Diabetes Care Disparities: The Impact of Lifestyle, Medication Access, and Medical Conditions on Glycemic Control in Rural and Urban Settings
Abstract
Background: Optimal glycemic control in Type 2 diabetes remains a critical public health challenge. This study was aimed at elucidating the sociodemographic, lifestyle, and clinical factors associated with poor glycemic control among Iranian patients.
Materials and Methods: A cross-sectional study was conducted on 546 patients with Type 2 diabetes at Imam Hossein Hospital, Tehran, from July 2023 to December 2024. Participants were categorized into good (HbA1c < 7%) and poor (HbA1c ≥ 7%) glycemic control groups. Data were collected using structured checklists and validated tools. Missing data were handled with multiple imputation, and logistic regression identified predictors of glycemic control.
Results: Rural residence significantly increased the odds of poor glycemic control (OR = 3.04, 95% CI: 1.98–4.65). Limited medication access was a major determinant; participants reporting “rarely” or “sometimes” having access to prescribed medications had markedly higher risks (OR = 5.16, 95% CI: 2.41–11.06, and OR = 3.75, 95% CI: 2.20–6.40, respectively). Engaging in moderate and high physical activity (based on metabolic equivalent) was protective (OR = 0.49 and OR = 0.40, respectively). Additionally, each extra day per week of fatty food consumption increased the odds of poor control by 26% (OR = 1.26, 95% CI: 1.09–1.46). A 10 mg/dL increase in HDL cholesterol reduced risk (OR = 0.86, 95% CI: 0.75–0.98, p = 0.04), while longer durations of oral medication use were associated with a modest risk increase (OR = 1.05, 95% CI: 1.01–1.10, p = 0.02).
Conclusions: These findings underscore the significance of geographic disparities, medication access, lifestyle practices, and dietary behaviors in diabetes management. Targeted interventions tailored to these factors are essential to improve glycemic outcomes in Type 2 diabetes.
1. Introduction
Diabetes ranks among the top 10 global causes of death [1] and is associated with a significantly elevated risk of severe health complications, leading to increased healthcare costs, diminished quality of life, and higher mortality rates [2].
The prevalence of diabetes and impaired glucose tolerance among adults has been steadily increasing in recent decades [3]. In 2017, diabetes affected an estimated 8.4% of adults aged 18–99 years, with projections indicating a rise to 9.9% by 2045. This widespread prevalence poses significant social, economic, and developmental challenges, particularly in low- and middle-income countries [4].
Inadequate glycemic control in patients with Type 2 diabetes mellitus (T2DM) represents a significant public health concern and a key risk factor for the development of diabetes-related complications [5]. A cross-sectional study revealed that over two-thirds (74%) of patients had poor glycemic control [6]. Previous studies assured that poor glycemic control correlated with an enlarged risk of visual impairment [5], an enlarged risk of kidney failure [6], and an enlarged risk of cardiovascular disease [7].
Globally, poor blood sugar control is becoming increasingly prevalent. Studies report alarmingly high rates in countries such as India (91.8%), Saudi Arabia (74.9%), and Bangladesh (76%). These statistics underscore substantial challenges in diabetes management, driven by factors such as unhealthy lifestyle changes, inadequate diets, physical inactivity, and limited access to healthcare services [8]. Additional contributing factors include a lack of awareness, time constraints, insufficient healthcare workforce, poor adherence to treatment, and, most critically, the absence of appropriate guidelines and diabetes education for both caregivers and patients [9, 10].
Previous studies have reported that suboptimal glycemic control could cost diabetes patients more care requirements, associated healthcare costs, and loom the complications [11, 12]. Numerous studies have investigated the risk factors and determinants of poor glycemic control. However, many of these studies have failed to account for critical confounding factors, such as access to treatment, insulin dosage, and the types of medications used by patients—particularly in observational studies. Furthermore, previous research has often focused on a single aspect, such as clinical factors, lifestyle-related factors, or self-care behaviors, without providing a comprehensive analysis. To address these gaps, this study was designed to investigate the factors associated with poor glycemic control among patients with Type 2 diabetes in Iran in a more holistic manner.
