Antimicrobial Activity of Gels Supplemented With Nanoparticles as Intracanal Medication in Endodontics: A Systematic Review and Meta-Analysis of In Vitro, In Vivo, and RCT Studies
Abstract
Background: The compalete eradication of microorganisms within the root canal system remains a challenge in endodontic treatment. Gels supplemented with nanoparticles, particularly silver nanoparticles (AgNPs), have shown promise in enhancing antimicrobial efficacy. This systematic review and meta-analysis aimed to evaluate the antimicrobial activity of these nanoparticle-supplemented gels as intracanal medication in endodontics.
Methods: A comprehensive search was conducted in PubMed, Web of Science, and SCOPUS databases up to September 2024 using specific keywords related to nanoparticles, gels, and antimicrobial activity in endodontics. Only studies published in English were included. In vitro, in vivo, and randomized clinical trials (RCTs) comparing nanoparticle gels with conventional antimicrobial agents were selected based on predefined inclusion and exclusion criteria. Data extraction and risk of bias assessment were performed by independent reviewers. A random-effects model was employed for the meta-analysis to estimate the overall antimicrobial effectiveness of the nanoparticle-supplemented gels. Heterogeneity among studies was evaluated using the I2 statistic.
Results: Out of 991 identified records, 752 were screened after duplicate removal, and 24 articles underwent full-text review. Six studies met the eligibility criteria and were included in the final analysis, comprising three in vitro studies, one in vivo study, and RCTs. The meta-analysis revealed that gels supplemented with nanoparticles exhibited a statistically significant positive antimicrobial effect in in vitro studies (pooled effect size: 5.96; 95% CI: [0.92, 10.99]). However, high heterogeneity (I2 = 96%) was observed. In contrast, the meta-analysis of RCTs indicated no significant overall antimicrobial effect (combined effect: 1.04; 95% CI: [−0.06, 2.15]; I2 = 76%). Funnel plot asymmetry suggested potential publication bias.
Conclusions: Gels supplemented with nanoparticles show potential antimicrobial efficacy in vitro, but the evidence from clinical trials is inconsistent. High heterogeneity and potential publication bias indicate the need for more standardized clinical research to accurately assess the effectiveness of nanoparticle-supplemented gels in endodontic treatment.
1. Introduction
Endodontics, a specialized branch of dentistry, deals with diagnosing and treating diseases affecting the dental pulp and periapical tissues [1]. One of the significant challenges in endodontic treatment is the complete eradication of microorganisms within the root canal system [2]. Studies have shown that while mechanical and chemical cleaning procedures are effective, they often fail to achieve total microbial elimination, including bacteria, fungi, and viruses [3]. The mechanical debridement of the root canal system, despite its precision, can leave microorganisms behind, particularly in complex anatomical areas like isthmuses, lateral canals, and apical deltas. These areas are difficult to reach and clean effectively with mechanical tools alone. In mechanical disinfection, it is essential to remove infected tissue while preserving as much of the root canal’s original shape and structure as possible to maintain the dental organ’s structural integrity. This conservation is crucial because excessive removal of dentinal tissue can weaken the tooth, making it more susceptible to fracture. However, the need to preserve tooth structure can also limit the extent of mechanical cleaning, potentially leaving behind bacteria and other pathogens [4, 5].
To address these limitations, the use of chemical disinfection through intracanal medication becomes essential. As a result, various intracanal medications, such as calcium hydroxide, have been investigated for their disinfection properties [2]. Calcium hydroxide is widely used due to its high pH and antimicrobial effect, but research continues to seek more effective and biocompatible alternatives [6].
Nanomedicine has gained interest in this context because nanoparticles can penetrate hard-to-reach areas and release therapeutic agents in a controlled manner [7]. Silver nanoparticles (AgNPs) are among the most studied, known for their broad-spectrum antimicrobial properties, low toxicity at appropriate concentrations, and biofilm prevention capabilities. Their effectiveness against a range of microorganisms, including multi-drug-resistant bacteria, makes them a promising alternative for intracanal medication in endodontics [8].
