Optimization of Bio-Based Adhesive Synthesis From Gelatin and Euphorbia abyssinica Latex
Abstract
This investigation is an attempt to extract bio-based adhesives from gelatin and plant latex to overcome the negative effects of synthetic adhesives. Since synthetic adhesives (CM-43) have environmental impact due to resource depletion, production emission, toxicity and pollution, and nonbiodegradability character, bio-based adhesive utilized renewable resources, which has biodegradability, contains nontoxic chemicals, and has eco-friendly properties. So, this bio-based adhesive was extracted by mixing plant resins from Euphorbia abyssinica as additives with gelatin through optimization. The factors in the synthesis of bio-based adhesive were temperature, time, and concentration with levels of 50°C–60°C, 30–60 min, and 50%–75% gelatin; and the optimum level was found 50°C, 45 min, and 75%, respectively, with the optimum result of 282.12 g gel strength. The characteristics of the resulting bioadhesive were 6.25 cP, 6.85, and 11.53% of viscosity, pH, and moisture content values, respectively. The performance of the bioadhesive was examined in terms of peel and shear strength, and it has an average value of 6.645 N/mm and 198.1 N, respectively. So, this research is aimed at overcoming the environmental burden of synthetic adhesives by the production of alternative bioadhesives from gelatin and plant resin.
1. Introduction
According to [1], adhesives are any binding polymers that join two or more things together through adhesion and cohesion forces. Nowadays, adhesives are an effective transition agent with a very large application and market demand due to their wide application areas like packaging materials [2], automobile manufacturing [3], aerospace [4, 5], construction [6, 7], medicine and healthcare [8], and leather [9] and textile industries [10]. There are different types of adhesives and mostly grouped under synthetic and natural adhesives based on their source of origins [11], and mostly, industries nowadays utilize synthetic adhesives [12] where these types of adhesives are harmful [13], toxic [14], nonrenewable [15], and environmentally unfriendly [16] because they are derived from petroleum and natural gas–based chemicals [17, 18]. According to a report [19], the demand for synthetic adhesive is dramatically increased and worth $60 billion in FY 2021, and the effect on the environment and the workers also increased.
Recently, many scholars have investigated different findings to combat the negative effect of petroleum-based adhesive; and adhesive industries have turned their eyes toward bio-based adhesives [20]. Bio-based adhesives are extracted from different biosources like animals [21–23], plants [18, 24], and micro-organisms [22, 25]. Plant-based bioadhesives are mostly either starch [26, 27] based or cellulose [18, 28] based in nature and the extraction leads to extinction of the plant. The investigations in [29, 30] presented that animal-based adhesives are extracted from the collagenous part of an animal like from the gut, skins/hides, and intestine [31, 32] and animal glues are one of the well-known animal-based adhesives extracted from the hydrolysis of animal structural protein called collagen through alkaline [33], acidic [34], enzymatic [35], or combined treatments of the collagen structure.
As researchers compare and contrast synthetic and bio-based adhesive in terms of environmental concepts, synthetic adhesives are typically petroleum-based products [13] which leads to significant greenhouse gas emissions during production, depletion of nonrenewable resources, and potential toxicity and persistence in the environment due to their nonbiodegradability and containment of volatile organic compounds (VOCs) contributing to air pollution. However, bio-based adhesives are derived from renewable resources (e.g., gelatin, plant starches, proteins), resulting in reduced reliance on fossil fuels, lower greenhouse gas emissions during production, and enhanced biodegradability at end of life [20]. In the environmental perspective, bio-based adhesives are much better than synthetic adhesives [36].
Even though many researchers extract animal glues from the skins of animals, the extracted glue has limitations on microbial susceptibility [37], weaker in bonding strength [38], and its shelf life will be affected and this limits its applications and performance. Investigations have been conducted by different researchers [39–41] to modify the physical, chemical, and antimicrobial properties of animal glue, but all modifications use chemicals that are costly, harmful, and harsh to the environment.
The present study is a continuous research from [42] and focuses on the production and optimization of bio-based adhesive from gelatin extracted previously from tannery solid waste mixing with latex from Euphorbia abyssinica plant to provide an alternative adhesive for the hazardous synthetic adhesive. The aim of using plant latex is to improve the antimicrobial properties and performance of the bioadhesives and as a result to improve the shelf life of the adhesive. This work has been posted as a preprint shown in [43] to get feedback from scholars, but it has not been peer-reviewed or published anywhere.
