Optimising the Decarboxylation of Anacardic Acid by Exploring the Temperature Effects
Abstract
Cashew nut shell liquid (CNSL) is one of the raw materials extracted from cashew nut shells (CNS). This liquid can be processed to produce cardanol through the decarboxylation of anacardic acid, a significant isolate from CNSL. The decarboxylation process involves heating anacardic acid to a favourable reaction temperature. This study investigated the ideal temperature required for this process. Fourier-transform infrared (FTIR) spectroscopy characterised CNSL and cardanol isolates. Anacardic acid was separated, purified and heated to temperatures ranging from 140°C to 152°C after being isolated from CNSL. At each temperature, the decarboxylation yield was calculated. The results indicated that a temperature of 145°C is optimal for the decarboxylation of anacardic acid at standard pressure, with a maximum yield of 66% observed. These findings contrast with other studies, which suggested optimal temperatures of 140°C and 180°C–200°C for the decarboxylation of anacardic acid. The main advantage of the identified temperature in the anacardic acid decarboxylation reaction is the increased yield and overall process efficiency by promoting the synthesis of desired products over potential side reactions (selectivity). Furthermore, reactions at lower temperatures can be more energy-efficient than those at higher temperatures, thus reducing costs in industrial processes. Lastly, it is generally safer to work with lower temperatures, as there is a reduced risk of thermal degradation and thermal runaway reactions.
1. Introduction
Agricultural wastes and by-products are the most adaptable bio-based renewable materials, especially in the search for functional materials and chemicals derived from renewable resources. Accordingly, cashew nut shells (Anacardium occidentale) are typical agricultural by-products of cashew nut processing that produce a versatile liquid known as cashew nut shell liquid (CNSL). This dark brown, viscous liquid is found inside the honeycomb structure of the cashew nut shell [1]. CNSL comprises phenolic compounds (anacardic acid, cardanol, cardol and methyl cardol) with a long unsaturated C15 chain substitution at the meta position. The functional groups in these natural lipids, including alkene, phenol and carboxylic acid, allow for the conversion of the constituents of CNSL into a variety of substances, and speciality chemicals [2].
CNSL has versatile uses, including forming special polymers such as fire retardant polymers, resin synthesis, additives and varnishing agents [3]. CNSL consists of four major phenolic components (Figure 1): anacardic acid, cardanol, cardol and 2-methyl cardol. The composition of these constituents depends on the method of extraction, whether natural (cold extraction) or technical (hot extraction). The natural CNSL contains anacardic acid (46%–70%), cardanol (5%–22%), cardol (15%–31%) and a trace amount of methyl cardol and polymeric materials. The technical CNSL encompasses (1%-2%) anacardic acid, (60%–95%) cardanol, (4%–19%) cardol, (1.2%–4.1%) 2-methyl cardol and a trace of polymeric materials.
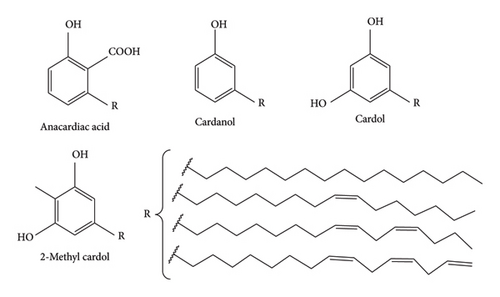
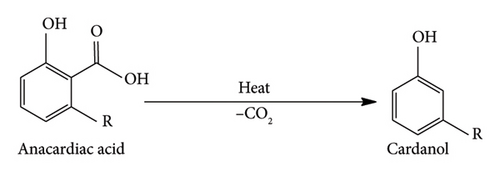
Moreover, phenolic groups in these compounds enable change through esterification processes and interactions with aldehydes such as formaldehyde [1]. Scientists have established that CNSL is a naturally occurring, affordable and renewable source of phenolic compounds, exhibiting adhesive qualities [6]. Among the four components, cardanol is the most adaptable bio-based renewable material that can replace phenol in its reaction with equal or superior performance to phenol [4]. Cardanol’s presence of a phenolic hydroxyl group in its structure led to its adoption as a replacement for phenol in phenolic-based adhesives [3, 7]. Furthermore, compared to other phenolic compounds, cardanol has been used industrially without causing contamination since it has a low volatilisation rate, a high boiling point and no objectionable aroma [6]. The synthesis of cardanol relies on the decarboxylation reaction of anacardic acid. It has been reported that this reaction occurs at a temperature between 180°C and 200°C [5, 6, 8]. On the flip side, few studies have reported that it appears at 140°C for 1 h [4]. A comparison of the reported temperature values indicates that the variation is significant; hence, exploring the effects of temperature on the decarboxylation of anacardic acid is an exciting research topic. Therefore, this study aimed to isolate cardanol from anacardic acid through a decarboxylation process at varying temperatures to determine the optimal temperature to maximise cardanol yield.