2. Methods
This cross-sectional study was aimed at evaluating glycemic control in two groups of patients with Type 2 diabetes, categorized based on their HbA1c levels. Patients with poor glycemic control (HbA1c ≥ 7%) were compared to those with good glycemic control (HbA1c < 7%) in order to identify demographic, clinical, and behavioral factors associated with glycemic outcomes. The study was conducted at the diabetes clinic of Imam Hossein Hospital in Tehran. Informed consent was obtained from all participants prior to enrollment. Ethical approval for this study was granted by the Research Ethics Committees of the School of Public Health & Safety, Neuroscience Research Center, Shahid Beheshti University of Medical Sciences (Approval ID: IR.SBMU.PHNS.REC.1402.036).
2.1. Study Population
- •
Diagnosis of Type 2 diabetes.
- •
Age ≥ 18 years
- •
Disease duration of at least 1 year
- •
Diagnosis of Type 1 diabetes or gestational diabetes
- •
Age < 18 years
- •
Disease duration of less than 1 year
- •
Lack of informed consent to participate
- •
Diagnosis of anemia
2.2. Data Collection
Demographic and clinical data were collected through a structured checklist during in-person visits. Data collection was done during 1 July 2023 to 30 December 2024 using a convenience sampling method. Variables included age, gender, weight, height, systolic and diastolic blood pressure, presence of diabetic foot ulcers, smoking status, disease duration, treatment accessibility, type of medication and dose of insulin, and residence location (urban/rural). Laboratory data, including HbA1c, LDL, and HDL, were extracted from patients’ medical records.
2.3. Assessment Tools
- •
Physical activity (PA): The International Physical Activity Questionnaire (IPAQ) assessed activity levels over the past 7 days, with results expressed in MET-minutes per week. The Persian version’s validity and reliability have been confirmed [13].
- •
Perceived stress: The Cohen’s 10-item Perceived Stress Scale (PSS) assessed stress levels over the past month. Higher scores indicated greater perceived stress. The tool’s Persian version has demonstrated adequate validity and reliability [14].
- •
Dietary habits: Dietary habits, including fruit and vegetable intake, consumption of high-fat foods, and adherence to dietary recommendations, were evaluated using items from the Tobert Self-Care Questionnaire. The Persian version has been validated with Cronbach’s alpha of 0.79 in Boogar’s study [15].
2.4. Statistical Analysis
2.4.1. Handling Missing Data
Missing data were addressed using multiple imputation to mitigate bias and maintain statistical power. Predictive mean matching (PMM) was used for imputing continuous variables, while the random forest method was applied for categorical variables. These techniques were implemented using the mice package in R, generating five imputed datasets [16]. The missing data mechanism was assumed to be missing at random (MAR), meaning that the probability of missingness could be explained by observed data. However, the potential for missing not at random (MNAR) was acknowledged as a limitation, as no formal tests were conducted to distinguish between MAR and MNAR mechanisms.
2.4.2. Descriptive Analysis
Descriptive statistics were reported for both categorical and continuous variables. For categorical variables, chi-square tests or Fisher’s exact tests (if expected cell counts were < 5) were used to compare distributions. For continuous variables, the Shapiro–Wilk test was employed to assess normality. If the data followed a normal distribution, means and standard deviations were reported, and comparisons were made using independent t-tests. If the data were nonnormally distributed, medians and interquartile ranges were reported, and comparisons were performed using the Mann–Whitney U test.
2.4.3. Inferential Analysis and Rubin’s Rules
- 1.
Pooled estimate of coefficients (β):
- 2.
Total variance (T):
- 3.
95% CI: The CI for each pooled estimate is calculated as
3. Results
3.1. Multiple Imputation Results
Following multiple imputation, the distributions of continuous variables in the imputed datasets closely resembled those in the original data. Variables such as HDL levels, duration of diabetes, number of cigarettes smoked per day, and presence of diabetic foot showed high consistency between the original and imputed datasets. Figure 1 illustrates the alignment of distributions, indicating adequate preservation of data patterns across key variables.