2. Materials and Methods
2.1. Materials
2.1.1. Gelatin
Gelatin was taken from the result of previous research which was extracted through an alkaline extraction method from Bahir Dar tannery solid waste, Bahir Dar, Ethiopia.
2.1.2. Euphorbia abyssinica
Euphorbia abyssinica resin was collected from around Bahir Dar City, Bahir Dar, Ethiopia, by incision of the plant parts.
2.2. Machinery and Equipment
The BI-PH-710L model digital pH meter (with pH range 0.00~14.00pH, pH accuracy ±0.05pH, and resolution 0.01pH), digital viscometer (Brookfield, Spindle No. 3) (at 60 rpm at room temperature), Brookfield texture analyzer (distance 4 mm, speed 0.5 mm/s, and spindle R3), dryer machine (with max. temp 250°C, min. temp ambient + 30°C, volume 28–128 L), PerkinElmer differential scanning calorimetry (DSC) (temperature range of 20°C–250°C), and PerkinElmer Fourier transform infrared spectroscopy (FTIR) in the Ethiopian Institute of Textile and Fashion Technology (EiTEX) laboratory (with minimum 1000 and maximum 4000 wavelength) were the equipment and machinery that the researcher utilized during the research. In addition, a bottle, a stirrer, a polyester fabric, bikers, a sharp knife, a round bottom flask, an Erlenmeyer flask, a water bath, a shaker with a heating incubator, a refrigerator, a digital scale, and a mixer were utilized.
2.3. Methods
In this research, the extracted and characterized gelatin was taken from the earlier study and plant latex was collected. The plant latex was characterized in terms of its moisture content, pH, and chemical constituents through FTIR. Then, bioadhesive synthesis was conducted where concentration, temperature, and time were independent variables influencing the dependent variable gel strength. After the synthesis process, the physicochemical characteristics of purified bioadhesive were determined. Finally, the performance of the extracted adhesive was evaluated at the EiTEX leather construction laboratory. Figure 1 depicts the general methodology of the research.
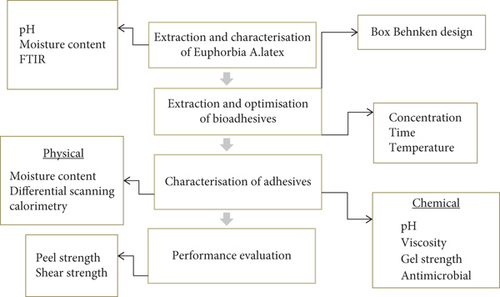
2.4. Research Design
The experimental research design was applicable by using the Box–Behnken design (BBD) through Design-Expert software with three independent variables having maximum and minimum levels and one dependent variable. The reason why BBD was selected is due to its offer of balanced approach to experimental design, providing efficient, flexible, and robust methods for optimizing processes while minimizing resource use and no aliasing of effects.
2.5. Characterization of Gelatin
Since this study is a proceeding study from [42], extraction and characterization of gelatin were known. Even though the viscosity, gel strength, moisture content, color, bacterial percent of reduction, and pH characteristics of gelatin were known, the researcher in this study also conducted characterizations to assure the previous result.
2.6. Extraction and Characterization of Plant Resin
The liquid resin is easily collected from around Bahir Dar, Ethiopia, by incision of the cover or part of the plant by manual method of hurting the plants with a cutter. The milky like resin was then easily collected and its physical and chemical properties characterized like pH, moisture content, and chemical nature.
2.6.1. FTIR Analysis of the Latex
The nature of the chemical structure, functional group, and bonding information of plant latex was examined by PerkinElmer FTIR models with FTIR analysis where its spectrum was arranged between 4000 and 250 cm−1 at Bahir Dar Institute of Technology laboratory.
2.6.2. Moisture Content
The moisture content of the plant latex was measured in ETADRY machines with drying capacity of 50–150 kg and temperature range of 30°C–120°C by taking a 79.905-g sample and heating at 105°C for 1 h.
2.6.3. pH
The pH of the solution was measured by the BI-PH-710L model digital pH meter (with pH range 0.00~14.00pH, pH accuracy ±0.05pH, and resolution 0.01pH) by taking five trials to determine the charge character of the plant resin.
