00 | 1.63 | 0.01 ± 0.00 | 0.00 | 33.33 | 0.25 ± 0.00 | 0.00 | 0.00 |
| Metals/minerals | Sampling sites/locations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zala Galma (ZG) | Demba Gofa (DG) | Sawla town (ST) | Geze Gofa (GG) | |||||||||
| Well water | SEM | RSD | Well water | SEM | RSD | Well water | SEM | RSD | Well water | SEM | RSD | |
| Na (mg/L) | 0.04 ± 0.03 | 0.02 | 76.31 | 0.44 ± 0.20 | 0.12 | 45.74 | 0.63 ± 0.06 | 0.04 | 10.08 | 17.04 ± 0.27 | 0.15 | 1.56 |
| K (mg/L) | 0.003 ± 0.00 | 0.00 | 33.33 | 0.01 ± 0.00 | 0.00 | 50 | 0.09 ± 0.07 | 0.40 | 75.22 | 0.004 ± 0.00 | 0.00 | 50 |
| Mg (mg/L) | 0.94 ± 0.40 | 0.23 | 42.06 | 0.44 ± 0.05 | 0.03 | 12.02 | 1.37 ± 0.11 | 0.06 | 7.66 | 0.68 ± 0.11 | 0.06 | 15.56 |
| Ca (mg/L) | 0.38 ± 0.03 | 0.02 | 8.15 | 0.80 ± 0.11 | 0.06 | 13.90 | 1.90 ± 0.48 | 0.28 | 25.15 | 0.37 ± 0.06 | 0.03 | 15.67 |
| Cr (mg/L) | BDL | BDL | BDL | BDL | ||||||||
| Fe (mg/L) | 0.65 ± 0.11 | 0.06 | 16.23 | 0.60 ± 0.04 | 0.02 | 7.06 | 0.60 ± 0.00 | 0.00 | 0.66 | 0.62 ± 0.01 | 0.01 | 2.27 |
| Co (mg/L) | 0.06 ± 0.00 | 0.00 | 3.12 | 0.04 ± 0.00 | 0.00 | 1.16 | 0.01 ± 0.00 | 0.00 | 4 | 0.26 ± 0.00 | 0.00 | 0.78 |
| Cu (mg/L) | 0.28 ± 0.01 | 0.00 | 2.18 | 0.013 ± 0.00 | 0.00 | 3.84 | 0.16 ± 0.00 | 0.00 | 0.62 | 0.14 ± 0.00 | 0.00 | 2.89 |
| Zn (mg/L) | 0.26 ± 0.02 | 0.01 | 6.22 | 0.02 ± 0.00 | 0.00 | 5.88 | 0.17 ± 0.00 | 0.00 | 1.16 | 0.46 ± 0.00 | 0.00 | 0.43 |
- Abbreviations: BDL, below detection limit; RSD, relative standard deviation; SEM, standard error of the mean.
| Parameters | Organizations | ||||
|---|---|---|---|---|---|
| WHO (2008, 2011) | KEBS (2010) | ESA (2013) | Spring water in this study | Well water in this study | |
| Na | 200 mg/L | 200 mg/L | 200 mg/L | 1.00 mg/L | 4.53 mg/L |
| K | 12 mg/L | 50 mgL | 1.5 mg/L | 0.93 mg/L | 2.28 mg/L |
| Mg | 300 mg/L | 100 mg/L | 50 mg/L | 0.95 mg/L | 0.86 mg/L |
| Ca | 400 mg/L | 100 mg/L | 75 mg/L | 0.54 mg/L | 0.87 mg/L |
| Cr | 0.002 mg/L | 0.002 mg/L | 0.05 mg/L | BDL | BDL |
| Fe | 0.3 mg/L | 0.3 mg/L | 0.3 mg/L | 1.08 mg/L | 0.62 mg/L |
| Co | < 2 mg/L | 2 mg/L | 2 mg/L | 0.01 mg/L | 0.09 mg/L |
| Cu | 2 mg/L | 1 mg/L | 2 mg/L | 0.11 mg/L | 0.15 mg/L |
| Zn | 5 mg/L | 5 mg/L | 5 mg/L | 0.10 mg/L | 0.23 mg/L |
- Abbreviations: ESA, Ethiopian Standard Agency; KEBS, Kenyan Bureau of Standards; WHO, World Health Organization.
