A Structural, Morphological, and Corrosion Study of 316L Stainless Steel Coated With Hydroxyapatite, Chitosan, Nano-MgO, and 3-Aminopropyl Trimethoxysilane via Electrophoretic Deposition
Abstract
This study presents the electrophoretic deposition (EPD) of hydroxyapatite (HAP), chitosan (CS), magnesium oxide (MgO) and silane coupling agent (KH-550) coatings on 316L stainless steel substrates to improve their corrosion protective properties for the intended biomedical applications. In addition, this work differentiates itself by analyzing the effects of varying anodization times (5, 10, and 15 min) on the surface characteristics, with a particular focus on the roughness, zeta potential of the suspension, and coating thickness. The results show that the root-mean-square roughness (Sq) and the arithmetic mean roughness (Sa) escalated from 78.18 nm to 67.14 nm on the uncoated base metal to 421.7 nm and 364.7 nm, respectively, after 15 min of anodization. Stable suspension was indicated by the zeta potential of −39.8 mV. As anodization time increased, coating thickness reached 14.14 μm after 15 min. The atomic force microscopy (AFM) analysis was used to assess the adhesion bond strengths of HAP–CS–MgO composites coated at different times on a stainless steel surface. The composite coated for 10 min exhibited the most substantial adhesion force at −0.5271 nN, indicating the highest reliability and stability in bonding interactions. The KH-550 concentration of 0.0793 g/mL demonstrated the lowest corrosion rate at 0.02933 mm/year, indicating superior corrosion resistance, along with the highest adhesion force of −1.0 nN and a coating thickness of 117.5 μm.
1. Introduction
One of the most significant current discussions in nanotechnology is biomaterial coating that applies on the metal surface to enhance the biocompatibility and healing time between metal implants and human tissues [1, 2]. The electrophoretic deposition (EPD) process stands out among various coating techniques because of its capability of using metal, polymer, ceramics, or hybrid combination materials for its suspension [3, 4]. It has been widely utilized in bioengineering applications due to its unique characteristics, such as low cost, simple apparatus, and short deposition time. Recent developments in the EPD process have heightened the need for improving coating’s quality by optimizing the process parameters, which are suspension composition, deposition time, and applied AC [5].
316L stainless steel has good mechanical properties; therefore, it is widely used for biomedical implantation. This material possesses strength and prolonged resistance to fatigue, and it is also difficult to corrode. Although this material can be employed for short periods when exposed to biological fluids, there are some disadvantages, which include pitting corrosion, ions’ release from the material, and probable cell toxicity which can trigger unwanted biological effects. These issues are of particular concern in long-term medical implants. The surface of 316L stainless steel has been improved by the application of biocompatible hydroxyapatite (HAP), chitosan (CS), or nanoparticles to increase its resistance to corrosion and ion erosion. Studies have shown that these coatings act as a limiting factor in the degradation of the materials over time, which would improve the biological property’s longevity of such materials. As a result, the use of coatings is essential to overcome these limitations and ensure the safety and longevity of 316L stainless steel in medical applications [6–8].
HAP is a ceramic material consent of bioactive calcium phosphate, and it has a chemical formula (Ca10 [PO4]6 [OH]2) [9]; it has unique crystallographic and chemical properties to natural bone and teeth. Therefore, it has been proven that it is a biocompatible and bioactive material. As a result, CS is an another important biomaterial utilized in dental implants and engineered scaffolds, which are crucial for implantation due to its antibacterial, biocompatible, and biodegradable properties as an organic material [10, 11]. CS comprises two monomers: d-glucosamine and N-acetyl-d-glucosamine units, with glucosamine predominant [12]. It has conclusively been shown that HAP has a similar composition to human bones, and it has proven a promising bioactivity and compatibility when used in bio-implantation. However, it has been noticed that it has poor bonding strength [13]. Consequently, it has been suggested that CS and gelatin would be worth integrating with HAP to enhance the bio-implant coating strength when applied to stainless steel [14, 15]. Combining this composite coating would improve the hydrophilic stability and prove more protein attachment during implantation, which shows the significant relationship between biopolymers and bioceramics composite EPD coating to prevent leaching of ions from metals such as 316L, widely used in orthopedic devices [16–18].
Coating stainless steel 316L with HAP and CS enhances its suitability for biomedical applications by improving its corrosion resistance, thereby reducing the release of metal ions into the body and enhancing biocompatibility to mitigate inflammation and rejection risks [19–21]. HAP, which is chemically similar to bone minerals, promotes osteoconductivity, enabling bone cells to adhere and proliferate, which is essential for orthopedic and dental implants [22]. Natural antibacterial properties of CS reduce postsurgery infection risk [23, 24]. Furthermore, using KH-550 (γ-aminopropyl triethoxysilane) in the process of EPD enhances the effectiveness of the coating [25]. KH-550 chemically bonds organic CS with inorganic HAP, enhancing their adhesion and resulting in a more uniform and long-lasting coating. KH-550 binds with HAP and perhaps CS via siloxane and covalent bonds, improving organic–inorganic compatibility, and this leads to enhance coating hardness and wear resistance [26, 27]. Physically, it aids in the dispersion of HAP within the CS matrix, enhancing the coating’s mechanical integrity and its ability to evenly distribute stress, thus improving overall toughness. The silane agent, KH-550, can react with the inorganic HAP, and covalent bonds can be established. The ethoxy groups on the silane molecule hydrolyze to form silanol groups which can further react with the hydroxyl groups available on the surface of the HAP particles. As a result, a durable siloxane bond (Si-O-Si) is formed, which effectively attaches the silane to the HAP. In addition, the amide group (NH2) of KH-550 has the ability to form hydrogen bonds or covalent bonds with the OH and amino groups of CS [28–33]. This interaction enhances the cohesion of organic CS and inorganic HAP [34]. When utilizing KH-550, it aids in achieving a more uniform distribution of HAP particles throughout the CS material [34, 35], and this can result in improved overall performance. The molecular entanglement and potential cross-linking of KH-550 result in enhanced mechanical strength of the composite coating. Enhanced fiber entanglement distributes mechanical stress more uniformly across the coating, enhancing durability and reducing corrosion risk. The coating with strong biomaterials makes stainless steel 316L a highly suitable option for long-term implants in medical applications.