Incorporating nanoparticles into gels offers several advantages. Their high specific surface area allows for more efficient interaction with microorganisms in the root canals. Additionally, their nanoscale size enables penetration into dentinal tubules, and these areas are often inaccessible with conventional irrigation techniques and other medications [9]. These properties can enhance the antimicrobial efficacy of gels, reducing the failure rates of endodontic treatments [10–12].
Several studies, particularly in vitro, have shown that these nanoparticle-supplemented gels can effectively inhibit the growth of microorganisms like Enterococcus faecalis, a common bacterium associated with persistent endodontic infections. Moreover, recent research suggests that combining AgNPs with other antimicrobial agents can produce a synergistic effect, further enhancing disinfection [10].
Despite these advances, using nanoparticles in clinical endodontics faces challenges. Concerns about potential cytotoxicity to human cells, microbial resistance, and the long-term stability of nanoparticle-supplemented gels necessitate further research. Evaluating the biocompatibility and establishing safe, effective clinical protocols remain essential. Exploring effective and biocompatible alternatives in endodontic treatment is imperative due to the limitations of mechanical methods in achieving complete disinfection. The potential of nanoparticle-supplemented gels as intracanal medication in endodontics aligns with the ongoing need to enhance microbial eradication capabilities and develop safer, more effective treatment modalities for managing root canal infections. These fundamentals justify the detailed investigation of the antimicrobial activity of nanoparticle-enhanced gels, highlighting the importance of integrating advanced chemical agents in intracanal medication to complement mechanical debridement in endodontic therapy [13].
The objective of this article was to evaluate the antimicrobial activity of gels supplemented with nanoparticles as intracanal medication in endodontics. This review covers the existing literature, including in vitro, in vivo, and randomized clinical trial (RCT) studies comparing the efficacy of these gels with other conventional intracanal medications. By exploring the effectiveness and mechanisms of these nanoparticle-supplemented gels, the study aims to inform future research and clinical practices in endodontic disinfection.
2. Materials and Methods
A comprehensive search was performed in electronic databases, including PubMed, Web of Science, Google Scholar, and SCOPUS, up to September 2024, using key terms such as (Figure 1) (“nanoparticles”) AND (“gels”) AND (“endodontics” OR “root canal” OR “dental treatment”) AND (“antimicrobial” OR “antibacterial” OR “biofilm”) AND (“intracanal medication” OR “root canal medicament” OR “intracanal disinfectant” OR “endodontic medicament”)
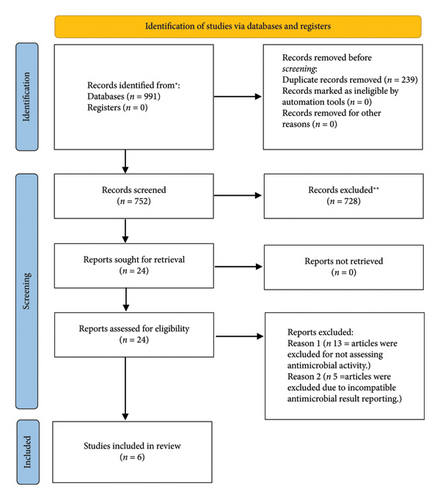
Only articles published in English were included, while gray literature was excluded to minimize the risk of publication bias. No similar systematic reviews or meta-analyses were found in PROSPERO. The PROSPERO registration number of this paper is CRD42024598368.
2.1. Inclusion Criteria
- •
In vitro, in vivo, and RCT studies on the antimicrobial effectiveness of gels with nanoparticles as intracanal medication in endodontics.
- •
Use of gels with nanoparticles compared to conventional antimicrobial agents.
- •
Measurement of antimicrobial activity using standardized methods.
- •
Articles in peer-reviewed journals.
- •
Articles in English.
- •
Published up to September 2024.
- •
Full-text available.
2.2. Exclusion Criteria
- •
Reviews, case reports, opinions, etc.
- •
Studies not addressing antimicrobial activity.
- •
Articles in languages other than English.