The natural source of sodium (Na) and potassium (K) in groundwater was from the weathering of rocks, but the elevated quantities in contaminated water may be attributed to the release of wastewater [73]. Na is a metallic element found in less quantity in water. The normal quantity of Na+ ions in the human body prevents many fatal diseases such as hypertension, kidney damage, and headache. The majority of water supply in most countries bears less than 20 mg/L, while, in some others, the level of Na in drinking water exceeds 250 mg/L [74]. The findings show that the mean concentration of Na in the spring water samples ranged from 0.06 ± 0.02 to 2.64 ± 0.07 mg/L, while in well water samples, sodium level was in the range of 0.04 ± 0.20 to 17.04 ± 0.27 mg/L. A higher amount was observed in BGWW8, and a lower level was investigated in the ZWW2, respectively. The mean values of Na in both spring and well-drinking water samples were below the WHO and ESA permissible limit of 200 mg/L (Table 5). K is necessary for all living organisms functioning and hence found in all human and animal tissues, particularly in plant cells. The K levels recorded in the water samples of study areas were very low, ranging from 0.003 ± 0.00 mg/L to 2.08 ± 0.08 mg/L in spring water and from 0.003 ± 0.00 mg/L to 0.09 ± 0.70 mg/L in well water (Tables 3 and 4). The concentration of K obtained from all spring and well water samples was far below the maximum permissible limit of WHO (10 mg/L) and KEBS of 50 mg/L for drinking water (Table 5). However, the concentration of K in spring water from ZSW1 and SSW5 was below the recommended limit of ESA (1.5 mg/L) (Table 5).
Calcium (Ca) and magnesium (Mg) ions are the major constituents of various types of rock, and they are the most common constituents present in natural waters ranging from zero to several hundred mg/L [75]. Generally, Ca has no effect on human health in drinking water, but it can cause hardness risk problems that are directly related to the hardness of water [7]. A higher level of calcium (1.90 ± 0.48 mg/L) of the well water was obtained at SWW6, whereas a low amount (0.37 ± 0.06 mg/L) was found in BGWW8 samples (Table 4). The amount of Ca recorded in spring water ranged from 0.35 ± 0.01 mg/L in DGSW3 to 0.64 ± 0.01 mg/L in GGSW7 (Table 3). The result shows that in all the spring and well-drinking water, Ca ions recorded were within the acceptable limit of WHO, KEBS, and ESA standards for drinking water (Table 5). The level of Mg ions in the well water varied between 0.44 ± 0.05 mg/L and 1.37 ± 0.11 mg/L, while in spring water, Mg concentration varied between 0.37 ± 0.07 and 1.24 ± 0.29 (Table 4). A high concentration of Mg was found in the GGSW7 and SWW6, whereas low levels were observed in the DGSW3 and DGWW4 samples. The results show that Mg content in all spring and well water samples was below the permissible guideline value of WHO, KEBS, and ESA (Table 5).
Chromium (Cr) exists naturally as an element in types of rocks, soils, plants, animals, and volcanic emissions. It was found in drinking water in trivalent form (Cr(III)) and hexavalent (Cr(VI)) principal forms. The mean concentrations of chromium in the study areas were below the detection limit (BDL) in both spring and well-drinking water.
Iron (Fe) exists in its natural form as ores of magnetite, taconite, and hematite in the rocks, soils, and minerals, making about 5% of the Earth’s crust. Fe is dark gray when in pure form and exists in groundwater as a ferric hydroxide [48, 76]. The concentration of iron measured in both spring water samples varied from 0.42 ± 0.01 mg/L to 1.94 ± 0.03 mg/L (Table 3), whereas in well-drinking water, it was in the range of 0.60 ± 0.04 to 0.65 ± 0.11 mg/L (Table 4). The findings deduced that all spring and well-drinking water samples had iron concentrations above the guideline values (0.3 mg/L) of WHO, KEBS, and ESA (Table 5). A higher concentration of Fe was observed in the GGSW7, and a low level was observed in the DGWW4, respectively. This may be due to the weathering of minerals, soil type, and sediments which are iron-rich materials [77]. Fe may not pose any health hazards but gives a bitter taste to the water when present in large concentrations. People consuming water sources with high concentrations of Fe were suffering from taste, color, corrosion of plumbing systems, and liver diseases. However, those exposed to low concentrations would be highly susceptible to anemia [66, 78].