The objectives of this study are to decrease the corrosion rate and surface properties of 316L stainless steel substrates with HAP/CS/MgO and silane coupling agent KH-550 by EPD technique with the purpose of improving their biocompatible and self-sustaining characteristics for biomedical uses. With regard to the objective of this research, this study focuses on determining the influence of different EPD durations on morphological characteristics including roughness and thickness which are influential in dictating the performance and durability of medical implants. The identified problem is that 316L stainless steel is easily prone to corrosion and exhibits low bioactivity which hinders implants’ performance. It is possible to state that the challenges, mentioned above, can be solved by the research, which aims at the optimization of EPD parameters and results in more uniform, stable, and biocompatible coating of biomedical implants that can last longer in their functions.
2. Experimental Procedure
2.1. Materials
The CS used in this study was obtained from Glentham Life Sciences Ltd. Based on viscosity measurements of 1000–2000 cps, it is ascertained that the molecular weight is over 1,500,000 with a purity of greater than 90%. Afa Nanomaterials Chemistry Ltd, USA, supplied the MgO nanoparticles. HAP, a naturally occurring calcium apatite mineral in the form of a fine white powder with particle sizes under 37 microns, was obtained from Sunkoo Ltd., South Korea. The glycidoxypropyltrimethoxysilane (KH-550) which was used in this study was obtained from Chengdu Kelong Chemical Reagent Factory, Chengdu, China.
2.2. Suspension Preparation
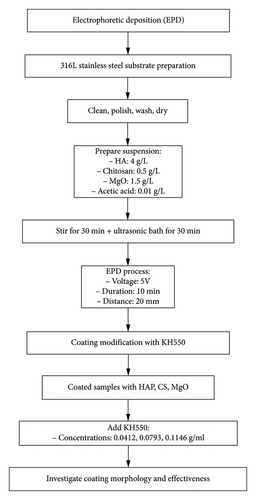
In this current work, it has been done in two steps. The first step, 316L stainless steel was used as substrate to be coated by the EPD process (as shown in Figure 1). The suspension used for EPD coating consisted of HAP 4 g/L, CS 0.5 g/L, MgO 1.5 g/L, and the concentration of acetic acid about 0.01 g/L [17, 36]. The substrate samples were cut into rectangular shapes measuring with a dimension of 15 × 33 × 1.5 mm. Then, the samples were cleaned with water, polished using SiC sheets of 400-, 500-, and 600-μm grits, washed with methanol and deionized water, and air-dried. The initial step involved the CS biopolymer dissolution in ethanol, acetic acid, and distilled water. CS was completely dissolved before adding HAP and MgO powders into the solution. For uniformity, the mixture was magnetically stirred for a period of 30 minutes. To prevent any particle clustering, further dispersion of the solution was carried out in an ultrasonic bath for 30 minutes at 40 W, all conducted at room temperature. The samples were made including suspension material concentration and process parameters detailed in Table 1. The samples were prepared while maintaining constant process parameters, including the suspension material concentration: HA (4 g/L), chitosan (0.5 g/L), MgO (1.5 g/L), and acetic acid (0.01 g/L). The distance between the electrodes was set to 20 mm. In the second phase of the experiment, 316L stainless steel, previously coated with a mixture of HAP, CS, and MgO using EPD at 5 V for 10 min, was further modified by incorporating the silane coupling agent KH-550 at varying concentrations of 0.0412, 0.0793, and 0.1146 g/mL into the HAP–CS–MgO solution. This step was undertaken to investigate how different levels of KH-550 concentration influence the morphology and effectiveness of the coating. A schematic illustration of the EPD process demonstrating the coating preparation on the sample.
| No. | Voltage (v) | Time (min) | CS (g/L) | MgO (g/L) | HAP (g/L) |
|---|---|---|---|---|---|
| 1 | 5 | 5 | 0.5 | 1.5 | 4 |
| 2 | 5 | 10 | 0.5 | 1.5 | 4 |
| 3 | 5 | 15 | 0.5 | 1.5 | 4 |
| Suspension zeta potential (mV) | −39.8 | ||||
| Suspension particles mobility (μ/s)/(V/cm) | −078 | ||||
In order to perform the EPD process, a regular DC-power supply has been used at room temperature, and the distance between the two electrodes was kept to a value of 20 mm. In the second phase of the experiment, 316L stainless steel, previously coated with a mixture of HAP, CS, and MgO using EPD at 5 V for 10 min, was further modified by incorporating the silane coupling agent KH-550 at varying concentrations of 0.0412, 0.0793, and 0.1146 g/mL into the HAP–CS–MgO solution. This step was undertaken to investigate how different levels of KH-550 concentration influence the morphology and effectiveness of the coating. A schematic illustration of the EPD process demonstrates the coating preparation on the sample.