- •
Articles with unclear methodology.
- •
Articles without peer review.
2.3. Elimination Criteria
- •
Duplicate articles found in different databases.
- •
Nonquantitative results or nonextractable formats for meta-analysis.
- •
P (Population): Adult patients with root canal infection.
- •
I (Intervention): Use of intracanal gels supplemented with nanoparticles.
- •
C (Comparison): Use of calcium hydroxide or another intracanal medication commonly used in endodontics.
- •
O (Outcome): Reduction in intracanal bacterial load.
PICO Question: “In adult patients with root canal infection (P), is the use of intracanal gels supplemented with nanoparticles (I) more effective than the use of calcium hydroxide or another commonly used intracanal medication in endodontics (C) in reducing intracanal bacterial load (O)?”
Four independent reviewers screened titles and abstracts to identify relevant studies. Duplicates were manually removed. Then, a full-text review was conducted to apply the inclusion and exclusion criteria. Only studies that met all eligibility criteria were included in the systematic review and meta-analysis.
Two reviewers independently performed data extraction, collecting information on study design, types of nanoparticles used, gel concentrations, methods for evaluating antimicrobial activity, and reported outcomes. Discrepancies were resolved by consensus or by a third reviewer.
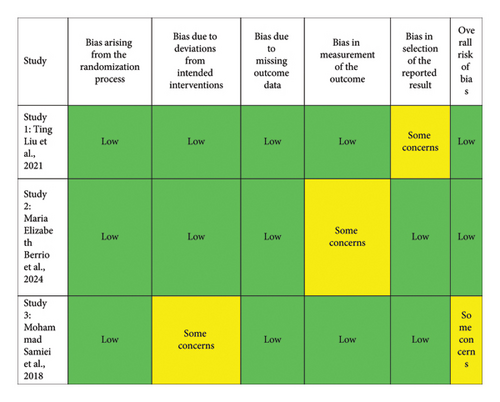
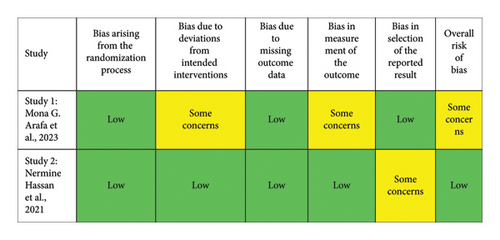
A risk of bias assessment was conducted on the included studies using the Risk of Bias 2.0 (RoB 2.0) tool for clinical trials and an adapted version for in vitro studies. This tool evaluates several domains of risk of bias, including (1) bias arising from the randomization process, (2) bias due to deviations from intended interventions, (3) bias due to incomplete outcome data, (4) bias in measurement of the outcome, and (5) bias in selection of the reported results. Each domain was classified as “low,” “some concerns,” or “high” based on the methodological quality and the information available for each study. The evaluation was carried out for a total of five studies.
A meta-analysis was performed using a random-effects model to estimate the average effectiveness of nanoparticle-enhanced gels compared to other conventional antimicrobial agents. Heterogeneity among studies was assessed using the Chi-square test and the I2 statistic. The meta-analysis and associated funnel plots were conducted using OpenMeta software, a robust and widely used software for meta-analytic studies. OpenMeta was chosen for its flexibility in handling diverse datasets, user-friendly interface, and ability to support various statistical models. The WebPlotDigitizer application was employed to extract data from graphs when results were not presented in clear numerical formats.