Cobalt (Co) is not detected in DGSW3. The mean concentration of Co obtained in spring water was in the range of BDL to 0.23 ± 0.00 mg/L, and in well-drinking water samples, the level of Co was from 0.01 ± 0.00 mg/L to 0.26 ± 0.00 mg/L. Its value is high in the GGSW7 and BGWW8; however, its value is low in the DGSW3, respectively. The results revealed that the mean concentration of Co in water samples was below the guideline value of the WHO (≤ 2 mg/L) (Table 5).
Copper (Cu) metal is one of the essential dietary requirements; however, a 2 mg/L level of Cu in drinking water can cause astringent tastes. At levels above 2.5 mg/L, Cu in drinking water imparts an undesirable bitter taste to water; at higher levels, the color of the water is also impacted [79]. The major sources of Cu in water bodies are agricultural activities and municipal solid wastes, pesticides, herbicides, etc. Intake of a high amount of Cu in humans can cause nausea, vomiting, diarrhea, and damage to our liver and kidneys and can even cause death [80]. The mean concentrations of Cu in spring and well water samples of this study ranged from 0.06 ± 0.00 to 0.28 ± 0.01 mg/L. A high level was detected in the ZWW2 (0.28 mg/L), and low in the DGSW3 (0.06 mg/L), respectively. All water samples in this study were below the permitted limit of the WHO (1 mg/L) and are recommended for drinking and other home consumptions.
Zinc (Zn) in surface water and groundwater normally does not exceed 0.01 and 0.05 mg/L, respectively. Its concentration in tap water can be much higher as a result of the dissolution of Zn from pipes. Commonly, Zn is found at low concentrations in many rocks and soils, principally as sulfide ores and lesser degree as carbonates. Mostly Zn is introduced into water by artificial pathways such as byproducts of steel production or coal-fired power stations or from the burning of waste materials from fertilizer which may leach into groundwater.
The mineral distribution pattern between water samples from all sample sites was investigated using principal component analysis (PCA). The PCA was used to compare the mineral contents and other physicochemical parameters in spring and well-drinking water samples. The analyzed parameters into PCA expressed a 55.33% distinction among the four spring and four well-drinking water samples. PC1 explains 30.97% of the total variability, while PC2 explains only 24.36% of the total variation. As shown in Figure 3, the GGSW7, ZSW1, SSW5, and SWW6 waters tend to occupy the positive PC2 side while BGWW8, DGWW4, and ZWW2 well waters and DGSW3 water occupy the opposite sides of PC2. The parameters pH, K, F−, Fe, NO3−, and SO42− were positively correlated with each other and higher in GGSW7, ZSW1, and SSW5 spring water samples. SWW6 water samples were characterized by higher Mg, Ca, temperature, and EC.
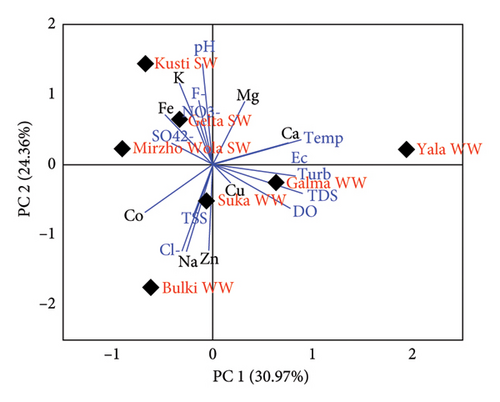
Computation of Pearson correlation coefficients indicated that Co and Na had a significant positive correlation across samples from the eight sites (Table 6). A significant correlation was also found between Zn-Na (r = 0.74), Zn-Co (r = 0.68), and Zn-Cu (r = 0.65) in the samples (Table 6). Also, significant positive correlations found between temperature, EC, DO, turbidity, and F− are higher in SWW6 water samples (Figure 3 and Table 7). Positive correlations in the concentration of the minerals are an indicator of having a common source, mutual dependence, and identical behavior during transportation [41, 81].