2.3. Characterization of the Coating
The study used the Zeta Plus Signal Processing utilizes electrophoretic light scattering (ELS) to achieve a precision of ±3%, which may vary depending on the salt concentration. The system is equipped with a standard 35 mW red diode laser with a nominal wavelength of 640 nm. The zeta potential of the suspension was measured at 25°C using a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, Worcestershire, UK).
The surface roughness, adhesion force, and mechanical performance (Young’s modulus and stiffness) of the coating were evaluated using the CoreAFM 2023 from Nanosurf AG, Switzerland.
To identify the phases of the coating layer, the X-ray diffraction apparatus (XRD) (Plate Number 1, NF type) with Cu target (K1 of 1), model: XRD-6000 X-ray Tube: Cu, NF type with Cu target was utilized. Also it has an X-ray leakage less than 2.5 Sv/h. After that, the peaks were compared to the standard peaks individually for the substance using Joint Committee on Powder Diffraction Standards (JCPDS) cards. The Scherrer formula was used to determine the crystallite size (particle size) of the HAP powder and HAP/CS composite coating.
Field-emission scanning electron microscopy (FESEM) technique and energy-dispersive X-ray spectroscopy (EDS) analysis (Inspect F 50 FEISEM, Eindhoven, the Netherlands) were used to investigate the thickness of coating, surface morphology, and element characterization of the coating on the surface of 316L stainless steel.
3. Results and Discussion
3.1. Morphological Analysis of Surface of 316L Stainless Steel Coated With KH-550-HAP–CS–MgO
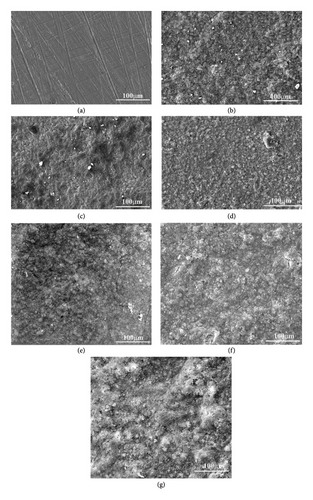
Figure illustrates the morphological structures of the FESEM images of (HAP–CS–MgO) composites on the surface of stainless steel (top surface of coating) prepared via electrochemical deposition at 5 V for various durations: (a) base metal, (b) 5 min, (c) 10 min, and (d) 15 min. It could be seen that for the three values of coating time, there is a good homogeneity between suspension compounds with dense, free of cracks, and smooth layers. It is notable that by increasing the coating time to 15 min, more densely packed layers, and free of voids when compared to 10-min coating time, some pores were seen in dark black color. It is worth mentioning that the presence of HAP dispersed in the CS with a small number of light particles of MgO, and it can be argued this smooth dispersing of the particles in the composite coatings is due to a stable suspension of the colloid that was prepared in this study. These findings including the structure of the HAP dispersed in the CS are consistent with which have been reported. Remarkably, coating layers with crack-free and less porosity in their structure leads to enhanced biocompatibility of the coated implant as demonstrated.
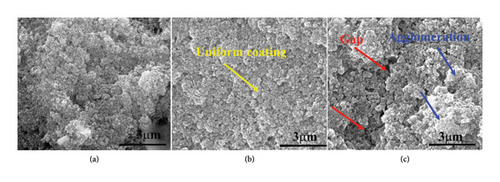
Figures 2(e), 2(f), and 2(g), and Figure 3(a), 3(b), and 3(c) reveal the influence of the silane coupling agent KH-550 concentration on the surface characterization of HAP, CS, and MgO coating on surface of the stainless steel. At a KH-550 concentration of 0.0412 g/mL (Figures 2(e) and 3(a)), the coating appears relatively uniform with minimal agglomeration. Raising the concentration up to 0.0793 g/mL (Figures 2(f) and 3(b)) leads to a more even distribution of the coating particles within the solution. However, at the highest concentration of 0.1146 g/mL (Figures 2(g) and 3(c)), there were signs of particle agglomeration and pore formation. The availability of silane molecules at increased concentrations increases the incidence of particle interactions and the likelihood of clustering. The changes of surface energy with the augment of the KH-550 also disrupt the force equilibrium where particles are pulled toward each other, resulting in agglomerations.
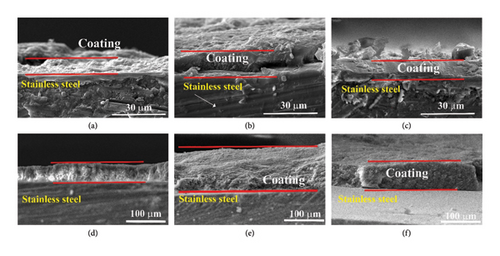
The SEM analysis was performed to measure the thickness of the coating layer that was deposited on the substrate, (SEM) cross section of selected samples at 5V for different durations. Figures 4(a) and Table 2 illustrate the 5 min, (b) 10 min, and (c) 15 min of deposition. These images of the cross section showed that the coating thickness of produced layers for 5, 10, and 15 min have thickness layers’ values of 9.1, 12.1, and 14.14 μm, respectively. Meanwhile, Figure also shows densely and uniformly packed layers of the HAP with CS, and MgO mixture, a good interaction between the substrate and the coating layer can be seen, and the results of the adhesion force further confirmed these findings. This observation agreed with the results in Javidi et al. [37]; they stated that the thickness of HAP coatings increased along with the increase in deposition time. They noted that as the deposition time was increased, the coatings continued to get thicker and thicker. This is one of the essential elements of the EPD process—time, which affects various factors including the final characteristics of the coating.