3. Results
The systematic search identified a total of 991 records from electronic databases. After removing 239 duplicates, 752 unique records were screened based on titles and abstracts. During this screening phase, 728 records were excluded for not meeting the inclusion criteria, such as lacking relevance to the antimicrobial activity of nanoparticle-supplemented gels in endodontics. This left 24 records eligible for a full-text review. Subsequently, the full-text assessment of these 24 articles resulted in 8 studies that were further evaluated for inclusion. Of these, 2 studies were excluded: one did not specifically evaluate antimicrobial activity, and the other presented its antimicrobial results in a format that was not compatible with the quantitative analysis required for the meta-analysis. Following the application of the inclusion and exclusion criteria, 6 studies were ultimately included in the review. These studies comprised different research designs, with 3 being in vitro studies (Table 1), 1 mixed study (in vitro and in vivo study) (Table 2), and 2 RCTs (Table 3). The in vitro studies provided data on the antimicrobial properties of nanoparticle-supplemented gels under controlled laboratory conditions. The in vivo study contributed insights into the effectiveness of these gels in a biological system, while the RCTs offered evidence of their clinical efficacy in real-world endodontic treatments. This diverse mix of study designs allowed for a comprehensive evaluation of the antimicrobial potential of nanoparticle-supplemented gels in various experimental and clinical contexts.
| Author and year | Study design | Nanoparticle type | Nanoparticle size (nm) | Study groups | Control group | Sample size per group | Antimicrobial activity evaluation method | Main result |
|---|---|---|---|---|---|---|---|---|
| Samiei et al., 2018 [14] | In vitro study comparing ZO, ZO/Ag, and CHX with Ca(OH)2 | Zinc oxide (ZO) and zinc oxide/Silver (ZO/Ag) | ZO: 50 ppm, ZO/Ag: 1 ppm |
|
Normal saline | 11 per group | Colony count, SEM | Ca(OH)2 + CHX was significantly more effective than ZO and ZO/Ag. No significant difference between ZO and ZO/Ag |
| Liu et al., 2021 [15] | In vitro | Silver nanoparticles | ∼21.6 |
|
|
15 per group | CLSM analysis, bacterial sampling with paper points, SEM | AgNPs-PL groups significantly reduced E. faecalis compared to CH; 32 μg/mL AgNPs-PL most effective |
| Berrio et al., 2024 [16] | In vitro study | Silver (AgNPs) and copper (CuNPs) | 17 nm |
|
PVA/PVP/PEG gel without nanoparticles | 3 per group | Confocal microscopy, colony counting, SEM, TEM, AFM | CuNP gels demonstrated higher antibacterial activity, while AgNPs gels showed higher cytotoxicity. CuNP gels had better biocompatibility, promoting HPDL cell proliferation |
| Author and year | Study design | Nanoparticle type | Nanoparticle size (nm) | Study groups | Control group | Sample size per group | Antimicrobial activity evaluation method | Main result |
|---|---|---|---|---|---|---|---|---|
| Cheng et al., 2024 [17] | In vitro and in vivo studies on biofilm treatment in apical periodontitis | Octenidine nanoparticles (OCT/PECT@OCT) | 162 | OCT/PECT@OCT, OCT/PECT@OCT + ALK, Ca(OH)2, CHX | Saline | 10 per group | Colony counting, SEM, CLSM | OCT/PECT@OCT + ALK was the most effective in reducing biofilm and showed better clinical operability compared to Ca(OH)2 |
| Author and year | Study design | Nanoparticle type | Nanoparticle size (nm) | Study groups | Control group | Sample size per group | Antimicrobial activity evaluation method | Main result |
|---|---|---|---|---|---|---|---|---|
| Arafa et al., 2023 [18] | Randomized clinical trial on patients with endodontic infections | PLGA nanoparticles with chitosan | N/A | F1: CIP-PLGA-chitosan and F2: free CIP gel | Ca(OH)2 | 11 per group | Colony counting, biofilm inhibition assays | F1 gel demonstrated the best antibacterial and biofilm inhibition effect compared to F2 and Ca(OH)2, with significant healing results in the clinical trial |
| Hassan, Diab, and Ahmed 2021 [19] | Randomized controlled clinical study | Silver nanoparticles (AgNPs) | 17 nm | Ca(OH)2 + AgNPs, AgNPs | Ca(OH)2 | 10 per group | Colony-forming units (CFUs) on agar plates, culture | Combined Ca(OH)2 + AgNPs reduced intracanal bacterial counts significantly but increased interappointment pain |
The risk of bias assessment included five studies, presented in two figures. Figure 2 evaluated three in vitro studies. Most domains in these studies indicated a low risk of bias, except for specific concerns in the third study, particularly in “bias due to deviations from intended interventions” and “bias in the selection of reported results.” Overall, two studies were classified as low risk, while one had “some concerns” for overall bias. Figure 3 focused on two clinical studies: one on silver nanoparticles gel and another on PLGA nanoparticles gel. The silver nanoparticles gel study showed “some concerns” in “bias due to deviations from intended interventions” and “bias in the measurement of outcomes,” leading to an overall classification of “some concerns.” In contrast, the PLGA nanoparticles gel study had a low risk in most domains, with “some concerns” only in “bias in the selection of reported results,” resulting in an overall low-risk classification. In conclusion, the studies generally presented a low risk of bias, with some areas of concern, providing a solid methodological basis for interpreting and comparing the results.