2. Materials and Methods
2.1. Materials, Reagents and Instrumentation
The cashew nut shells, discarded as waste after kernel removal, were sourced from the Xinnuo International Ltd factory located in Mtwara, a port town on southern Tanzania’s coastline. Before analysis, all chemicals and reagents were obtained from Sigma-Aldrich (South Africa) and prepared to the required concentration. A single reflection ATR accessory of the Fourier transform infrared (FTIR spectrophotometer model manufactured in 2011 by Bruker Optics GmbH) was used to characterise CNSL and cardanol in the laboratories at the Nelson Mandela African Institution of Science and Technology (NM-AIST) in Arusha, Tanzania.
2.2. Extraction of CNSL




2.3. Isolation of Anacardic Acid From CNSL
Anacardic acid (2-hydroxy-6-[(8Z,11Z)-8,11-pentadecadien-1-yl] benzoic acid) was isolated through the calcium anacardate route, and cardanol was subsequently obtained by decarboxylating the isolated anacardic acid. To achieve this, approximately 50 g of the CNSL was dissolved in 300 mL of a 5% aqueous methanol solution, and 5 g of calcium hydroxide was added slowly while stirring the mixture for 20 min [5]. The reaction temperature was raised and maintained at 50°C for 3 h. The formed precipitates were filtered using a suction filtration pump. During the filtering process, they were rinsed three times with 500 mL of methanol solution to ensure that other compounds, aside from calcium anacardate salt, were washed away. Finally, the precipitates were dried in an oven overnight and weighed on an analytical balance.
About 35 g of dried calcium anacardate salt was transferred to a beaker containing a 450-mL mixture of 6 M hydrochloric acid (250 mL) and ethyl acetate (200 mL) and stirred until all the precipitates dissolved. The resulting solution was then transferred to a separating funnel, shaken well and left for 1 h for the layers to form. After an hour, the organic layer was extracted, washed with 200 mL of distilled water, dried over anhydrous sodium sulphate, filtered and concentrated under reduced pressure using a rotary evaporator. The characterisation of anacardic acid was performed using FTIR with similar procedures described for CNSL.
2.4. Determination of the Density of Anacardic Acid
The density of anacardic acid was determined using a hydrometer calibrated at 29°C before measurement. The anacardic acid solution was taken, ensuring it was at the desired temperature. The hydrometer jar was then placed on a level surface and carefully filled with the liquid sample to allow any bubbles to escape. The hydrometer was lowered into the liquid jar, ensuring it was free to move and not touching the container’s side. Finally, the scale on the hydrometer was read at the point where the liquid surface (meniscus) intersects with the hydrometer scale. This reading corresponded to the liquid density.
2.5. Determination of the pH of Anacardic Acid
A digital pH meter was used to measure the pH of anacardic acid. The meter was calibrated using a standard buffer solution with known pH values of 4.0 for acidic pH and 7.0 for neutral pH. Shortly after calibration, it was employed to measure the pH of anacardic acid.
2.6. Determination of the Viscosity of Anacardic Acid
A Krebs Stormer-BDG 186 model viscometer was used to measure the viscosity of anacardic acid. The viscometer was calibrated using standard reference fluids with known viscosities, following the manufacturer’s instructions. Since viscosity can be temperature-dependent, the sample solution was thoroughly homogenised before measurement. The anacardic acid sample was placed precisely in the viscometer setup, rotation commenced, and measurements were recorded.