| Sample | Duration of coating (min) | Thickness (μm) |
|---|---|---|
| a | 5 | 9.1 ± 5.1 |
| b | 10 | 12.1 ± 3.3 |
| c | 15 | 14.14 ± 4.6 |
| KH-550 concentration (g/mL) | Thickness (μm) | |
| d | 0.0412 | 46.2 ± 3.4 |
| e | 0.0793 | 117.5 ± 8.9 |
| f | 0.1146 | 76.2 ± 9.7 |
Figure 4(d), 4(e), and 4(f) and Table 2 demonstrate the effect of varying concentrations of KH-550 on the coating thickness of HAP, CS, and MgO on 316L stainless steel substrates. The KH-550 concentrations tested were 0.0412, 0.0793, and 0.1146 g/mL. As depicted in Figures 4(d), 4(e), and 4(f), successful coatings were achieved on the metal substrate, with the KH-550 concentration significantly impacting the coating thickness. At a KH-550 concentration of 0.0412 g/mL, the coating thickness was measured at 46.2 microns. Increasing the concentration to 0.0793 g/mL resulted in a coating thickness of 117.5 microns. However, further increasing the concentration to 0.1146 g/mL reduced the thickness to 76.2 microns. The observed variation in coating thickness with increasing KH-550 concentration during the EPD process can be attributed to several factors. Initially, increasing the KH-550 concentration from 0.0412 to 0.0793 g/mL enhances the dispersion and adhesion of the coating materials (HAP, CS, MgO) to the 316L stainless steel substrate, leading to a more uniform and thicker coating layer. However, beyond this optimal concentration (0.0793 g/mL), excess KH-550 may cause agglomerates or clusters to form in the coating solution, resulting in a less uniform deposition and potential defects or gaps in the coating layer. Additionally, higher concentrations of KH-550 can increase the solution’s viscosity, hindering the effective movement of particles during the EPD process and producing a less dense and thinner coating. Consequently, the coating thickness increases from 46.2 microns at 0.0412 g/mL to 117.5 microns at 0.0793 g/mL, but decreases to 76.2 microns at 0.1146 g/mL.
The EDS characteristics of HAP–CS–MgO composites on the stainless steel surface via electrochemical deposition at 5V for different durations are shown in Figure 5, and the base metal (a) serves as a reference point, showing elemental peaks characteristic of stainless steel, predominantly iron (46.3%), chromium (12.8%), and nickel (6.8%). The spectra show the presence of the constituent elements of the deposited HAP–CS–MgO composites such as calcium and phosphorus originating from HAP; nitrogen from CS; and magnesium from MgO as the deposition time is changed to 5 min (b), 10 min (c), and 15 min (d). After 5 min of deposition, the elemental composition changes drastically where Fe is reduced to 0. 7%, Ni to 0.1%, and Cr to 0.1%. Elements’ characteristics of the composite material, such as Ca, P, O, N, and Mg, start to appear. After 10 min of deposition, Fe drops to 0.3%, Ni stays at 0.1%, and Cr is undetectable (0.0%), while Ca, P, O, N, and Mg peaks grow more pronounced. Fe, Ni, and Cr reach 0.4%, 0.2%, and 0.1% at 15 min of deposition, with persistent composite element peaks. The intensity and emergence of new peaks in parts (b), (c), and (d) suggest successful deposition of the composite materials with varying degrees of thickness and uniformity, reflecting the deposition time’s effect on the coating’s composition and structure. Longer deposition times show increased intensity of these composite-specific peaks, indicating thicker or more uniform coatings.
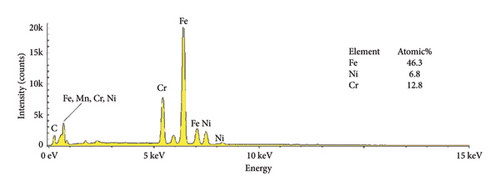
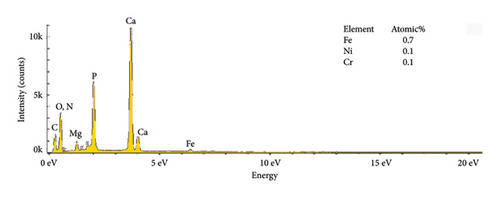
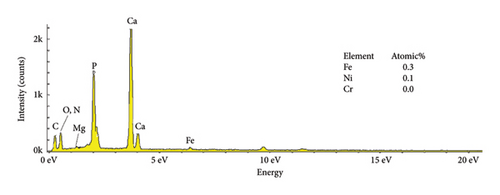
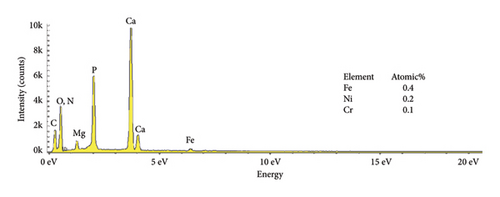
The reduction or elimination of nickel (Ni) and chromium (Cr) after coating the surface of base metal with HAP–CS–MgO brings notable benefits to biomedical applications. Since Ni and Cr can cause allergic reactions and toxicity, their reduction enhances the biocompatibility of the material. HAP, like bone mineral, biocompatible CS, and MgO create a durable, protective barrier. It resists corrosion and blocks harmful ions. This coating also improves bioactivity and osteointegration, which are essential for implant–bone integration. Ni and Cr are reduced in the coating, reducing allergic responses and toxicity.
