Selective Synthesis of cis and trans o-Hydroxychalcone Derivatives for Metal Sensing Applications
Abstract
This study explores a novel synthetic approach for obtaining cis- and trans-isomers of o-hydroxychalcone derivatives and their metal-sensing capabilities. Four chalcones (CH-1 to CH-4) were synthesized using an optimized Claisen–Schmidt condensation reaction. This method efficiently yielded the desired products by combining o-hydroxyacetophenone with various substituted benzaldehydes in the presence of NaOH. The key innovation lies in the reaction conditions that enable the selective synthesis of both cis- and trans-isomers. Analysis of the allylic protons in CH-1, CH-2, and CH-3 using 1H NMR revealed a coupling constant (JHα-JHβ) of 15.60 Hz, confirming their trans configuration. Conversely, CH-4 displayed a coupling constant of J = 8.40 Hz, indicating a cis configuration. Further structure optimization was performed using the B3LYP method with a DFT B3LYP/Lan2DZ level of theory. The metal-sensing properties and selectivity of the synthesized chalcones were evaluated using UV-Vis spectroscopy in an ethanolic solution containing various metal ions (Cu2+, Fe3+, Zn2+, K+, Mn2+, and Co2+). Interestingly, CH-1 and CH-2 exhibited specific interactions with Cu2+ ions. In contrast, CH-3 and CH-4 demonstrated the ability to sense both Cu2+ and Fe3+, highlighting their potential for dual metal ion detection. This work presents a novel synthesis method for controlling cis-trans isomerism in o-hydroxychalcones and unveils their promising uses in selective metal ion–sensing applications.
Summary
- •
Synthesis of o-hydroxychalcone derivatives via Claisen–Schmidt condensation reaction.
- •
Structural characterization using 1H NMR revealing trans and cis configurations of allylic protons in the synthesized chalcones.
- •
Structural optimization performed using the density functional theory (DFT) B3LYP method with Lan2DZ level of theory.
- •
Evaluation of metal-sensing properties using UV-Vis spectroscopy in ethanolic solutions.
- •
CH-1 and CH-2 demonstrated selective interaction with Cu2+ ions.
- •
CH-3 and CH-4 exhibited sensing capabilities for both Cu2+ and Fe3+ ions.
1. Introduction
Chalcones are a type of natural flavonoid compounds which have a characteristic chemical structure consisting of two aromatic rings, an α, β-unsaturated ketone group and a carbon–carbon double bond in the side chain. Chalcones are widely distributed in the plant kingdom [1]. The general formula for chalcones is C15H12O. Chalcones are important intermediates [2] in the biosynthesis of flavonoids family [3–5], ant, antimicrobial, cytotoxic, analgesic, and antipyretic properties. They are considered precursors of flavonoids and isoflavonoids [6–9]. Chalcones exhibits a wide range of biological activities [10]. The general structure of a chalcone is shown in Scheme 1.

The structure of chalcones can be described as consisting of two aromatic rings (A and B) connected by an α, β-unsaturated carbonyl group [11]. The A ring is typically a benzene ring, while the B ring can be a variety of different aromatic rings, including phenyl, pyridine, or furan [12]. The α, β-unsaturated carbonyl group is responsible for the characteristic yellow color of chalcones, which is due to the presence of a conjugated system of double bonds [12, 13]. The stereochemistry of chalcones is important for their biological activity [10, 14], as it can affect their ability to interact with biological targets [15]. In particular, the E isomer is generally more biologically active than the Z isomer [10]. Chalcone and its derivatives have shown to exhibit a wide range of biological activities, such as antioxidant [15], anti-inflammatory [16], antimicrobial [17], anticancer [18], antihypertensive [19], antimalarial [20], antiulcer [21], antiviral [22], and antiprotozoal activities [23]. Additionally, they have been reported to exhibit cardiovascular effects [24] and mutagenic properties [25]. Chalcones and bis-chalcones are not only biologically active compounds themselves but they also serve as useful precursors for the synthesis of various bioactive heterocycles, including pyridine, pyrimidine, pyrazoline, and isoxazoline derivatives [26, 27]. Both natural and synthetic chalcones have been found to exhibit strong antiproliferative effects on primary and established ovarian cancer cells [18, 28], as well as on gastric cancer cells (HGC-27) [29]. Isoliquiritigenin and chalcones containing hydroxyl group have been identified as potent inhibitors [30]. These chalcones are believed to exert their remarkable biological potential by interacting with various proteins involved in cell proliferation and apoptosis [31]. The recent research indicates that chalcones induce apoptosis in various cell types, including breast cancer cells [32]. Chalcones can exhibit fluorescence if their benzene ring has suitable functional groups that pull and push electrons, owing to their conjugated structure [33]. This property makes them useful chemical probes for mechanistic studies and imaging/diagnostic applications [34, 35]. Moreover, fluorescent chalcones have been identified as potential probes for investigating cellular targets [36]. By utilizing the intermolecular charge transfer (ICT) process, chalcone and its derivatives with electron-donating and accepting units can increase their quantum yields by modifying the polarity of solvents [37]. Therefore, the flexibility of the chalcone skeleton for molecular modification is an attractive feature for researchers. Moreover, there has been considerable interest in exploring the potential of numerous chalcone derivatives for sensing applications and conducting related investigations [34, 38–42].