2.7. Bioadhesive Synthesis and Characterization
2.7.1. Bioadhesive Synthesis
The bioadhesive synthesis process was optimized by using BBD with concentration, temperature, and mixing duration as control variables having 50%–75% gelatin, 50–60, and 30–60 min levels, respectively, and the response was gram strength. There were 13 numbers of experiments which was proposed by the software as shown in Table 1.
| No. of run | Factors | Gram strength | ||
|---|---|---|---|---|
| Percentage | Time | Temp. | ||
| 1 | −1 | −1 | 0 | — |
| 2 | −1 | 1 | 0 | — |
| 3 | −1 | 0 | −1 | — |
| 4 | −1 | 0 | 1 | — |
| 5 | 1 | 0 | 1 | — |
| 6 | 1 | 0 | −1 | — |
| 7 | 1 | 1 | 0 | — |
| 8 | 1 | −1 | 0 | — |
| 9 | 0 | 1 | −1 | — |
| 10 | 0 | −1 | −1 | — |
| 11 | 0 | 1 | 1 | — |
| 12 | 0 | −1 | 1 | — |
| 13 | 0 | 0 | 0 | — |
2.7.2. Characterization of Bioadhesive
After obtaining the bioadhesive, the physicochemical characteristics were examined because those characteristics determine the properties and quality of the final glues. As the literature in [36] shows gel strength and viscosity are the two main characteristics of bioadhesive that determine the characteristics of the final adhesive, that is why the response in this experiment was gel strength.
2.7.2.1. Viscosity
In this work, the resulting glue’s viscosity was determined by using a digital viscometer (Brookfield, spindle No. 3) (at 60 rpm at room temperature). The viscometer’s speed, type of spindle, and revolution time were set at 60 rpm and R3 for 15 min, respectively, in the Bahir Dar Institute of Technology laboratory.
2.7.2.2. pH
The pH of the bioadhesive was examined by the BI-PH-710L model digital pH meter (with pH range 0.00~14.00pH, pH accuracy ±0.05pH, and resolution 0.01pH) by taking five trials.
2.7.2.3. Moisture Content
2.7.2.4. Thermal Analysis of Bioadhesive
The thermal nature of the resulted adhesive was determined by using PerkinElmer DSC (temperature range of 20°C–250°C) by taking 13 mg of the sample at the temperature range of 20°C–250°C and the peak, delta H, and area were analyzed and the graph was expressed in terms of heat flow versus temperature.
2.7.2.5. Microbial Test
2.8. Application and Quality Assessment of Bioadhesive
After the synthesis of the adhesive, the quality was assessed compared with the conventional glue which is available in the EiTEX leather construction laboratory.
2.8.1. Shear Test
A single-lap shear test method was applicable referring to ASTM D1002 standard because of its working simplicity as well as accuracy in result by using HD-B615-S model universal tensile testing machine at the EiTEX textile laboratory.
2.8.2. Peel Test
The T peel test method was applicable, and the sample was prepared based on the STMD5170 standards. This test was conducted by using the HD-B615-S model universal tensile testing machine at the EiTEX textile laboratory.
3. Result and Discussions
3.1. Characteristics of Gelatin
The gelatin has a viscosity, gel strength, moisture content, and pH of 6.85 centipoises (cP), 245-g gel strength, 12.11%, and 7.2, respectively [42]. The gelatin percent of bacterial reduction was 3.17% for gram-positive bacteria and 1.41% for gram-negative bacteria, and these show that gelatin is highly susceptible to bacterial action. The color L, a, and b values of the gelatin were 78.94, 5.2, and 14.64 which indicates that gelatin has a yellowish color.
3.2. Characterization of Plant Resin
3.2.1. FTIR Analysis of Extracted Plant Resin
The FTIR analysis result of plant latex is presented in Figure 2.