The EDS analysis of the composites modified by KH-550 is shown in Figure 6 to map the chemical composition of HAP–CS–MgO composites based on the concentration of KH-550. The result of chemical analysis showed that the nitrogen (N) and silicon (Si) were detected in the composites as KH-550 was successfully grafted on the fiber surface. Notably, at a KH-550 concentration of 0. 0793 g/mL, the three usual metal elements contained in stainless steel metal including Fe, Cr, and Ni could not be detected; this means that KH-550 works in a way that it has a barrier effect or passivate the said metal components, which provides advantages for use in certain applications where stability and low emission of metal ions are critical.
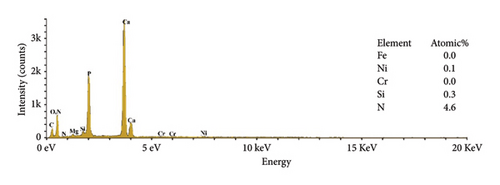
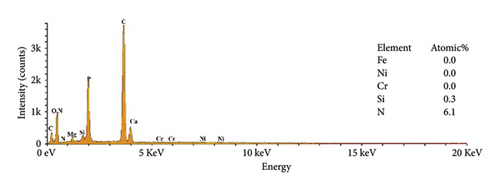
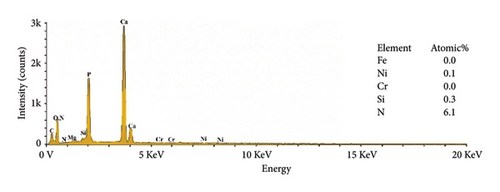
The XRD pattern for 316L stainless steel coated with HAP, CS, and MgO via the EPD process at different coating times (5, 10, and 15 min) along with the base metal is depicted in Figure 7. The base metal peaks at 2θ values of 43.0°, 50.2°, 73.8°, and 91.9° correspond to the planes (300), (422), (444), and (820) (reference code: 00-033-0395) of 316L stainless steel. The coatings at 5, 10, and 15 V show peaks for the composite materials at 26.0° (130), 28.7° (0012), 31.7° (−115), 39.3° (2014), and 45.8° (206) (reference codes: 00-054-2272, 01-087-1582), marked with diamond symbols, indicating the presence of HAP, CS, and MgO. The maximum intensity increases with the increasing voltage, and the highest value was realized for 15 min, suggesting a thicker deposition at higher coating time. The presence of stainless steel peaks despite the coating indicates that the X-rays penetrate through the thin coating layer, confirming a successful but thin-layered deposition.
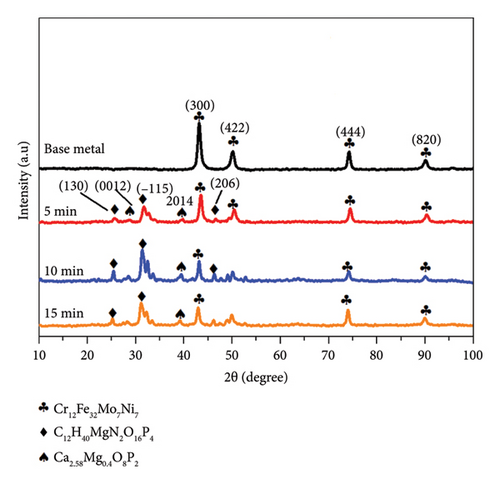
According to Figure 7 XRD patterns, two phases C12H40MgN2O16P4 and Ca2.58Mg0.4O8P2 can be found which can be related to the growth of composite materials based on HAP and MgO. One explanation of this is that HAP and MgO possibly interacted or reacted chemically during the EPD. HAP has a calcium phosphate structure, and it is clear that MgO can either act as a stabilizer or interact with the phosphate ions in HAP. It is likely that while coating or proceeding with the heating step, Mg2+ from the MgO used may substitute Ca2+ from HAP lattice, leading to mixed phases like Ca258 Mg0.4O8P2. Additionally, the condensation of C12H40MgN2O16P4 may also result from the stimulation with CS that contains phases of nitrogen, organic matter, magnesium, and the phosphate which were present in the EPD process. This phase is probably a hybrid compound formed as a result of CS, magnesium, and phosphate reaction. These results suggest that the deposition process triggers chemical reactions between the materials, leading to the formation of new phases that could affect the properties of the coating.
In the present investigative work, it was observed that the surface roughness of the prepared HAP–CS–MgO-coated 316L stainless steel alloys increased significantly with anodization time of 5, 10, and 15 min both by using roughness parameters Sq and Sa and from AFM micrographs and Table 3. The root-mean-square roughness (Sq) and the arithmetic mean roughness (Sa) escalated from 78.18 nm to 67.14 nm on the uncoated base metal to 421.7 nm and 364.7 nm, respectively, after 15 min of anodization. AFM Figures 8(a), 8(b), 8(c), and 8(d) clearly illustrate a gradual increase in the texturing of the surface at the micro–nano-level, and Figure 8(d) shows the maximum roughness and increased topographical features. This trend indicates that when deposition time increases, more time is provided to deposit a thicker and perhaps more agglomerated layer of the HAP–CS–MgO composite and thus rougher surface profile. Such changes in the surface topography might be a result of the buildup of material and morphological changes in the coating microstructure over time. The higher value of Ra could give better mechanical interlocking between the coating/substrate system, which is beneficial for biomedical applications since better cell adhesion and proliferation are used for biomedical applications. Our results are in good agreement with the findings presented by Cantaragiu et al. [38], highlighting a similar trend where longer deposition times lead to increased surface roughness during the EPD of HAP on stainless steel substrates. The findings indicated the deposition time and surface roughness are directly proportional. The reason for this increase in roughness is the larger deposition and clustering of nanoparticles and development of additional features on the coating, such as cracks and polygonal shapes, associated with longer processing times. These findings incorporate wider materials science principles in which, as processing time is increased, more film growth and alteration of surface coatings’ morphology take place.