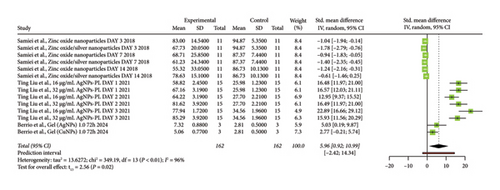
The forest plot (Figure 4) presents the results of a meta-analysis of in vitro studies on the antimicrobial activity of gels supplemented with nanoparticles in endodontics. Each study is listed with its specific treatment and evaluation time points, evaluating different nanoparticles such as zinc oxide, silver (AgNPs), and copper (CuNPs). The horizontal axis shows the standardized mean difference (SMD) with a 95% confidence interval (CI). Most studies show positive SMDs, indicating a beneficial antimicrobial effect of the nanoparticle gels. The pooled effect size is represented by the black diamond at the bottom, with a value of 5.96 (95% CI: [0.92, 10.99]), which does not cross the line of no effect, signifying a statistically significant positive antimicrobial effect. However, there is high heterogeneity among the studies (I2 = 96%), suggesting considerable variation in the effect sizes, likely due to differences in study designs, nanoparticle types, concentrations, or methodologies. The prediction interval ([−2.42, 14.34]) crosses zero, indicating some uncertainty about the effect in future studies. Overall, the plot suggests that nanoparticle-supplemented gels have a positive antimicrobial effect, but the variability across studies calls for cautious interpretation.
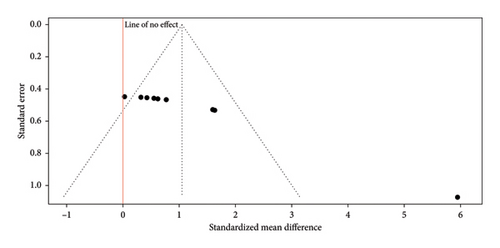
The funnel plot (Figure 5) presented shows the results of a meta-analysis on the antimicrobial activity of gels supplemented with nanoparticles in endodontics. The distribution of the studies in the plot is not symmetrical, as most of the points are concentrated on the right side, indicating a tendency toward a positive effect on antimicrobial activity (reflected by a positive SMD). This asymmetry suggests the possible presence of publication bias, as studies with negative results or with less difference in antimicrobial activity may not have been published or included in the analysis. The positive effect size is evident, showing that gels with nanoparticles have a significant impact. On the vertical axis, the upper points with smaller standard errors represent studies with larger sample sizes and more precise results, while the lower points, with larger standard errors, correspond to studies with smaller sample sizes and less precision. In summary, the plot indicates that gels supplemented with nanoparticles in endodontics have significant antimicrobial efficacy, although the asymmetry suggests a potential publication bias.