| Na | K | Mg | Ca | Fe | Co | Cu | Zn | |
|---|---|---|---|---|---|---|---|---|
| Na | 1 | |||||||
| K | −0.28 | 1 | ||||||
| Mg | −0.32 | 0.41 | 1 | |||||
| Ca | −0.28 | −0.12 | ∗0.52 | 1 | ||||
| Fe | −0.19 | 0.14 | 0.35 | −0.07 | 1 | |||
| Co | ∗0.67 | −0.16 | −0.14 | −0.55 | 0.49 | 1 | ||
| Cu | −0.01 | −0.36 | 0.5 | 0.03 | 0.36 | 0.30 | 1 | |
| Zn | ∗0.74 | −0.46 | 0.13 | −0.13 | 0.04 | ∗0.68 | ∗0.65 | 1 |
- ∗Significant correlation at α = 0.05.
| Temp | pH | EC | TDS | DO | Turb | TSS | Cl− | SO4−2 | NO3− | F− | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp | 1 | ||||||||||
| pH | 0.20 | 1 | |||||||||
| EC | ∗0.82 | 0.09 | 1 | ||||||||
| TDS | 0.40 | −0.23 | 0.15 | 1 | |||||||
| DO | ∗0.63 | −0.39 | 0.48 | 0.34 | 1 | ||||||
| Turb | ∗0.83 | −0.10 | ∗0.60 | 0.19 | ∗0.81 | 1 | |||||
| TSS | −0.41 | −0.51 | −0.55 | −0.23 | 0.33 | 0.12 | 1 | ||||
| Cl- | −0.35 | −0.42 | −0.10 | 0.33 | −0.18 | −0.39 | −0.12 | 1 | |||
| SO4−2 | −0.34 | 0.05 | −0.08 | −0.31 | −0.53 | −0.35 | −0.08 | −0.11 | 1 | ||
| NO3− | 0.18 | 0.52 | 0.12 | −0.47 | −0.21 | 0.32 | −0.05 | −0.33 | 0.30 | 1 | |
| F− | 0.03 | ∗0.74 | 0.04 | −0.28 | −0.05 | −0.10 | −0.08 | −0.36 | 0.01 | 0.27 | 1 |
- ∗Significant correlation at α = 0.05.
3.4. Water Quality and Health Risk Assessment
The average MPI and HEI were calculated using Equations (1) and (2), respectively, and their values are shown in Table 8. According to the calculated mean MPI, the drinking water in ZSW1, DGSW3, DGWW4, and SWW6 can be categorized as pure water because the values lie between 0.3 and 1. The drinking waters are considered highly polluted based on the mean HEI values of > 10 [82, 83]. Hence, SSW5, GGSW7, and BGWW8 are considered highly polluted drinking waters and are not recommended for drinking purposes.
| Sample site/drinking water | MPI | HEI |
|---|---|---|
| ZSW1 | 0.883 | 6.563 |
| DGSW3 | 0.860 | 0.265 |
| SSW5 | 1.150 | 11.419 |
| GGSW7 | 1.279 | 24.290 |
| Average | 1.042 | 10.634 |
| ZWW2 | 1.057 | 6.961 |
| DGWW4 | 0.904 | 4.610 |
| SWW6 | 0.985 | 0.945 |
| BGWW8 | 1.102 | 26.992 |
| Average | 1.012 | 9.877 |
- Abbreviations: SW, spring drinking water; WW, well-drinking water.
Health risk assessments were not performed for Cr, as the concentrations in water samples were BDLs of the method. As presented in Tables 9 and 10, the average CDI values of heavy metal levels for adults and children were in the order of Fe > Co > Cu > Zn. CDI values were higher in children compared to adults exposed to drinking spring water. However, in well-drinking water samples, the average CDI values of heavy metals for both adults and children were in the order of Fe > Zn > Cu > Co. Similarly, higher CDI values were identified in children than in adults.