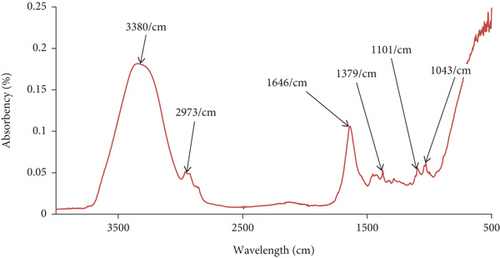
Based on the absorbance versus wavelength graph of FTIR shown in Figure 2, the plant resin absorbs at 3380, 2973, 1646, 1379, 1101, and 1043 cm−1 wavelength/centimeter and that wavelength represents O-H (alcohol) which has broader peaks, O-H (carboxyl acid), C=O (amide), C-O (ether), alkyl ketone, and ether, respectively. As FTIR analysis shows, the plant latex has an amide group, carboxyl acid, ether, alkyl ketone, and alcohol and this makes the latex acidic in nature and the ketone group increases its antimicrobial effect. Based on the presence of abundant (wide) hydroxyl groups in the graph, the resin exhibits acidic characteristics. The FTIR graph suggests that the sample contains a mix of functional groups, including hydroxyl groups, aliphatic hydrocarbons, carbonyl groups, and possibly aromatic or unsaturated components.
Since gelatin is a protein rich in amide (-CONH-) groups and hydroxyl (-OH) groups from its constituent amino acids, the plant resin is likely to interact with gelatin primarily through hydrogen bonding and hydrophobic interactions.
3.2.1.1. Moisture Content
3.2.1.2. pH
The pH of the latex was determined by taking five trials (6.5, 6.3, 6, 5.9, and 6.1), and the mean was 6.16 which is weak acidic due to the presence of carboxylic acid and ether; but the pH of gelatin was near to neutral. So there may be a tendency for neutralization.
3.3. Synthesis of Bioadhesive
The result of the experiment in this study is presented in Tables 2 and 3 with the independent variables, dependent variable, and the number of runs based on the BBD.
| Std | Run | A : temperature (°C) | B : time (min) | C : concentration (%) | Gram strength (g bloom) |
|---|---|---|---|---|---|
| 1 | 1 | 50 | 30 | 62.5 | 268 |
| 13 | 2 | 55 | 45 | 62.5 | 257 |
| 9 | 3 | 55 | 30 | 50 | 246.65 |
| 3 | 4 | 50 | 60 | 62.5 | 260 |
| 11 | 5 | 55 | 30 | 75 | 271.55 |
| 2 | 6 | 60 | 30 | 62.5 | 251.15 |
| 5 | 7 | 50 | 45 | 50 | 252 |
| 10 | 8 | 55 | 60 | 50 | 243.89 |
| 15 | 9 | 55 | 45 | 62.5 | 256 |
| 8 | 10 | 60 | 45 | 75 | 268 |
| 6 | 11 | 60 | 45 | 50 | 238.45 |
| 4 | 12 | 60 | 60 | 62.5 | 250.35 |
| 7 | 13 | 50 | 45 | 75 | 282.12 |
| 14 | 14 | 55 | 45 | 62.5 | 257.25 |
| 12 | 15 | 55 | 60 | 75 | 274.45 |
- Abbreviation: Std, standard.
| Source | Sum of square | Df | Mean square | F value | p value | |
|---|---|---|---|---|---|---|
| Model | 2033.04 | 3 | 677.68 | 122.35 | < 0.0001 | Significant |
| A—temperature | 366.04 | 1 | 366.80 | 66.22 | < 0.0001 | Significant |
| B—time | 9.37 | 1 | 9.37 | 1.69 | 0.2199 | Not significant |
| C—concentration | 1656.80 | 1 | 1656.80 | 299.13 | < 0.0001 | Significant |
| Residual | 60.93 | 11 | 5.54 | — | — | — |
| Lack of fit | 60.05 | 9 | 6.67 | 15.25 | 0.0630 | Not significant |
The adhesion and cohesion strength is dependent on its gel strength [42], and it was the dependent variable in this experiment. As shown in Table 2, the maximum result was obtained in Run No. 13 with an optimum gram strength value of 282.12 g bloom. The concentration of gelatin is directly related to the gram strength of bioadhesives [44]. As presented in Table 2, we are 97% confident that the increment of concentration from 50–75 increases the gel strength of the adhesive from 238.45 to 282.12 g bloom due to the increase of cohesive and adhesion force but the effect of temperature was inversely proportional to gel strength due to the degradation of bonds in the protein content of gelatin. Time has no significant effect on the bioadhesive synthesis, but increased temperature with minimum time had better gel strength and vice versa. The gel strength at 60°C and 30 min has better results than that at 60°C and 60 min which is 251.15 g greater than 250.35 g. However, we are 97% confident that the rise of gelatin concentration reduces the bacterial-resistant properties of bioadhesives. Generally, both harsh and inadequate extraction condition affects the quality and quantity of bioadhesives.