| Sample | Duration of coating (min) | Roughness | |
|---|---|---|---|
| (Sq) nm | (Sq) nm | ||
| a | 0 | 78.18 | 67.14 |
| b | 5 | 203.8 | 158.3 |
| c | 10 | 402.8 | 317.2 |
| d | 15 | 421.7 | 364.7 |
| KH-550 concentration (g/mL) | (Sq) nm | (Sq) nm | |
| e | 0.0412 | 374.3 | 301.5 |
| f | 0.0793 | 106.1 | 84.6 |
| g | 0.1146 | 114.0 | 96.24 |
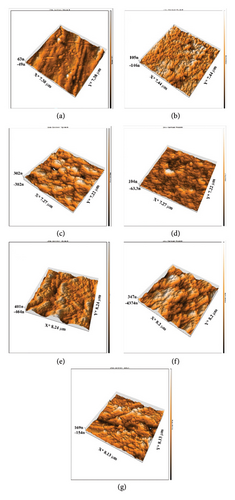
In addition, the AFM analysis highlighted features an adhesion map that depicts the adhesion forces’ mapping within a range of 3000 nm × 3000 nm and color coded with 50 μV blue for weaker adhesion force and 400 μV red for stronger adhesion force (Figure 9). The evaluation of HAP–CS–MgO composites on a stainless steel surface shows varying bonding strengths among the samples: Sample (a), coated for 5 min, exhibits an adhesion force of −0.30004 nN, a Young’s modulus of 7.981 MPa, and a surface stiffness of 0.3267 nN/nm; Sample (b), coated for 10 min, demonstrates the most substantial negative adhesion force at −0.5271 nN, along with the highest Young’s modulus of 8.253 MPa and the greatest surface stiffness of 0.4507 nN/nm, indicating the strongest and most stable bond; Sample (c), coated for 15 min, however, presents a positive adhesion force of 5.039 nN, a Young’s modulus of 3.853 MPa, and the least surface stiffness of 0.1561 nN/nm. This is commonly observed in AFM investigations, where negative adhesion forces and high values of stiffness are linked to strong adhesion. Negative contact adhesion measurements are attributed to attractive forces between the probe tip and the sample surface, and these forces comprise of van der Waals forces, electrostatic forces, or established chemical bonds where a large negative force typically characterizes stronger bonding between the surface and the tip of the probe in AFM [39–41].
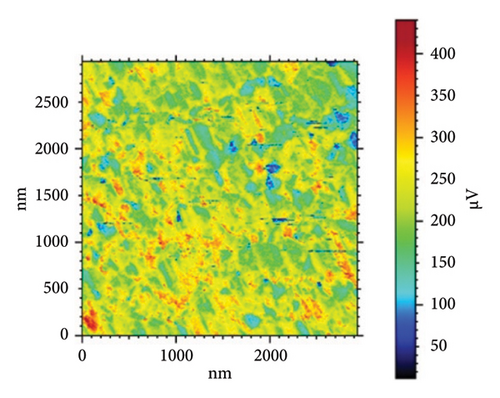
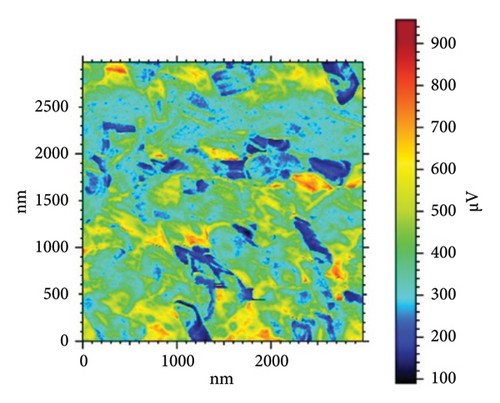
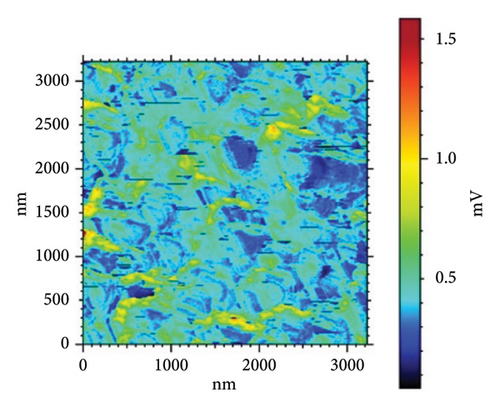
Table 4 and Figure 10 show the results of three sets of HAP–CS–MgO samples (a–d) where the samples have different levels of silane agent KH-550 (0, 0.0412, 0.0793, and 0.1146 g/mL) modified using the EPD technique at 5V for 10 min. Sample d, in which KH-550 concentration is the smallest, has the adhesion force of −0.7057 nN, while the maximum Young’s modulus at was 5.727 MPa and a surface stiffness of 0.5264 nN/nm. The raising of KH-550 concentration to 0.0793 g/mL yields the highest adhesion force of −1.004 nN; however, Young’s modulus is decreased to 5.423 MPa and the decrease of the surface stiffness up to 0.2614 nN/nm. Sample f with the highest content of KH-550 has low adhesion force adhesion with a value of −0.5405 nN, the lowest Young’s modulus at 4.621 MPa, and a surface stiffness of 0.2856 nN/nm. From the above results, it can be concluded that in spite of the fact that the adhesion augments as concentration of KH-550 increases up to the specific point as is exemplified by Sample e, the Young’s modulus as well as surface stiffness prove to be reduced. It implies within the context of the composite matrix, KH-550 may be playing a role of a plasticizer where it is contributing to the mobility of the polymer chains. The presence of the ethereal groups (–Si–O–Si–) allows the chain of CS to be more mobile and, therefore, has a lower stiffness and Young’s modulus. Therefore, the concentration has to be at 0.0793 g/mL (Sample e), which gives the highest adhesion force evidently at the cost of mechanical strength on the ascendency of the force value and implies room for fine-tuning the force adhesion and mechanical strength.