2.7. Synthesis of Cardanol
The anacardic acid obtained was converted into cardanol through a decarboxylation reaction, as shown in Scheme 1. Since a significant number of works in the literature have established that the decarboxylation reaction occurs at a temperature between 180°C and 200°C for two hours, and a few reported that it happens at 140°C, this study tested both higher and lower temperature ranges for decarboxylation to unveil the existing contradictions. Initially, the decarboxylation at temperatures between 180°C and 200°C at intervals of 5°C was studied, followed by the decarboxylation of anacardic acid at different temperatures between 140°C and 152°C at intervals of 1°C. The decarboxylation was conducted for one and a half hours at each temperature, with the resulting yields recorded. The optimal temperature for the maximum yield of cardanol was determined from these temperatures by extrapolation through a temperature yield curve. Subsequently, the optimal temperature was tested in the laboratory for its performance efficiency.
2.8. Determination of the Boiling Point of Cardanol
Approximately 2 mL of cardanol solution was initially placed in the boiling tube. The capillary tube was inserted into the boiling tube with the end closed upwards. The boiling tube was then attached to the thermometer. A 100-mL beaker was filled with paraffin oil and placed on the hot plate. The boiling tube and thermometer assembly were immersed in the beaker, ensuring that the boiling tube was halfway submerged in the oil. The hot plate was allowed to heat slowly, and as the liquid neared its boiling point, a few bubbles were observed emerging from the end of the capillary tube. The hot plate was turned off once the stream of bubbles appeared. Finally, when the liquid began to flow into the capillary tube, the temperature of the liquid was recorded as the boiling point of the anacardic acid at 1 atmosphere.
2.9. Determination of Density, pH and Viscosity of Cardanol
The density, pH and viscosity of cardanol were determined using the same procedures outlined in Sections 2.4, 2.5 and 2.6, respectively.
3. Results and Discussion
3.1. The Yield of CNSL
In the 4000 g of CNS soaked in 12,000 mL of acetone, 1600 mL, equivalent to 1475.56 g of liquid, was recovered. The percentage yield of the CNSL was 36.89% (w/w). This yield is relatively higher than the 20%–35% by weight of the CNS documented in other studies [11, 12]. The variation in yield can be attributed to several factors, including the geographical location where the cashew nut shells were sourced and the extraction protocols. Specifically, the cashew nut shells used in this study were obtained from the Mtwara region of Tanzania, known for their favourable climatic conditions and fertile soil, which contribute to the higher oil content of the nuts. Additionally, the homogeneity achieved through continuous agitation with an orbital shaker machine ensured the solvent was evenly distributed and in consistent contact with all parts of the ground cashew shells. This reduced the chances of local saturation and enhanced overall extraction efficiency. Therefore, the combination of ground CNS, shaking the mixture and geographical location may account for the higher liquid yield observed in this study.
3.2. FTIR Spectra for CNSL
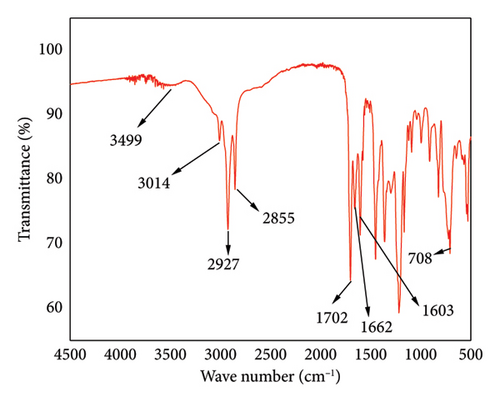
The FTIR spectrum for CNSL is shown in Figure 3. The strong absorption bands at 3499 cm−1 indicated the presence of phenolic OH-stretch [13]. Other prominent bands are attributed to the unsaturated hydrocarbon portion, C-H asymmetric and symmetric stretching vibrations of the most extended alkyl side chains at 3014 cm−1, 2927 cm−1 and 2855 cm−1, respectively. The sharpest peak at 1702 cm−1 revealed the C=O vibration of the carboxylic acid. The additional C=C aromatic stretching bands were observed at 1662 cm−1 and 1449 cm−1. The sharp peaks at 992 cm−1 and 911 cm−1 indicated the presence of conjugated cis and trans double bonds and a terminal vinyl group, respectively [13]. These findings on the FTIR analysis agreed with other research works by Kyei et al. [10], Pandian et al. [14] and Gaitán-Jiménez et al. [15].