3.4. Analysis of Bioadhesive Synthesis
3.4.1. Analysis of Variance (ANOVA) of Bioadhesive Synthesis Optimization
ANOVA was used to validate the model for the response of the experiment, and its result is presented in Table 3.
As it is presented in Table 3, the model is significant with a value of 0.0001 which indicates we can be 97% sure that the selected variables are adequate for the experiment. Temperature and concentration were significant with values of 0.0001, but time was not significant. The nonsignificant lack of fit shows that the variance due to effects included in the model is significantly lower than the variation in the randomized experiment and possible to conclude that the researchers are 97% confident that they use the right model (experiment) which means data in the model is described correctly. Time is not significant which means it has not much effect on the response, but temperature and concentration have a great effect on the dependent variable.
3.4.2. Fit Statistics
| Std. dev. | 2.35 | R2 | 0.9709 |
| Mean | 258.46 | Adjusted R2 | 0.9630 |
| CV% | 0.9106 | Predicted R2 | 0.9423 |
| Adeq precision | 34.82 |
The coefficient of determination (R2) is a statistical measure of the percentage of variance of the dependent variable described by one or more independent variables in the regression model, and it describes how the variance of one variable explains the variance of the second variable. Therefore, if R2 of the model is 0.50, about half of the observed variation can be explained by the input of the model, but as it is shown in the table, the value of R2 is 0.97 or 97% which is the best value and indicates that the model has the fraction by which the variance of the errors is less than the variance of the dependent variable. Adjusted R2 is always smaller than R2, but the difference is usually very small unless you are trying to estimate too many coefficients from too small a sample in the presence of too much noise so this research has adjusted R2 less than R2 values which is 0.9630 < 0.9709 and their difference has smaller values which is 0.0079. In this research, the adjusted and predicted R2 has reasonable agreements that their difference is less than 0.2 which is 0.0207.
3.4.3. Regression Analysis
As shown in the regression equation and formula, gram strength has a direct relation with concentration but negatively correlates with time and temperature. As the coefficient of the independent variable indicates concentration and temperature have greater values, this shows the variables have a high effect on the response. As many literatures [45, 46] state, animal-based adhesives with high concentration values have high molecular weight and increase the gram strength of bioadhesives. Another important factor in bioadhesive synthesis is temperature; the increment of values decreases the values of the dependent variable due to the degradation of the protein peptide bonds and dissociation of the protein molecules by the heat energy [47].
3.5. Characterization of Bioadhesives
The physicochemical characterization of the adhesive is presented below.
3.5.1. Viscosity
The resulting bioadhesives from a combination of plant resin and gelatin have an optimum viscosity of 6.25 cP. Investigation in [48] showed that the viscosity of animal-based glues ranges from 2 to 7 cP and the lower viscosity values of animal glue affect the gel strength of the solution which affects the adhesion strength of the final glues and makes low-grade glue. The larger viscosity value indicates that the solution has more molecular weight and this increases the cohesive force of the molecules in the adhesive which improves the bond strength of the bioadhesives [49].
3.5.2. Percent of Moisture Content
The moisture content of the synthesized bioadhesive has less moisture content values than gelatin which is due to evaporation of some water content during mixing as well as experimental running with action of heat [50].
3.5.3. Microbial Test
Based on the observation and qualitative examination, gelatin extract has more bacterial growth within 24 h and it was observed that the bacteria accumulated on the petri dish but the bioadhesive obtained was reduced. The numbers of bacteria counted in the bioadhesive after incubation for E. coli and S. aureus were 3.5 and 2.9 cfu/mL, so based on this information, the percent of reduction was concluded and the result is 50.7% and 54%, respectively, which indicates that the plant resin can improve the microbial resistance of bioadhesives. The percent of bacterial reduction for the gelatin alone was 3.17% for gram-positive bacteria and 1.41% for gram-negative bacteria. But, the percent of bacterial reduction of bioadhesive was 50.7% and 54% for gram-negative and gram-positive bacteria, respectively, which is much better than gelatin. Based on this result, the addition of plant resin can improve the microbial resistance and shelf life of gelatin and this makes bioadhesive durable and last long. The shelf life of bioadhesive was determined by checking and observing different characteristics of the bioadhesive after some days, weeks, and months. The bioadhesive was manufactured on 23/10/2022 GC in the EiTEX textile laboratory, and the test of shelf life was conducted for a year in terms of gel strength, viscosity, pH, and moisture content after 2 weeks, a month, and a year at 07/11/2014, 24/11/2014, 09/12/2022, and 08/12/2023 GC, respectively, and the result is presented in Table 4.