| Sample site/drinking water | CDI ingestion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Co | Cu | Zn | CDItotal | ||||||
| Adult | Children | Adult | Children | Adult | Children | Adult | Children | Adult | Children | |
| ZSW1 | 0.021 | 0.049 | 0.003 | 0.007 | 0.003 | 0.008 | 0.003 | 0.008 | 0.030 | 0.071 |
| DGSW3 | 0.021 | 0.049 | — | — | 0.003 | 0.007 | 0.003 | 0.007 | 0.027 | 0.064 |
| SSW5 | 0.078 | 0.181 | 0.005 | 0.012 | 0.003 | 0.007 | 0.001 | 0.001 | 0.087 | 0.202 |
| GGSW7 | 0.097 | 0.227 | 0.012 | 0.027 | 0.013 | 0.030 | 0.013 | 0.030 | 0.134 | 0.312 |
| Sample site/drinking water | CDI ingestion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Co | Cu | Zn | CDItotal | ||||||
| Adult | Children | Adult | Children | Adult | Children | Adult | Children | Adult | Children | |
| ZWW2 | 0.033 | 0.076 | 0.003 | 0.008 | 0.014 | 0.032 | 0.013 | 0.030 | 0.063 | 0.146 |
| DGWW4 | 0.030 | 0.069 | 0.002 | 0.005 | 0.001 | 0.002 | 0.001 | 0.002 | 0.034 | 0.078 |
| SWW6 | 0.030 | 0.070 | 0.0003 | 0.001 | 0.008 | 0.019 | 0.009 | 0.020 | 0.047 | 0.110 |
| BGWW8 | 0.031 | 0.072 | 0.013 | 0.030 | 0.007 | 0.016 | 0.023 | 0.054 | 0.074 | 0.172 |
The maximum daily intake rate for children and adults through ingestion paths in spring and well-drinking water was observed as Fe (0.227 and 0.097 mg/kg/day) in children and (0.076 and 0.033 mg/kg/day) in adults, respectively, and this is higher than tap drinking water for Akaki Kality of 0.00198 and 0.00108 mg/kg/day, respectively [84]. According to Belew et al. [85], the mean daily intake values (children and adults) of Fe (0.97 and 0.83 mg/kg/day), Zn (0.091 and 0.091 mg/kg/day), and Cu (0.072 and 0.061 mg/kg/day) in the Jigjiga city were higher than the determined values for the drinking water samples of this study. On the other hand, Dessie et al. [84] reported that the mean daily intake values (children and adults) of Zn (0.0037 and 0.0020 mg/kg/day) and Cu (0.0023 and 0.0013 mg/kg/day) in the Gullele subcity of Addis Ababa were nearly in line with the determined values for the drinking water samples in this study. However, similar studies conducted by Mohammadi et al. [86] in Iran and Nawab et al. [87] in Pakistan indicated that the mean daily intake rate of Zn and Cu was lower than the values investigated in this study.
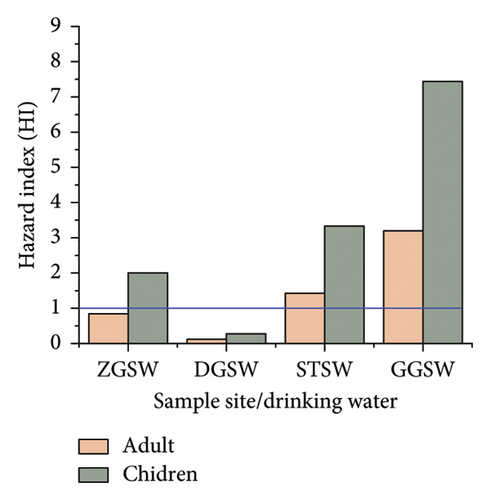
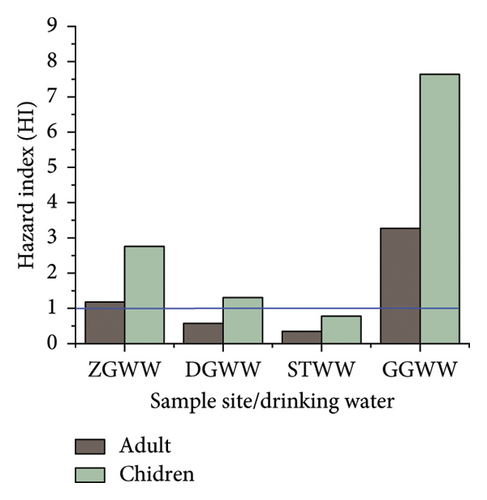
HQs and HIs were used to assess the non-carcinogenic risks for Fe, Co, Zn, and Cu in adults and children in the study area. The results are shown in Tables 11 and 12, and Figure 3. The HQ and HI values for the heavy metals (Fe, Co, Cu, and Zn) were also higher in children than in adults for the spring and well-drinking water source of the study sites. A similar study was also reported by [84–86, 88]. The HQ values for Fe, Cu, and Zn were within the acceptable range of less than one. A HQ value of > 1 suggests a level of concern. The values of Co in spring and well-drinking water sources for both adults and children in the study area were above the acceptable limit and required attention. The HI values were > 1 in all sites indicating high health risk on long-term exposure, and the noncancer effect is of concern and seeks attention (Figure 4). The HIs for children were higher compared to those of the adults indicating that children could be more likely to have noncancer risks than adults.