The pervasive presence of heavy metals throughout the environment poses a significant global threat due to the resulting pollution [43, 44]. Our world, from living organisms to nonliving environments, is filled with metal ions. These can be broadly categorized into two groups: essential metals and nonessential hazardous metals. Essential metal ions, such as iron, copper, zinc, and manganese, play a vital role in biological processes and enzyme function [45]. Some essential metals, such as copper, along with other metals such as gold and platinum, have shown to possess anticancer properties [46]. In contrast, metal ions such as mercury, zinc (at high levels), and cadmium can be detrimental to living organisms and the environment [47, 48]. The human body relies on trace amounts of certain metal ions for various biological functions. These essential metals include zinc, iron, nickel, manganese, copper, cobalt, and even gold. However, excessive amounts of these essential metals can become toxic [49]. For example, lead accumulation can lead to arthritis, kidney damage, and neurological problems. Similarly, mercury exposure can cause lung damage, memory loss, and cancer. Modern lifestyles contribute to various forms of pollution, with water pollution being a major concern. This contaminated water poses a serious threat to all living organisms in the environment. Industrial waste often contains heavy metals, which disrupts the ecological balance. As a result, there is a growing need for methods to effectively detect and measure the concentration of these harmful metal ions [50]. The objective of this study is to selective synthesis of substituted 1,3-diaryl-prop-2-en-1-ones, cis and trans isomer shown in Table 1 and their metal sensing properties shown in Figure 1. Structures were confirmed by various spectroscopic techniques, including elemental analysis, UV-Vis, FTIR, and 1H NMR. The metal-sensing properties of the synthesized compounds were examined by subjecting them to UV-visible spectroscopy. On the other hand, the structures were optimized using DFT, and the excitation energy was calculated by TD-DFT B3LYP/Land2DZ method. The synthesized substituted 1,3-diaryl-prop-2-en-1-ones were titrated with different metals ions such as Cu2+, Fe3+, Zn2+, K+, Mn2+, and Co2+ and investigated their selectivity and sensing capabilities using UV-Vis absorption spectroscopy.
 |




2. Experimental Section
The initial materials and reagents were procured without undergoing additional purification steps. The determination of the melting points of the synthesized compounds was carried out using the Stuart Melting Point SPM 30 model apparatus. The UV-Vis spectra of the samples were recorded using a UV-180 SHIMADZU spectrophotometer. For the measurement of FTIR spectra, an IRTracer-100 instrument from SHIMADZU, Japan, was employed. The 1H-NMR spectra of the samples were acquired using an AVANCE Bruker NMR spectrometer operating at 400 MHz. CDCl3 was utilized as the solvent, and TMS served as the internal standard. Elemental analysis was conducted via a CE-440 elemental analyzer manufactured by Exeter Analytical, Inc.
2.1. General Procedure for the Synthesis of Chalcone Derivatives, CH-1 and CH-2
For the synthesis of CH-1 and CH-2, o-hydroxyacetophenone and a substituted benzaldehyde in equimolar quantities were dissolved in an ethanol solvent. A 40% NaOH solution was gradually added to the mixture with continuous stirring. Throughout, the reaction temperature was maintained within the range of 20°C–25°C. The advancement of the reaction was monitored using thin-layer chromatography (TLC). Following the completion of the reaction, a diluted HCl solution was cautiously added to neutralize the reaction mixture. The resulting precipitate was filtered, subsequently sequential washing with n-hexane and cold water to eliminate any remaining initial substances. The crude product was air-dried and purified through recrystallization [51–55]; all the synthesized compounds are shown in Scheme 2.
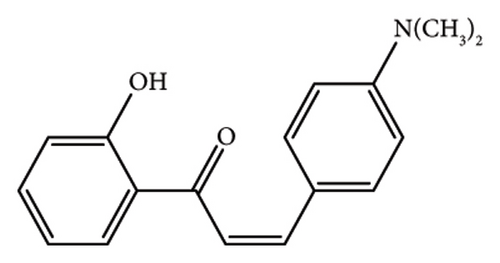
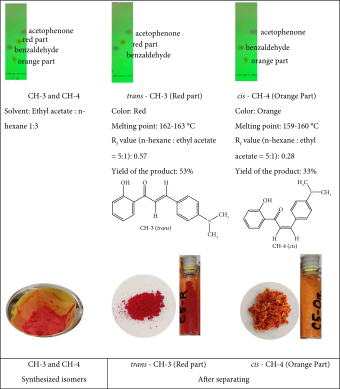
To synthesize the CH-3 and CH-4 compounds, a modified procedure was employed. An equimolar combination of o-hydroxyacetophenone and p-dimethylaminobenzaldehyde was subjected to stirring for a duration of 3 h at ambient temperature in the presence of NaOH. Subsequently, the mixture was refluxed for 2 h using a hot water bath. The remaining steps closely resembled those outlined in the previous protocol. Consequently, two distinct products were yielded, and these were isolated through filtration and purified via recrystallization.