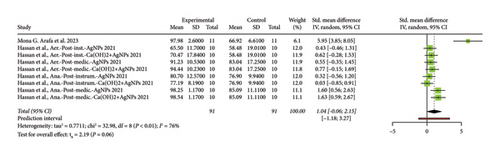
The forest plot (Figure 6) presents the results of a meta-analysis of RCTs evaluating the antimicrobial effect of gels supplemented with nanoparticles used as intracanal medication in endodontics. Each study is represented by a green square, with the size of the square reflecting the study’s weight in the meta-analysis. The horizontal lines through the squares indicate the 95% CIs for the SMD. Most of the studies have CIs that cross the line of no effect (0), suggesting that their results are not statistically significant. The diamond at the bottom represents the combined effect of all studies, with a total value of 1.04 (95% CI: [−0.06, 2.15]). Since this CI crosses the line of no effect, the overall effect is not statistically significant. The meta-analysis shows considerable heterogeneity, with I2 = 76%, indicating significant variability among the studies. This variability could be due to differences in nanoparticle types, methodologies, and experimental conditions. The prediction interval of (−1.18, 3.27) further highlights the uncertainty of the effect in future studies, as it crosses zero. In conclusion, while some individual studies show positive effects, the overall analysis does not support a consistently significant antimicrobial effect of nanoparticle-supplemented gels when used as intracanal medication in endodontics.
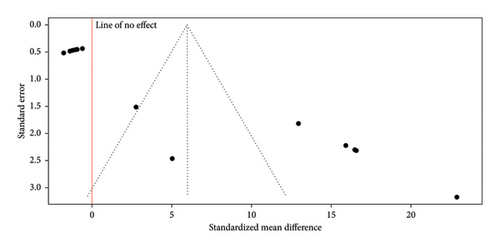
The funnel plot (Figure 7) presents the results of RCTs evaluating the antimicrobial effect of nanoparticle-supplemented gels used as intracanal medication in endodontics. The plot shows some asymmetry, with most studies clustered to the right of the line of no effect, suggesting that the majority of the trials report a positive antimicrobial effect of the nanoparticle gels. Additionally, one study appears as an outlier with a significantly stronger effect. This asymmetry may indicate potential publication bias, as studies showing positive effects are more likely to be published than those with negative or nonsignificant results. The plot’s y-axis represents the standard error, with studies near the top having smaller standard errors and, therefore, more precision due to larger sample sizes. Most studies are positioned near the top, suggesting relatively precise estimates, but the horizontal spread reveals variability in reported effect sizes. Overall, while there is a trend toward positive outcomes, the asymmetry and potential bias mean these findings should be interpreted with caution.
4. Discussion
The in vitro studies included in this review used different controls to assess the antimicrobial activity of nanoparticle-supplemented gels. Some studies employed untreated samples as controls, while others utilized calcium hydroxide or similar commonly used intracanal antimicrobial agents as the control. For the meta-analysis, the control group was standardized to include calcium hydroxide or another agent frequently recognized as the “gold standard” in endodontic intracanal medication [20].
This choice of control for the meta-analysis significantly influenced the interpretation of the results. When the nanoparticle-supplemented gels were compared to untreated controls, they appeared to exhibit a much greater antimicrobial effect, suggesting high efficacy. However, when the comparison was made against calcium hydroxide or other similar antimicrobial agents commonly used in endodontics, the difference in antimicrobial activity was less pronounced. This indicates that while the gels show some promise, their efficacy over the gold standard treatments is not as significant as it may seem when compared to an untreated baseline. This emphasizes the need for more comparative studies using consistent and clinically relevant controls to accurately assess the potential of nanoparticle-supplemented gels in endodontic treatment [21, 22].
A key limitation of this meta-analysis is the small sample size in many of the included studies, which compromises the robustness and generalizability of the findings. Small samples increase variability, reduce statistical power, and heighten susceptibility to biases, such as selective reporting and random error, potentially skewing the pooled results. In clinical studies, limited cohorts may fail to represent diverse populations, while in vitro studies, despite offering controlled conditions, often oversimplify the complex clinical environment, neglecting factors like host immune responses and anatomical variability. These issues may lead to over- or underestimation of the antimicrobial efficacy of nanoparticle-supplemented gels. To strengthen future research, larger, well-powered studies, both in vitro and clinical, are needed to provide more reliable and generalizable insights, incorporating realistic conditions and diverse microbial and patient variables [23].