| Sample site/drinking water | HQ ingestion | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Co | Cu | Zn | |||||
| Adult | Children | Adult | Children | Adult | Children | Adult | Children | |
| ZSW1 | 0.030 | 0.069 | 0.721 | 1.721 | 0.08 | 0.188 | 0.011 | 0.025 |
| DGSW3 | 0.030 | 0.071 | — | — | 0.078 | 0.178 | 0.010 | 0.024 |
| SSW5 | 0.111 | 0.258 | 1.233 | 2.884 | 0.08 | 0.185 | 0.002 | 0.005 |
| GGSW7 | 0.139 | 0.324 | 2.698 | 6.279 | 0.318 | 0.74 | 0.042 | 0.098 |
| Sample site/drinking water | HQ ingestion | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Co | Cu | Zn | |||||
| Adult | Children | Adult | Children | Adult | Children | Adult | Children | |
| ZWW2 | 0.047 | 0.109 | 0.744 | 1.744 | 0.345 | 0.803 | 0.043 | 0.1 |
| DGWW4 | 0.043 | 0.09 | 0.512 | 1.163 | 0.018 | 0.038 | 0.003 | 0.007 |
| SWW6 | 0.043 | 0.101 | 0.070 | 0.140 | 0.205 | 0.475 | 0.029 | 0.067 |
| BGWW8 | 0.044 | 0.103 | 2.977 | 6.954 | 0.173 | 0.403 | 0.077 | 0.180 |
4. Conclusion
In this study, the levels of selected physicochemical parameters and metallic minerals status of 24 spring and well water samples of eight selected districts from the four woreda administrations of Gofa Zone, Ethiopia, were assessed. All water samples were analyzed for physicochemical parameters such as temperature, pH, DO, TDS, EC, Cl−, F−, NO3−, and SO42− and the status of Na, K, Mg, Ca, Cr, Co, Cu, and Zn using standard analytical procedures. The findings showed that the mean levels of TSS, turbidity, and Fe were above the standard permissible values of WHO, KEBS, and ESA. The high concentration of this heavy metal as well as the typical physicochemical parameters indicates the presence of water pollutants and poor drinking water treatments in the study areas, which have implications for the health of local people. According to the health risk assessment findings, Co metal showed high HIs compared with Fe, Cu, and Zn from eight study sites implying noncarcinogenic risks through prolonged intake. Moreover, MPI and HEI values indicating the SSW5, GGSW7, and BGWW8 water sources are considered highly polluted drinking waters and not recommended for drinking purposes.
Hence, it recommended that the government authorities and responsible bodies should use the findings to appropriately address health matters by creating awareness focusing on the dangers of waterborne diseases and the benefits of utilizing safe drinking water for the health of local inhabitants. In addition, periodic monitoring and disinfection of drinking water spots before being consumed by the communities have been encouraged. To ensure that community health is better protected, further research investigations recommended on the exposure to other various toxic heavy metals, such as Pb, As, and Mn, can have effects on human health, seasonal, and geographical variation, and groundwater flow systems study, including the presence of organic constituents and microbiological tests of water sources that are not addressed in this study due to limited resources and finance.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
There has been no significant grant or financial support received from any funding agency for this work that could have influenced its outcome.
Acknowledgment
The authors would like to thank the Wolaita Sodo University Department of Chemistry, for their material support.
Open Research
Data Availability Statement
The data and materials presented in this manuscript can be made available as per the editorial policy of the journal.