2.1.1. (E)-1-(2-Hydroxyphenyl)-3-(2-Methoxyphenyl)Prop-2-En-1-One (CH-1)
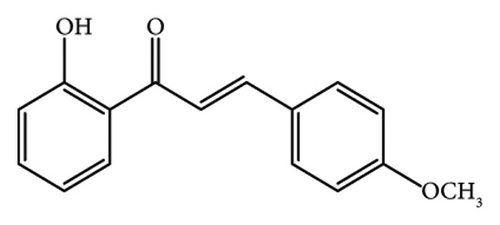
Yellow powder, mp 101°C, yield 89%. The IR (KBr) spectrum of the product (ν cm−1): 3450, 3062, 2958, 1641, 1579, 1483, 1022, and 746.1H NMR (400 MHz, CDCl3) δ: 12.94 (s, 1H, -OH), 8.24 (d, J = 15.60, 1H, allylic), 7.93 (d, J = 8.0, 1H, Ar-H), 7.80(d, J = 15.60, 1H, allylic), 7.65 (d, J = 8.0, 1H, Ar-H), 7.48 (t, J = 8.0, 1H, Ar-H), 7.40 (t, J = 8.4, 1H, Ar-H), 7.01 (d, J = 8.0, 1H, Ar-H), 6.91–6.99 (m, 3H, Ar-H), 3.83 (s, 3H, -OCH3). Anal. Calcd for C16H14O3 (254.28): C, 75.57; H, 5.50; O, 18.87. Found: C, 75.40; H, 5.87; O, 18.51.
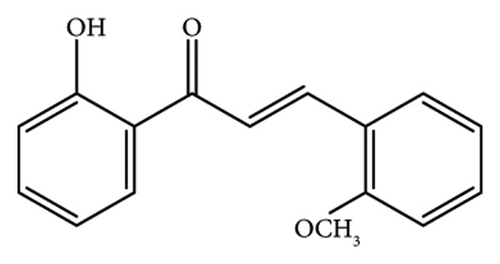
2.1.2. (E)-1-(2-Hydroxyphenyl)-3-(4-Methoxyphenyl)Prop-2-En-1-One, (CH-2)
Light orange powder, mp 85°C, yield 82%. The IR (KBr) spectrum of the product (ν cm−1): 3441, 3030, 2972, 2872, 1635, 1571, 1500, 1026, and 758.1H NMR (400 MHz, CDCl3) δ: 12.94 (s, 1H, -OH), 7.92 (d, J = 15.60, 1H, allylic), 7.88 (d, J = 10.8, 1H, Ar-H), 7.63(d, J = 8.80, 2H, Ar-H), 7.55 (d, J = 15.20, 1H, allylic), 7.48 (t, J = 8.40, 2H, Ar-H), 7.02 (d, J = 8.40, 1H, Ar-H), 6.93 (d, J = 8.80, 2H, Ar-H), 3.84 (s, 3H, -OCH3). Anal. Calcd for C16H14O3 (254.28): C, 75.57; H, 5.50; O, 18.87. Found: C, 75.01; H, 6.07; O, 18.92.
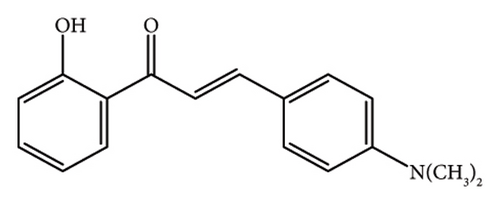
2.1.3. (E)-3-(4-(Dimethylamino)Phenyl)-1-(2-Hydroxyphenyl)Prop-2-En-1-One, (CH-3)
Red powder, mp 162°C, yield 53%. The IR (KBr) spectrum of the product (ν cm−1): 3446, 3062, 2916, 1610, 1525, 1323, 1163, and 765.1H NMR (400 MHz, CDCl3) δ: 13.12 (s, 1H, -OH), 7.93 (d, J = 7.20, 1H, Ar-H), 7.89 (d, J = 8.40, 1H, allylic), 7.61 (d, J = 8.40, 2H, Ar-H), 7.50 (d, J = 15.20, 1H, allylic), 7.46 (t, 2H, Ar-H), 7.01 (d, J = 8.40, 2H, Ar-H), 6.85 (d, 1H, Ar-H), 3.07 (s, 6H, 2 × CH3). Anal. Calcd for C17H17NO2 (267.32): C, 76.38; H, 6.40; N, 5.23; O, 11.96. Found: C, 76.57; H, 6.20; N, 5.03; O, 12.19.
2.1.4. (Z)-3-(4-(Dimethylamino)Phenyl)-1-(2-Hydroxyphenyl)-Prop-2-En-1-One, (CH-4)
Orange powder, mp 159°C, yield 33%. The IR (KBr) spectrum of the product (ν cm−1): 3441, 2916, 1597, 1529, 1332, 1166, and 815.1H NMR (400 MHz, CDCl3) δ: 13.12 (s, 1H, -OH), 7.93 (d, 1H, Ar-H), 7.92 (d, 1H, allylic), 7.60 (d, 1H, allylic) 7.44–7.70 (m, 4H, Ar-H), 6.87–6.99 (m, 3H, Ar-H), 3.07 (s, 6H, 2 × CH3). Anal. Calcd for C17H17NO2 (267.32): C, 76.38; H, 6.40; N, 5.23; O, 11.96. Found: C, 75.98; H, 7.05; N, 5.79; O, 11.17.