| Dates (GC) | Parameters | Results | Deviations for the original |
|---|---|---|---|
| 07/11/2014 | Gel strength (g) | 282.1203 | −0.0003 |
| Viscosity (cP) | 6.2502 | −0.0002 | |
| pH | 7.4395 | 0.0005 | |
| Moisture content (%) | 11.5295 | 0.0005 | |
| 24/11/2014 | Gel strength (g) | 282.1259 | −0.0059 |
| Viscosity (cP) | 6.2596 | −0.0096 | |
| pH | 7.42 | 0.02 | |
| Moisture content (%) | 11.525 | 0.005 | |
| 09/12/2014 | Gel strength (g) | 282.1266 | −0.0066 |
| Viscosity (cP) | 6.2599 | −0.0099 | |
| pH | 7.4175 | 0.0235 | |
| Moisture content (%) | 11.5212 | 0.0088 | |
| 08/12/2023 | Gel strength (g) | 282.1265 | −0.0065 |
| Viscosity (cP) | 6.2597 | −0.0097 | |
| pH | 7.4175 | 0.0235 | |
| Moisture content (%) | 11.5211 | 0.0087 | |
So, as it is shown in Table 4, the bioadhesive is not susceptible to microbial action and this makes the bioadhesive have good shelf life. But in the currently available glue which is made only from gelatin, it is highly susceptible to microbial susceptibility and its shelf life or storage condition is not good, if it gets wet.
3.5.4. pH
Five trials were taken with a value of 6.5, 6.75, 6.9, 7, and 7.1, and the average result was 6.85 which is less than the gelatin pH (7.2); this shows that the plant resin can neutralize extracted gelatin and reduce the pH from a neutral state to mild acidic condition because of the presence of weak acid in the plant.
3.5.5. Thermal Analysis of Extracted Bioadhesives
The result of thermal analysis is indicated with its peak, enthalpy, delta H, and area in Figure 3. The values of peak, enthalpy, and area were 108.15°C, 4190.3710 J/g, and 50,284.452 mJ, respectively. But the figure does not show Tg.
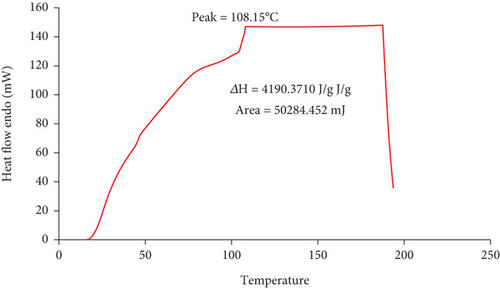
As Figure 3 shows, the melting temperature of bio-based adhesive was 108.15°C and the glass transition temperature starts from above room temperature and then changes its state around 200°C. Figure 3 provides the DSC thermogram, showing the heat flow during a temperature ramp. It displays an endothermic peak, indicating a phase transition. The peak temperature (108.15°C) is likely the melting point (Tm) of the bioadhesive, and the area under the curve represents the enthalpy change (ΔH) associated with melting. The sharp drop after the peak suggests crystallization or another phase change upon cooling. The relatively low melting point (108.15°C) suggests the bioadhesive could be applied at temperatures below this value. However, for applications requiring high thermal stability, this might be a limitation. If the application requires temperatures above 108.15°C, the adhesive would melt and lose its structural integrity.
3.6. Performance Evaluation of the Adhesive
3.6.1. Shear Test
In most cases, standard glues have > 200 N shear strength based on [30], and the glues extracted in this research have an average of 198.1 N shear strength by taking three trials (196.5, 199.45, and 198.35) which is comparable with the conventional glues.