In one of the clinical trials included in the meta-analysis, the antimicrobial activity of the gels was evaluated at multiple time points. However, for the purposes of the meta-analysis, only the data at 72 h were included. This decision was made because the earlier time points presented challenges in data extraction; the lines and points in the graph overlapped significantly, making it difficult to distinguish and accurately extract the necessary data for analysis [18]. This situation underscores a recommendation for future studies of this nature: authors should consider reporting these types of results in tables rather than solely in graphical form. Tables allow for a clearer and more precise presentation of numerical data, facilitating accurate extraction and comparison in meta-analyses. Providing data in tabular form enhances the quality of meta-analyses by allowing for a more thorough and detailed evaluation of results, thereby leveraging the advantages that meta-analyses offer in synthesizing evidence across multiple studies. This practice would greatly aid in improving data usability for subsequent analyses and research reviews [24].
The in vivo study focused on the development of a thermosensitive hydrogel aimed at combating bacterial infection in the treatment of apical periodontitis [17]. Apical periodontitis, characterized by inflammation caused by bacterial infections affecting the tissues around the dental apex, has traditionally been managed with root canal therapy [25]. However, conventional intracanal medications, such as calcium hydroxide (Ca(OH)2), have shown limited effectiveness and pose challenges in terms of complete removal from the root canal system [26]. To address these limitations, the authors proposed a hydrogel designed for the sequential release of two antimicrobial agents: hydrophilic octenidine for rapid release and hydrophobic octenidine for sustained, long-term release [27]. The hydrogel was formulated using a specific copolymer that allowed for the encapsulation of the antimicrobial agents into nanoparticles, facilitating controlled release. Furthermore, the incorporation of calcium hydroxide into the hydrogel maintained an alkaline environment, enhancing its antibacterial effect. The study included seven groups to evaluate the antibacterial effects of the hydrogels in vitro. The control group was treated with sterile saline (0.9%) as a baseline. The blank PECT hydrogel group tested if the hydrogel had inherent antibacterial properties. The OCT/PECT@OCT hydrogel group used a sequential release of hydrophilic and hydrophobic octenidine. The OCT/PECT@OCT + ALK group added calcium hydroxide to create an alkaline environment for enhanced antibiofilm efficacy. The PECT + ALK group used hydrogel with calcium hydroxide but without octenidine. The Ca(OH)2 paste and 2% CHX gel groups served as comparisons using traditional paste and chlorhexidine gel. The in vivo component included four groups of rats: the OCT/PECT@OCT + ALK gel, Ca(OH)2 paste, 2% CHX gel, and a control group without additional treatment [17].
The results indicated that this novel hydrogel outperformed traditional calcium hydroxide treatments in both experimental root canal models and in vivo rat models of apical periodontitis. Specifically, its application demonstrated a higher efficacy in eliminating bacterial biofilm and a greater capacity to prevent reinfection when compared to current standard treatments. Additionally, the ease of application and removal of this hydrogel was noted as a significant advantage for clinical practice. The in vitro part of this study could not be included in the meta-analysis of in vitro studies because the graphs reported a 100% bacterial reduction for the gels used without providing standard deviations. This lack of statistical data prevented its inclusion in the analysis [17].
One potential reason for the high heterogeneity (I2 = 76%) observed in the forest plot is the variability in the design of the RCTs. While all studies were clinical in nature, differences in protocols, sample sizes, and inclusion criteria could significantly impact the results. Some trials might have involved different patient demographics, stages of infection, or types of teeth (e.g., anterior vs. molar) which can influence the effectiveness of the antimicrobial treatment. Variations in clinical procedures, such as the method of gel application and duration of treatment, may also contribute to inconsistencies in the results [28].
The RCTs included in this analysis used various types of nanoparticles, such as silver nanoparticles (AgNPs), zinc oxide nanoparticles, and copper nanoparticles, in different concentrations. The antimicrobial effectiveness of these nanoparticles can vary based on their type, size, concentration [29], and interaction with the intracanal environment [30]. In a clinical setting, factors such as the presence of organic tissue, dentinal debris, and the dynamics of the root canal system can affect how nanoparticles interact with bacteria. Thus, some studies might report greater antimicrobial effects than others depending on the specific formulation and concentration of nanoparticles used [31].