2.2. Measuring the Metal-Sensing Properties of Synthesized Chalcone Derivatives
The metal-sensing capabilities of synthesized chalcones were examined concerning various metal ions including Cu2+, Fe3+, Zn2+, K+, Mn2+, and Co2+. For this purpose, different salts such as CuCl2, FeCl3, ZnCl2, KCl, MnCl2, and CoCl2 were dissolved in ethanol. Similarly, chalcones were also dissolved in ethanol. Solutions were meticulously prepared at a concentration of 10−5 M. Subsequently, the prepared solutions of metal ions and chalcones were mixed, and the resulting interactions were investigated utilizing UV-Vis spectroscopy.
3. Results and Discussion
The foundation for the Claisen–Schmidt condensation reaction, a key method for creating carbon–carbon bonds, was laid by this resection in their 1881 research [56]. All the compounds in this research were synthesized followed by Claisen–Schmidt condensation reaction, as shown in Scheme 3, from the o-hydroxyacetophenone and substituted benzaldehyde. This reaction shares a similarity with the aldol condensation reaction. Both processes involve the addition of enolate ions to organic compounds [15]. Although both the aldol condensation and the Claisen condensation reaction involve enolates, their target molecules differ. In the aldol condensation, enolates react with ketones or aldehydes.
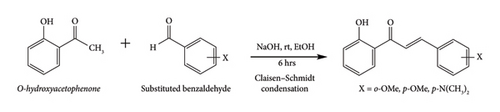
In contrast, the Claisen condensation reaction specifically uses enolates to react with esters [57]. When a base, such as sodium hydroxide, reacts with a ketone (a specific type of carbonyl molecule), it removes a hydrogen atom from the carbon next to the carbonyl group (α-carbon). This deprotonation leads to the formation of an enolate ion, a highly reactive molecule [58].
Adding a base to a carbonyl compound (containing a hydrogen atom on the carbon next to the C=O group) triggers the formation of an enolate ion, a highly reactive molecule. When the starting compound is an aldehyde, this enolate can react with another aldehyde molecule to form two products: either an aldol addition product (where both molecules remain separate) or an aldol condensation product (where a new carbon–carbon bond is formed) [59].
Synthesis and characterization of chalcone isomers: The Claisen–Schmidt condensation reaction proved to be an efficient method for synthesizing the desired chalcone derivatives [38]. The reaction involved the condensation of various substituted acetophenones and benzaldehydes under basic conditions [60]. Modifying the starting materials allowed for the introduction of different functional groups onto the chalcone backbone, leading to a diverse set of compounds (CH-1, CH-2, and CH-3).
- •
TLC: TLC successfully separated the reaction products. The distinct Rf values (0.57 for CH-3 and 0.28 for CH-4) in the n-hexane/ethyl acetate (5:1) solvent system confirmed the isolation of two individual compounds from the reaction involving 4-dimethylaminobenzaldehyde and 2-hydroxyacetophenone (CH-3 and CH-4).
- •
Melting Point: The measured melting points for each chalcone (162°C–163°C for CH-3 and 159°C–160°C for CH-4) provided valuable information for compound identification and purity assessment.
- •
UV-Visible and FTIR Spectroscopy: Functional groups and characteristic absorption spectra of the synthesized compounds were confirmed through FTIR and UV-visible spectroscopy (all UV-visible spectra for CH1–CH4 are given in Figures S1–S4 and FTIR spectra in Figures S5–S8).
- •
1H NMR Spectroscopy: 1H NMR spectroscopy played a crucial role in confirming the structures and stereochemistry of the synthesized chalcones. The coupling constant (J) between the α and β protons of the double bond (CH-1, CH-2, and CH-3) was around J = 15.60 Hz, indicating a trans configuration (opposite sides of the double bond) due to the large J value associated with a dihedral angle of approximately 180°C (all 1H NMR spectra are given in Figures S9–S16). This observation aligns with the expected outcome of the Claisen–Schmidt condensation, which typically favors the thermodynamically stable trans isomer.
However, the analysis of CH-4 revealed a distinct difference. The coupling constant for the corresponding protons in CH-4 was significantly lower (J = 8.40 Hz). This smaller J value suggests a cis configuration (same side of the double bond) for this specific isomer. This finding highlights the interesting observation that the reaction between 4-dimethylaminobenzaldehyde and 2-hydroxyacetophenone yielded a mixture of cis and trans isomers (CH-3 and CH-4, respectively). The different polarities of the isomers likely contributed to their separation using TLC.
Yields and Color: The isolated yields of the synthesized chalcones varied, with CH-3 achieving a yield of 53% and CH-4 at 33%. Further optimization of reaction conditions or purification techniques could potentially improve these yields. The synthesized compounds exhibited distinct colors (red for CH-3 and orange for CH-4, as shown in Table 1). The observed color variations can be attributed to the electronic properties of the substituents present on the aromatic rings.
This study successfully employed the Claisen–Schmidt condensation reaction to synthesize four chalcone derivatives. The characterization techniques confirmed the structures and stereochemistry of the obtained compounds. Notably, the reaction involving 4-dimethylaminobenzaldehyde and 2-hydroxyacetophenone yielded a mixture of cis and trans isomers.
3.1. Metal-Sensing Properties
Metal-sensing properties refer to the ability of certain metal ions or compounds to change color in response to changes in their chemical or physical environment. The metal-sensing response and selectivity of CH-1 was assessed by examining the effect of different metal ions such as Cu2+, Fe3+, Zn2+, K+, Mn2+, and Co2+ using UV-Vis spectroscopy, as shown in Figure 1.