| Sample | Duration of coating (min) | Coating properties | ||
|---|---|---|---|---|
| Adhesion force (nN) | Young’s modulus (MPa) | Surface stiffness (nN/nm) | ||
| a | 5 | −0.30004 | 7.981 | 0.3267 |
| b | 10 | −0.5271 | 8.253 | 0.4507 |
| c | 15 | 5.039 | 3.853 | 0.1561 |
| Sample | KH-550 concentration (g/mL) | Adhesion force (nN) | Young’s modulus (MPa) | Surface stiffness (nN/nm) |
| d | 0.0412 | −0.7057 | 5.727 | 0.5264 |
| e | 0.0793 | −1.004 | 5.423 | 0.2614 |
| f | 0.1146 | −0.5405 | 4.621 | 0.2856 |
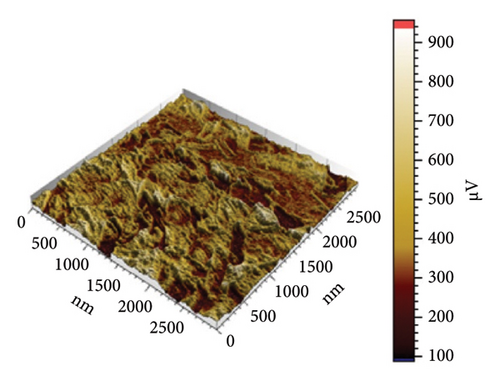
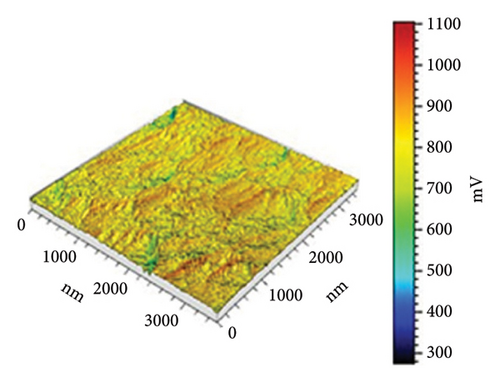
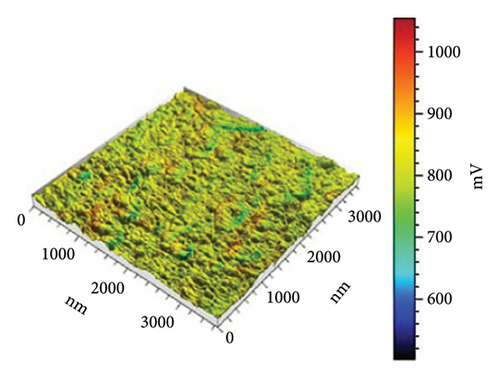
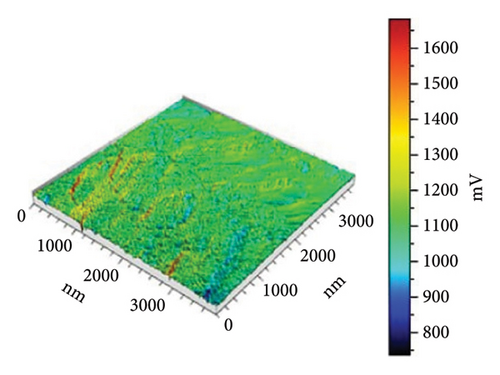
In Figure 10(a) with 0 g/mL KH-550, the rough surface exhibits height fluctuations and variable adhesion forces, as represented by the color scale (weight to brown) in microvolts (μV). When KH-550 concentration reaches 0.0412 g/mL in (b), the surface smooths and adhesion forces become more uniform. The color scale ranges from 300 mV to 1100 mV, ranging from blue to red in microvolts (μV). The color scale shows that 0.0793 g/mL KH-550 in (c) smooths and adheres better. The smoothest surface is obtained by raising the concentration to 0.1146 g/mL in (d); however, the color scale ranges up to 1600 mV due to adhesion force variability. The mV scale describes the size of the electrical signal measured with the help of the AFM. When near to the red mark, certain adhesion forces can be considered as high and for the other end of the scale which is the blue mark, the adhesion forces are considered to be low. The color fluctuation indicates strong and low adhesion forces on the surface. Equal colors indicate the same adhesion, whereas heterogeneous patches indicate different adhesion coefficients at different places. The mV readings give information as to how the AFM tip is interacting with the surface of the sample. These interactions are dependent on surface roughness, the type of material, and the use of additives such as KH-550. This implies that KH-550 enhances the surface properties and adhesion though there is a recommended amount of KH-550 additive around 0. 0793 g/mL as the optimal density for the best balance.