Clinical trials often involve patients with varying degrees of root canal infections, which can influence treatment outcomes [32]. Some RCTs might include patients with single-species infections, while others might deal with multispecies biofilms that are more resistant to treatment. The complexity of root canal infections, coupled with individual patient variability, such as differences in root canal anatomy and the patient’s immune response, could impact the efficacy of nanoparticle-supplemented gels. These clinical realities can introduce variability in the results, explaining the lack of a consistent effect across trials [33, 34]. Another source of variability could be the different treatment protocols used in these RCTs. The duration for which the nanoparticle-supplemented gels were left in the canal, the concentration of nanoparticles used, and the number of treatment sessions can all impact antimicrobial effectiveness. Additionally, variations in the methodology for bacterial assessment, such as culturing techniques, molecular methods, or direct sampling, could lead to differences in reported outcomes. Some trials may have employed more sensitive detection methods, capturing subtle differences in bacterial reduction that others did not [33]. Even though all studies were RCTs, biases inherent to clinical research, such as variations in operator skill, adherence to treatment protocols, and patient compliance, could influence the results. The skill level of the clinician performing the procedure can affect the thoroughness of canal debridement and gel application, impacting the treatment’s effectiveness. Furthermore, patient-related factors, such as the anatomical complexity of the root canal system and the presence of dentinal tubules, can affect the distribution and action of the nanoparticle gels [35].
The clinical environment presents unique challenges that do not exist in laboratory settings. In RCTs, factors such as the patient’s immune response, saliva contamination, and the presence of pre-existing conditions (e.g., diabetes and smoking) could affect the success of intracanal medications [36]. Nanoparticle gels need to perform in a dynamic environment that includes tissue fluids, enzymes, and the potential for reinfection, which might alter their antimicrobial efficacy compared to their expected performance in controlled settings [37, 38].
The root canal system’s anatomical complexity is a critical factor in clinical trials. Variations in root canal morphology, including lateral canals, isthmuses, and apical deltas, can limit the penetration and action of intracanal medications [39]. Nanoparticle-supplemented gels may have varying degrees of success depending on their ability to infiltrate these complex areas. The differences in anatomical challenges faced in each clinical trial may contribute to the observed heterogeneity in treatment outcomes [40].
The asymmetry observed in the funnel plot suggests that the results of the RCTs on nanoparticle-supplemented gels as intracanal medication in endodontics might be influenced by publication bias. This bias typically occurs when studies with positive or significant results are more likely to be published, while those with negative or nonsignificant outcomes remain underreported. Consequently, the positive trend seen in the plot may not fully represent the true effectiveness of these gels [41].
Another important aspect is the variability in bacterial biofilm composition in clinical cases [42]. In vivo, root canal infections often consist of multispecies biofilms with varying degrees of maturity and resistance to antimicrobial agents. These biofilms are more resilient and difficult to eradicate compared to the single-species biofilms commonly used in in vitro experiments [43, 44]. The biofilm’s complex extracellular matrix can hinder the penetration of nanoparticles, further limiting their antimicrobial action in clinical settings [45]. Furthermore, patient-related factors such as individual variations in immune response, salivary flow, and the presence of systemic conditions (e.g., diabetes and smoking) can influence the effectiveness of intracanal medications. These factors, present in RCTs but absent in in vitro studies, contribute to the inconsistency and reduced efficacy observed in clinical outcomes [46].
5. Conclusion
The analysis indicates that gels supplemented with nanoparticles, particularly silver nanoparticles, show a positive antimicrobial effect in endodontic disinfection. However, the findings vary due to differences in study designs, nanoparticle types, concentrations, and clinical conditions. Although in vitro studies demonstrated a significant antimicrobial activity, RCTs reported inconsistent results, highlighting the challenges in replicating these effects in real-world clinical scenarios. Further standardized research is necessary to confirm the potential of nanoparticle-supplemented gels as a viable intracanal medication in endodontics.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this research.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