The yield of calcium anacardate salt was about 36.7 g, equivalent to 73% of the initial CNSL. The yield of anacardic acid isolate was 29.5 g. This yield is equivalent to 59% of the initial CNS and aligns with previous studies that suggested natural CNSL contains approximately 60%–65% anacardic acid [16, 17, 18]. The calcium anacardate salt appeared as a brown cake (Figure 4(a)), and pure anacardic acid was obtained as a brownish liquid (Figure 4(b)).

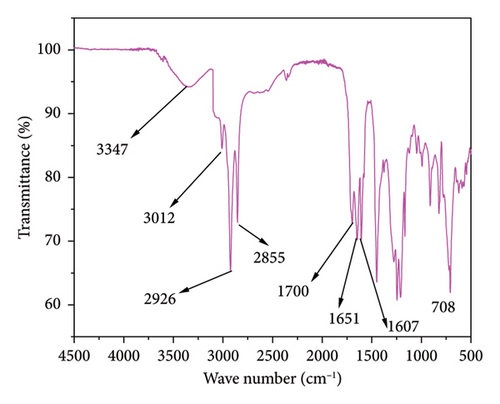
The FTIR major peaks for anacardic acid are presented in Figure 5. The broad peak at 3347 cm−1 corresponds to the hydroxyl group (OH) stretching. Other prominent bands at 3012, 2926 and 2855 cm−1 are attributed to the unsaturated hydrocarbon portion, C-H asymmetric and symmetric stretching vibrations of the most extended alkyl side chains, respectively [13]. The absorption peak at 1700 cm−1 is the characteristic vibration of the C=O of carboxylic acid. This absorption band was also seen in the study by Ribeiro et al. [16].
3.3. Decarboxylation of Anacardic Acid
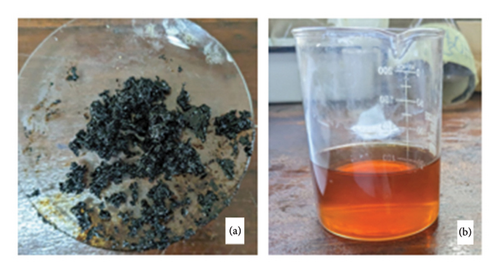
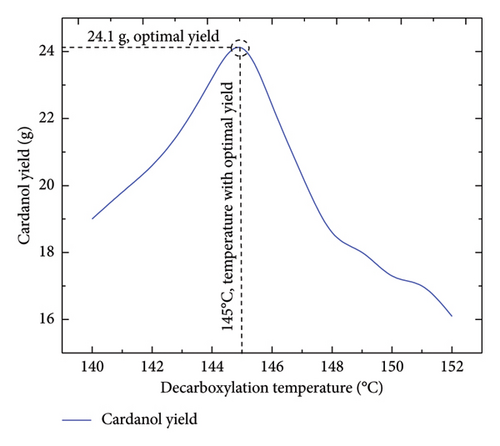
Initially, the decarboxylation of anacardic acid was performed at temperatures between 180°C and 200°C in increments of 5°C to ascertain the efficiency of this temperature range, as reported in various pieces of literature. Some dark brown residues are formed within this temperature range, as depicted in Figure 6(a). This was not the desired product, as cardanol was anticipated to be a liquid with a characteristic colour. After obtaining this product, a second round of decarboxylation was conducted at different temperatures between 140°C and 152°C in increments of 1°C. A curve for temperature versus yield was established to extrapolate the favourable temperature for optimal yield. The optimal yield for acid decarboxylation occurred at 145°C, as shown in Figure 7. The decarboxylation product at 145°C yielded cardanol, a viscous pale brown liquid with a mild sweet odour (Figure 6(b)).
3.4. Physical Properties of Anacardic Acid and Cardanol
Table 1 shows the physical properties of the two components, including their density and boiling point.
| Property | Anacardic acid | Cardanol | ||
|---|---|---|---|---|
| Experimental | Theoretical | Experimental | Theoretical | |
| Appearance | Dark brown | Dark brown | Pale brown | Brown |
| Density | 0.97 ± 0.06 g/cc | 1.0 ± 0.1 g/cc | 0.89 ± 0.06 g/cc | 0.93 g/cc |
| pH | 3.0 | 3.2 | 7.5 | 7.8 |
| Viscosity | 44.9 Cps | 40–57 Cps | 46.4 Cps | 40–60 Cps |
The density of anacardic acid obtained in this study was close to the theoretical value of 1.0 ± 0.1 g/cm3 [19]. This implies that the anacardic acid obtained in this study was relatively pure at 97% based on density. Similarly, the density of cardanol was also near the theoretical value of 0.93 g/cm3 [20], indicating that it was relatively pure at 96% based on density. In addition, the measured boiling point for cardanol was 222°C, which is also close to the theoretical value reported in various literature studies of 225°C [21].