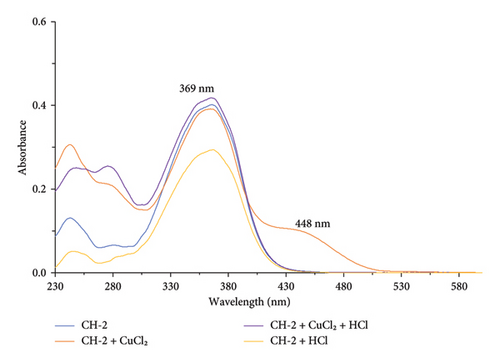
Upon addition of Cu2+ ion with CH-1 solution, the absorption maxima at 366 nm showed a hypsochromic shift by 5 nm, and another absorption band at 308 nm shifted to 276 nm, and a new absorption band appeared at 448 nm, as shown in Figure 2. This indicates that the carbonyl and hydroxyl groups present in the CH-1 molecule may interact with Cu2+ ion. The appearance of the shoulder peaks at 272 nm and 448 nm can be attributed to the formation of different species of the complex, which may have different electronic transitions and absorbance maxima.
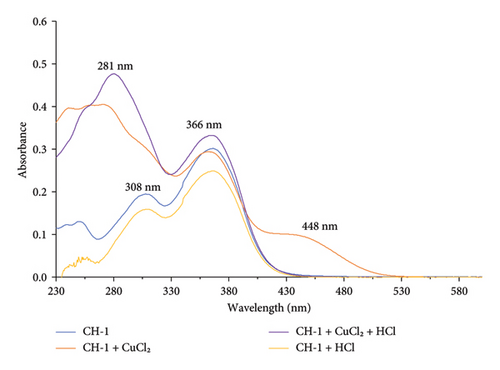
After adding HCl, the new absorption band at 448 nm was diminished and another absorption band showed a red shift from 276 to 281 nm. The decrease in intensity of the absorption band at 448 nm suggests that the protonation may have disrupted the coordination between the Cu2+ ion and the CH-1 molecule, resulting in the dissociation of the complex and the disappearance of the shoulder peak. The red shift of the absorption band from 276 to 281 nm may indicate that HCl interact with CH-1 molecule in the presence of Cu2+ ion. Here, Cu2+ ion acts as a catalyst because in the absence of Cu2+ ion, the absorption maxima of CH-1 remain unchanged with HCl.
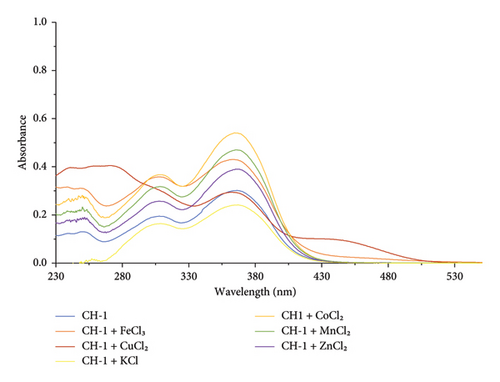
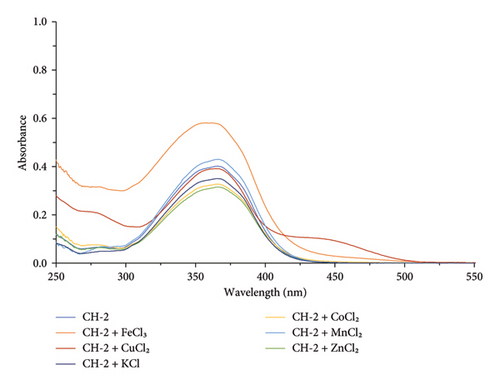
In the presence of other metal ions (K+, Fe3+, Zn2+, K+, Mn2+, and Co2+), CH-1 showed unchanged absorption maxima, as shown in Figure 3. This indicates that there was no interaction between CH-1 and these mentioned metal ions.
The metal-sensing response and selectivity of 1-(2-hydroxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (indicated as CH-2) was assessed by examining the effect of different metal ions using UV-Vis spectroscopy.
CH-2 solution in ethanol showed an absorption maximum at 369 nm which corresponds to a n-π∗ electronic transition within the conjugated system of the molecule. Upon addition of Cu2+ ion with CH-2 solution, the absorption maximum at 369 nm remains unchanged but the absorbance intensity increased with the appearance of the shoulder peak at 448 nm. Here, the Cu2+ ions may coordinate with the carbonyl and hydroxyl groups present in the CH-2 molecule. This coordination can result in a change in the electronic structure of the CH-2, which can increase the absorbance intensity. The appearance of the shoulder peaks at 448 nm can be attributed to the formation of different species of the complex, which may have different electronic transitions and absorbance maxima.
In the presence of other metal ions (K+, Fe3+, Zn2+, K+, Mn2+, and Co2+), CH-2 showed unchanged absorption maxima, as shown in Figure 4. This indicates that there was no interaction between CH-2 and these mentioned metal ions. The interaction in the UV-visible spectrum for CH-2 in the presence of various metal ions is shown in Figure 5.
The metal-sensing response and selectivity of (E)-3-(4-(dimethylamino)phenyl-1-(2-hydroxyphenyl)prop-2-en-1-one (indicated as CH-3) was assessed by examining the effect of different metal ions using UV-Vis spectroscopy, as shown in Figure 6.