3.6.2. Peel Test
The result of the average peel test was 6.645 N/mm which has some less deviation (1.54) when compared with conventional CM-43 glues.
The apparel and leather manufacturing industries in Ethiopia consume temporary glue like CM-43 in most of the time as a presewing attachment mechanism, but it is not environmentally friendly as its precaution indicates. For this reason, this bioadhesive can be considered as a solution and an alternative means for CM-43 and other temporary glues.
3.7. Comparison With Industry Standards
This section provides a thorough analysis of the bioadhesive’s performance in relation to established industry norms, extending beyond the CM-43 standard. This analysis is aimed at contextualizing our findings and demonstrating the competitiveness of the bioadhesive formulation in terms of adhesion strength, durability and aging, moisture resistance, and environmental impact.
Many traditional adhesives, such as epoxy and polyurethane including CM-43, achieve adhesion strengths measured in megapascal, often exceeding 10 MPa for structural applications. Even this bioadhesive is not applicable for structural bonding, it demonstrates adhesion strengths that are competitive within specific applications, particularly in the apparel and leather industries and for packaging materials, where it achieves strengths comparable to temporary synthetic adhesives like CM-43.
In the sustainability standards of adhesives, it is recommended that industry standards emphasize the environmental impact of materials, including biodegradability, eco-friendly production, and the use of renewable resources. The bioadhesive formulated in this research leverages renewable resources, aligning well with sustainability goals and potentially offering a lower environmental footprint compared to traditional adhesives and this is beyond CM-43. By comparing the bioadhesive’s performance to industry standards beyond CM-43, we identify both strengths and areas for improvement for further intervention to improve its quality. This thorough analysis not only highlights the competitiveness of our bioadhesive but also sets the stage for further optimization and development to meet or exceed industry expectations comprehensively.
4. Conclusion
The effect of synthetically produced carcinogenic, hazardous, nonrenewable, toxic, and harmful adhesives on the environment can be reduced by providing alternative bio-based adhesives. This work investigates the percentage of gelatin, time, and temperature that affect bioadhesive production, and the optimum level was 75% gelatin, 45 min, and 50°C with optimum gram strength. In this investigation, Euphorbia latex was utilized as an antibacterial agent and increased the bacterial resistance of the gelatin. The extracted bioadhesive has comparable properties with the standard glue including 282.12 g gel strength, 6.2 cP viscosity, 11.53% moisture content, and 108.15°C thermal property. The adhesives extracted from bio-based sources can be used as eco-friendly, nontoxic, safe, and sustainable products that can be applicable in the apparel industry. Generally, this research is climacteric for utilization of the gelatin and plant latex for the production of sustainable bio-based adhesives.
5. Recommendation
This study was undertaken as a pilot study and more rigorous experimental control, a greater number of samples, and statistical analysis of the data would have allowed drawing more definitive conclusions. It was all things considered set up that the physical and mechanical properties of collagen pastes can shift considerably with the sort of stick and with the way it is ready. Since this study had limitations on characterization due to lack of instruments, the researcher recommends the future researcher to analyze extracted gelatin as well as bioadhesive using those necessary equipment like HPLC for full information on the amino acid content. Future research should focus on studying the interaction or reaction of the plant resin and extracted collagen whether it has covalent, ionic, or coordination bonds happening during mixing of the two chemicals together. In this work, the researchers conduct the shelf life of the bioadhesive in constant environmental condition and recommend the future investigator to conduct the shelf life in various environmental settings of temperature, humidity, and pressure.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
L.G.A. experimented with the synthesis of bioadhesives, characterized them, and conducted a performance evaluation of the resulting adhesive. T.T. designed the experiment through Design-Expert software and did the tables and figures of the manuscript.
Funding
The authors declare that there were no external funding sources for this research project.
Acknowledgments
The authors in this article highly acknowledge the Ethiopian Institute of Textile and Fashion Technology and its laboratories that helped us to complete the work. In addition, the researchers acknowledge the Research Square where this manuscript has been posted (10.21203/rs.3.rs-3639930/v1) for feedback from different authors but it has not been peer reviewed.
Open Research
Data Availability Statement
The data collected and analyzed during this investigation are included in the manuscript and may also be obtained from the authors upon a reasonable request.