3.2. Corrosion Behavior of 316L Stainless Steel Coated With Silanized HAP–CS–MgO Nanocomposites
The effect of coating time on the corrosion rate of HAP–CS–MgO nanocomposites has been studied and the data of the samples (a, b, c and d) are presented in Table 5). Initially, the base metal (Sample a) exhibited a high corrosion rate of 0.4249 mm/year, as shown in Table 5 and Figure 11. After 5 min of coating (Sample b), the corrosion rate drops to 0.3825 mm/year, improving corrosion resistance. Increasing the coating period to 10 min (Sample c) lowers the corrosion rate to 0.3459 mm/year, demonstrating effective corrosion prevention. However, extending the coating time to 15 min (sample d) slightly increases the corrosion rate to 0.3582 mm/year, implying that excessively long coating times may not continue to enhance, and might even slightly reduce, corrosion resistance. Thus, it can be concluded that 10 min of coating application time seems to offer the greatest protection from corrosion.
| Sample | Duration of coating (min) | Coating properties | ||
|---|---|---|---|---|
| Io (A) | Equilibrium E (V) | Corrosion rate (mm/year) | ||
| a | Base metal | 1.407 × 10−5 | −0.830 | 4.249 × 10−1 |
| b | 5 | 1.267 × 10−5 | −0.880 | 3.825 × 10−1 |
| c | 10 | 1.146 × 10−5 | −0.899 | 3.459 × 10−1 |
| d | 15 | 1.186 × 10−5 | −0.829 | 3.582 × 10−1 |
| Sample | KH-550 concentration (g/mL) | Io (A) | Equilibrium E (V) | Corrosion rate (mm/year) |
| e | 0.0412 | 2.078 × 10−6 | −0.334 | 6.274 × 10−2 |
| f | 0.0793 | 9.715 × 10−7 | −0.348 | 2.933 × 10−2 |
| g | 0.1146 | 1.125 × 10−6 | −0.341 | 3.395 × 10−2 |
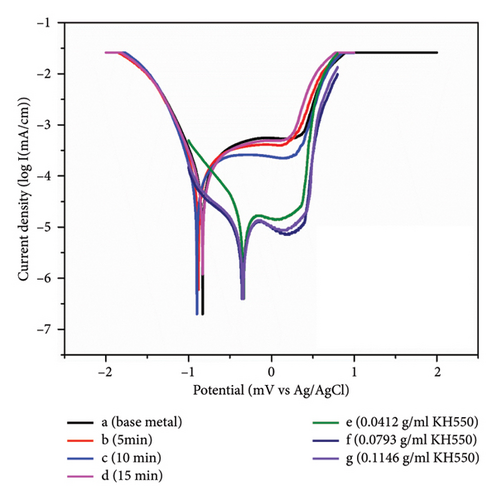
The incorporation of KH-550 at varying concentrations into HAP–CS–MgO, which was coated for 10 min at 5V (Sample c), also markedly impacts the corrosion rate, as illustrated by samples (e, f, and g). At a KH-550 concentration of 0.0412 g/mL (Sample e), the corrosion rate is 0.06274 mm/year. Increasing the concentration to 0.0793 g/mL (Sample f) greatly decreases corrosion to 0.02933 mm/year, improving corrosion resistance. Increasing KH-550 to 0.1146 g/mL (Sample g) slightly increases corrosion to 0.03395 mm/year. This implies that KH-550 enhances corrosion resistance up to 0.0793 g/mL, beyond which the advantages may plateau or reduce. KH-550’s coupling agent properties increase stainless steel corrosion resistance when applied to HAP, CS, and MgO coatings. The silane coupling agent KH-550 (γ-aminopropyl triethoxysilane) improves adhesion between coating materials (HAP, CS, and MgO) and metal substrates. Improved adherence generates a more uniform and thick covering that repels corrosion. In particular, KH-550 strengthens chemical connections between coating materials and stainless steel [42]. These stronger connections prevent coating delamination and micropores and microcracks, which may let water and oxygen in [43, 44]. This improves coating integrity and longevity, preventing corrosion. KH-550 also improves coating particle dispersion, creating a more homogeneous coating layer and reducing corrosion in stainless steel samples [45].
4. Conclusion
In this study, the silane coupling agent KH-550 and HAP–CS–MgO composites were electrophoretically deposited onto 316L stainless steel, resulting in improved corrosion resistance and enhanced surface morphology. The optimized anodization time of 10 min produced coatings that were rougher and denser, and exhibited stronger adhesion, as confirmed by AFM and SEM analyses. Corrosion resistance tests revealed that after 10 min of application, the HAP–CS–MgO composite reduced the corrosion rate to 0.3459 mm/year. The addition of KH-550 at an optimal concentration of 0.0793 g/mL further decreased the corrosion rate to 0.02933 mm/year, significantly enhancing corrosion protection. This optimal condition also improved adhesion force to −1.0 nN and yielded a coating thickness of 117.5 μm, demonstrating that KH-550 plays a crucial role in improving coating durability and protection.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Anees Kadhim Tayyeh: writing original draft, resources, and investigation. Ahmed F. Hasan: conceptualization, review and editing, supervision, project administration, data curation, methodology, and formal analysis.
Funding
No funding was received for this research.
Acknowledgments
The authors extend their appreciation to the Middle Technical University, Technical College Baghdad/School of Materials Engineering laboratories for their valuable support, with special thanks to Dr. Nabil Kadhim Taieh.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