CH-3 solution in ethanol showed absorption maxima at 438 nm which corresponds to n-π∗ electronic transition and at 277 nm which corresponds to π-π∗ electronic transition within the conjugated system of the molecule.
After the addition of dilute HCl with CH-3, it showed a hypochromic effect without changing absorption maxima. A possible explanation for the observed spectral changes is that HCl protonated the hydroxyl and amino groups on the CH-3 molecule and formed ammonium and oxonium ions, which decreased the nonbonding electrons. Thus, the absorbance intensity decreased at 438 nm.
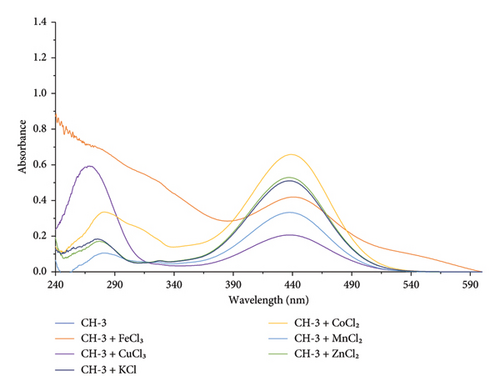
Upon addition of Fe3+ ion with CH-3 solution, the absorption maxima showed a red shift by 10 nm and formed two isosbestic points at 403 and 466 nm, and a new band appeared at 529 nm. This indicates that Fe3+ ions may coordinate with the carbonyl and hydroxyl groups present in the CH-3 molecule. This coordination can result in a change in the electronic structure of the CH-3, which can shift the position of the absorption maximum to a higher wavelength, as shown in Figure 6.
After adding dilute HCl in the mixture, the absorption maxima again shifted back to 438 and formed a new peak at 361 nm. The blue shift in the absorption maxima from 448 to 438 nm indicates that the addition of HCl caused a disruption or breakdown of the complex formed between Fe3+ ions and CH-3. The shift toward shorter wavelengths may be indicative of a decrease in the size of the conjugated system in the molecule. The appearance of a new band at 361 nm suggests the formation of a new product or intermediate in the reaction.
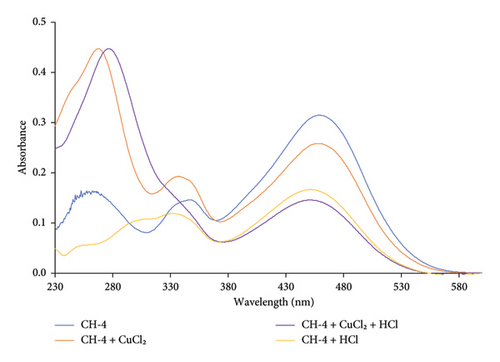
Upon addition of Cu2+ ion with CH-3 solution, the peak at 438 nm decreased its absorbance intensity while the other peak at 277 nm shifted to 269 nm with an increase in its absorbance intensity, as shown in Figure 7.
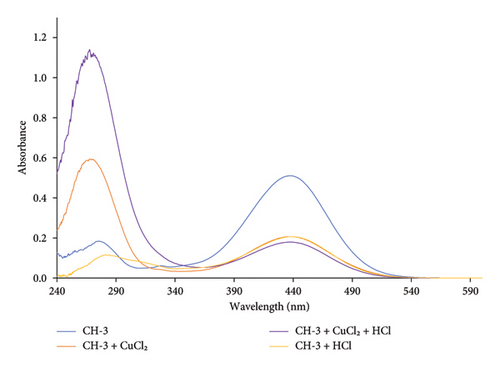
The peak at 438 nm is likely due to the n-π∗ transition of the CH-3 chromophore. When Cu2+ is added to the solution, it can coordinate with the oxygen atom of the hydroxyl group and the nitrogen atom of the amino group. This coordination can cause a reduction in the electron density on the CH-3 chromophore, leading to a decrease in the absorbance intensity at 438 nm. The shift of the peak at 277–269 nm with an increase in absorbance intensity suggests the formation of a new species with a different chromophore. This new species may be a complex between the Cu2+ ion and the CH-3, which has a different electronic structure and absorption spectrum compared to the CH-3 alone.
In the presence of other metal ions (K+, Fe3+, Zn2+, K+, Mn2+, and Co2+), CH-3 does not show any significant change in absorption maxima, as shown in Figure 8. This indicates that there was no interaction between CH-3 and these metal ions.
The metal-sensing response and selectivity of (Z)-1-(2-hydroxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (indicated as CH-4) was assessed by examining the effect of different metal ions using UV-Vis spectroscopy. CH-4 in ethanol solution showed absorption maxima at 458 nm which corresponds to n-π∗ electronic transition and at 347 and 257 nm which corresponds to π-π∗ electronic transition within the conjugated system of the molecule.
Upon introducing dilute HCl to CH-4, a hypochromic effect was evident at 458 nm. The alterations are attributed to the protonation of hydroxyl and amino groups on the CH-4 molecule, inducing modifications in its electronic structure. The protonation process likely leads to the emergence of new functional groups, such as ammonium and oxonium ions, consequently reducing the nonbonding electron density. As a result, the absorbance intensity at 458 nm is diminished, as shown in Figure 9.