3.5. Establishing Optimal Temperature for the Decarboxylation of Anacardic Acid
Figure 7 illustrates how the yield of cardanol increases with rising temperature, starting at 140°C. However, after the reaction reached the maximum yield of cardanol at the optimal temperature of 145°C, there was a sharp decrease in the cardanol yield from 146°C to 152°C because higher temperatures alter the reaction kinetics, potentially favouring side reactions [22, 23]. These adverse reactions lead to the formation of various products or by-products. The longest aliphatic side chain may undergo changes, such as double bond breaking, rearrangements or fragmentations, resulting in products other than cardanol. Thus, at an optimal temperature of 145°C, a maximum yield of 24.1 g, or 66% of the starting material, was achieved.
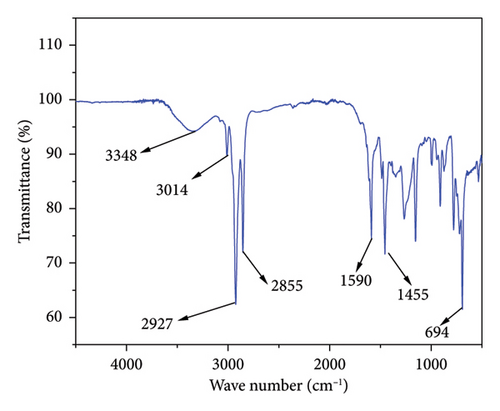
At the established optimal temperature, the product is evidenced by the FTIR spectra shown in Figure 8. An extensive absorption band at 3348 cm−1 is associated with the presence of the phenolic hydroxyl (OH) group. In addition, peaks at 3014, 2927 and 2855 cm−1 correspond to the unsaturated hydrocarbon part and C−H asymmetric and symmetric stretching vibrations of the most extended aliphatic side chains, respectively [14]. The cardanol spectra were found to lack a peak at 1700 cm−1, the characteristic vibration of the carboxylic acid’s C=O [24]. The absence of this peak in cardanol proves that the decarboxylation process was successful. As reported in the literature, carbon dioxide was removed as carbon dioxide.
The experimental findings for the decarboxylation of anacardic acid at 145°C are also close to those advocated by Buckner et al. [4], who conducted it at 140°C. The decarboxylation temperature between 180°C and 200°C for two hours, as supported by other researchers [5, 6, 8], resulted in dark solid materials (Figure 6(a)) instead of a brown viscous liquid. In contrast, the decarboxylation at 145°C yielded cardanol.
4. Conclusion
This study reports the optimal temperature for synthesising cardanol from anacardic acid via a decarboxylation reaction. The optimal temperature for this reaction is 145°C, yielding better results than the previously reported temperatures of 140°C and 180−200°C. Future research should focus on the optimal temperature suggested by these findings for the decarboxylation of anacardic acid, rather than relying solely on the temperatures reported in earlier studies. This approach will help researchers conserve energy resources, reduce production costs and save time while maximising yield.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Eva G. Nyoni: sampling, investigation, methodology, writing an original draft, project administration and funding acquisition. Aldo J. Kitalika: conceptualisation, investigation, methodology and review and editing. Petro E. Mabeyo: conceptualisation, investigation, methodology and review and editing.
Funding
This study was funded by Dar es Salaam University College of Education (DUCE) through an MSc-merit Scholarship for Best Female Student 2021.
Acknowledgements
The authors wish to thank the University of Dar es Salaam (UDSM-Merit Scholarship) for financial support and the Nelson Mandela African Institution of Science and Technology (NM-AIST) for the FTIR characterisation of the samples; special thanks go to Mr. Wilson Mahene and Ms. Geni Ng’helembi from NM-AIST for further technical assistance.
Open Research
Data Availability Statement
The data used to support the findings of this study are included in the article.