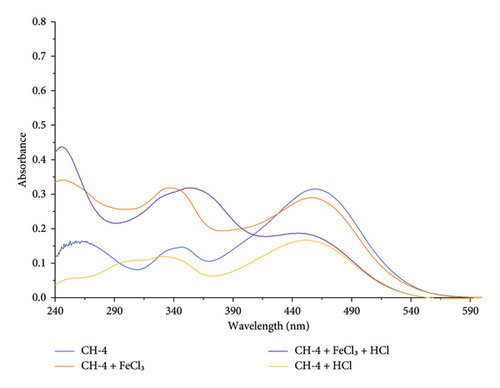
Upon addition of Fe3+ ion with CH-4 solution, absorbance intensity decreased at 458 nm and intensity increased at 347 nm, and the peak at 257 nm shifted to 250 nm. It also forms an isosbestic point at 427 nm. This indicates that Fe3+ ions may coordinate with the carbonyl and hydroxyl groups present in the CH-4 molecule. This coordination can result in a change in the electronic structure of the CH-4 and decrease the nonbonding electrons which results in decreasing the absorbance intensity at 458 nm.
Upon addition of Cu2+ ion with CH-4 solution, the peak at 458 nm decreased its absorbance intensity, while the absorbance intensity increased at 345 nm and another peak at 257 nm shifted to 272 nm with an increase in its absorbance intensity. It also forms an isosbestic point at 371 nm (shown in Figure 10. The peak at 458 nm is likely due to the n-π∗ transition of the CH-4 chromophore. When Cu2+ is added to the solution, it can coordinate with the oxygen atom of the hydroxyl group. This coordination can cause a reduction in the electron density on the CH-4 chromophore, leading to a decrease in the absorbance intensity at 458 nm. The shift of the peak at 257–272 nm with an increase in absorbance intensity suggests the formation of a new species with a different chromophore. This new species may be a complex between the Cu2+ ion and the CH-4, which has a different electronic structure and absorption spectrum compared to the CH-4 alone.
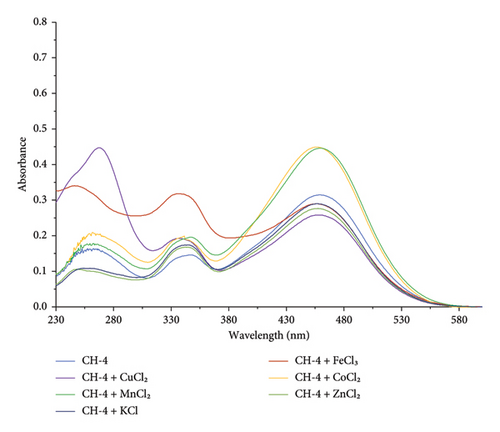
In the presence of other metal ions (K+, Fe3+, Zn2+, K+, Mn2+, and Co2+), CH-4 does not show any significant change in absorption maxima, as shown in Figure 11 This indicates that there was no interaction between CH-4 and these metal ions.
3.2. Ion Selectivity
The chalcone derivatives CH-1 and CH-2 exhibited selective interactions with Cu2+ ions, while CH-3 and CH-4 demonstrated dual-sensing capabilities for Cu2+ and Fe3+ ions in ethanolic solutions. For CH-1, a hypsochromic shift in the absorption maxima from 366 to 361 nm and the appearance of a new band at 448 nm indicated Cu2+ coordination with its carbonyl and hydroxyl groups. Similarly, CH-2 maintained its primary absorption maximum at 369 nm upon Cu2+ addition but showed an increased absorbance intensity and a shoulder peak at 448 nm, signifying complex formation. CH-3 displayed distinct redshifts and intensity changes; Fe3+ ions induced a new peak at 529 nm, along with isosbestic points at 403 and 466 nm, indicating Fe3+ complexation. Cu2+ ions caused the 438-nm peak to decrease in intensity and a shift from 277 to 269 nm, revealing electronic reorganization. CH-4 exhibited reduced absorbance at 458 nm for both Cu2+ and Fe3+ ions, with a redshift to 347 nm upon Fe3+ interaction, and Cu2+ addition shifted a peak from 257 to 272 nm with increased intensity, forming an isosbestic point at 371 nm. These findings highlight the metal ion selectivity and dual-sensing potential of the chalcone derivatives.
3.3. Computational Results
In order to perform computational calculations, various properties of the compound, such as its optimized structures, highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies (HOMO-LUMO energies), and molecular electrostatic potential (MEP) surface were investigated using the Gaussian 16W rev. B.01 [61] computation software. To achieve this, a theoretical method called DFT was employed. Specifically, the B3LYP functional was utilized along with the Lan2DZ basis set.
3.4. Frontier Molecular Orbitals
The HOMO and the LUMO are considered as the frontier orbitals of a molecule. Frontier molecular orbital analysis is a useful tool for predicting electronic transitions and assessing the reactivity of conjugated systems [62]. This approach allows for convenient examination of the system’s electronic properties and its potential for chemical reactions.
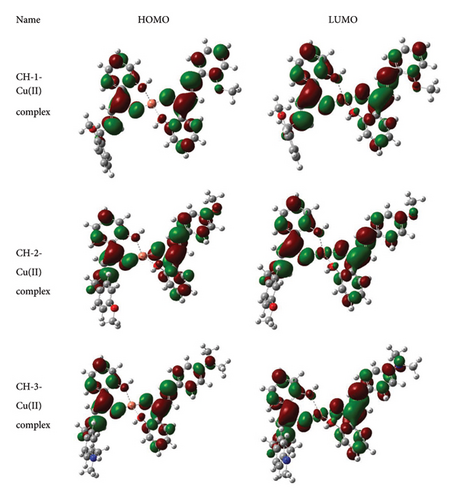
As shown in Figure 12, HOMO is located in the whole region except for one Ph ring and Cu atom. On the other hand, LUMO is less cloudily located in both Ph ring as well as Cu atom. Therefore, it exposed that intramolecular charge transfer predominantly happened during excitation of the molecules.
| Parameters | CH 1-Cu complex | CH 2-Cu complex | CH 3-Cu complex |
|---|---|---|---|
| EHOMO (eV) | −3.078 | −3.188 | −2.941 |
| ELUMO (eV) | −2.436 | −2.550 | −2.314 |
| Band gap, ∆E (eV) | 0.642 | 0.638 | 0.626 |
| Chemical hardness, h | 0.321 | 0.319 | 0.313 |
| Global softness, σ | 3.115 | 1.567 | 3.194 |
| Ionization potential, I | 3.078 | 3.188 | 2.941 |
| Electron affinity, A | 2.436 | 2.550 | 2.314 |
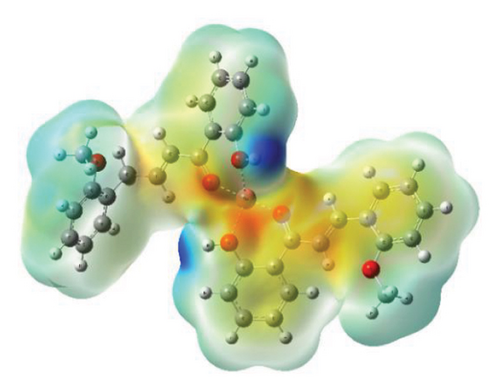
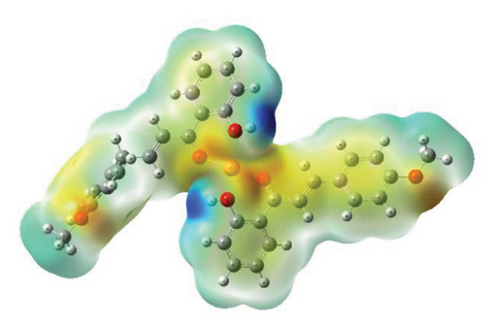
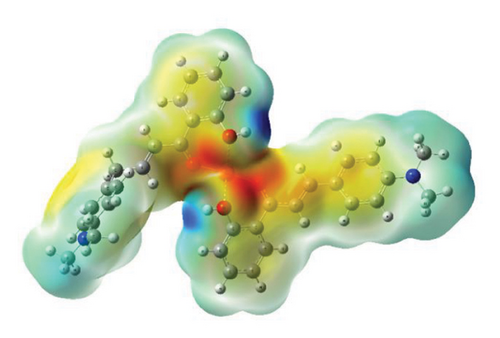
3.5. MEP
The MEP maps provide valuable insights into the three-dimensional charge distribution of molecules, revealing regions that can act as electron acceptors or electron donors [64]. These regions are depicted using different colors: red represents negative potential areas, blue represents positive potential areas, and green represents regions of zero potential [65].
MEP calculations of compounds were carried out using the DFT B3LYP/Lan2DZ method. Analyzing the MEP maps, as shown in Figure 13, it is evident that the highest negative potential regions align with the positions of oxygen (O) atoms, while the lowest negative potential regions correspond to the positions of hydrogen (H) atoms of the hydroxyl group (Cartesian coordinates of optimized structures are given in Tables S1–S3).
4. Conclusions
This study introduces a novel synthetic approach for obtaining both cis- and trans-isomers of o-hydroxychalcone derivatives. By carefully controlling reaction conditions, we successfully synthesized four chalcones (CH-1 to CH-4) using an optimized Claisen–Schmidt condensation reaction. The selective synthesis of these isomers was confirmed through the analysis of allylic protons using 1H NMR, and UV-Vis, FTIR, and elemental analyses were done for synthesized compounds. The data obtained from these analyses provided confirmation of the structures of the synthesized compounds. Additionally, the compounds’ structures were optimized using the B3LYP method, employing a Lan2DZ basis set. The metallochromic properties of the synthesized compounds were explored in the presence of various metal ions, including Cu2+, Fe3+, Zn2+, K+, Mn2+, and Co2+. The findings revealed that CH-1 and CH-2 displayed interactions with Cu2+, resulting in alterations in wavelength and forming new absorption bands in the UV-visible range. On the other hand, the spectral variation observed for CH-3 and CH-4 indicated their ability to detect both Cu2+ and Fe3+ ions in an ethanolic solution. Consequently, the metal-sensing properties exhibited by these chalcone compounds make them potentially useful in the development of test kits for the detection of Cu2+ and Fe3+ ions. These findings highlight the potential applications of these chalcones in selective metal ion sensing. Further exploration of their properties and optimization could lead to exciting advancements in this field.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was financially supported by Khulna University of Engineering & Technology.
Acknowledgments
The authors thank Khulna University of Engineering and Technology for financial support and necessary facilities.
Supporting Information
The UV-visible spectra for CH1–CH4 can be found in Figures S1–S4, the FTIR spectra in Figures S5–S8, and 1H NMR spectra in Figures S9–S16. Cartesian coordinates of optimized structures are in Tables S1–S3.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