3.2. Characteristics of Study Participants
A total of 546 individuals with Type 2 diabetes participated in this study, with 276 (50.55%) categorized as having good glycemic control (HbA1c < 7%) and 270 (49.45%) classified under poor glycemic control (HbA1c ≥ 7%). The mean age of participants was 56.46 years (SD = 11.82), with no significant age difference observed between the two groups (p = 0.67). Females represented a majority of the cohort (65.93%), and gender distribution was similar between the groups (p = 0.47, Table 1).
| Characteristic | Overall N = 546a | Good control N = 276a | Poor control N = 270a | p valueb |
|---|---|---|---|---|
| Gender | 0.47 | |||
| Female | 360 (65.93%) | 178 (64.49%) | 182 (67.41%) | |
| Male | 186 (34.07%) | 98 (35.51%) | 88 (32.59%) | |
| Education level | 0.25 | |||
| Illiterate | 235 (43.04%) | 119 (43.12%) | 116 (42.96%) | |
| Primary school | 163 (29.85%) | 76 (27.54%) | 87 (32.22%) | |
| Middle school | 73 (13.37%) | 36 (13.04%) | 37 (13.70%) | |
| High school | 56 (10.26%) | 36 (13.04%) | 20 (7.41%) | |
| University education | 19 (3.48%) | 9 (3.26%) | 10 (3.70%) | |
| Marital status | 0.18 | |||
| Single | 14 (2.56%) | 10 (3.62%) | 4 (1.48%) | |
| Married | 439 (80.40%) | 213 (77.17%) | 226 (83.70%) | |
| Divorced | 6 (1.10%) | 3 (1.09%) | 3 (1.11%) | |
| Widowed | 87 (15.93%) | 50 (18.12%) | 37 (13.70%) | |
| Residence area | < 0.001 | |||
| Rural | 242 (44.32%) | 78 (28.26%) | 164 (60.74%) | |
| Urban | 304 (55.68%) | 198 (71.74%) | 106 (39.26%) | |
| Medication access | < 0.001 | |||
| Never | 6 (1.10%) | 1 (0.36%) | 5 (1.85%) | |
| Rarely | 57 (10.44%) | 16 (5.80%) | 41 (15.19%) | |
| Sometimes | 154 (28.21%) | 59 (21.38%) | 95 (35.19%) | |
| Often | 173 (31.68%) | 91 (32.97%) | 82 (30.37%) | |
| Always | 156 (28.57%) | 109 (39.49%) | 47 (17.41%) | |
| Height | 0.75 | |||
| Mean (SD) | 161.55 (8.95) | 161.25 (8.17) | 161.84 (9.69) | |
| Median (Q1, Q3) | 160.00 (156.00, 167.00) | 160.00 (156.00, 166.00) | 160.00 (155.00, 168.00) | |
| Weight | < 0.001 | |||
| Mean (SD) | 73.37 (14.67) | 71.51 (14.55) | 75.27 (14.58) | |
| Median (Q1, Q3) | 72.00 (63.00, 81.00) | 70.00 (61.50, 79.00) | 75.00 (65.00, 84.00) |
- an (%).
- bPearson’s chi-squared test, Fisher’s exact test, Wilcoxon rank sum test.
Educational attainment did not differ significantly between groups (p = 0.25), with most participants having primary or lower levels of education. The majority were married (80.40%), with no statistically significant difference in marital status across glycemic control groups (p = 0.18). Similarly, family history of diabetes was not associated with glycemic control status (p = 0.33).
Residence area, however, showed a pronounced disparity. Participants residing in rural areas were significantly more likely to exhibit poor glycemic control (60.74%) compared to urban residents (39.26%, p < 0.001). This trend underscores potential geographic disparities in diabetes management.
Anthropometric comparisons revealed that participants with poor glycemic control had a higher average body weight (mean = 75.27 kg) than those with good control (mean = 71.51 kg, p < 0.001). No significant differences were observed in height (p = 0.75).
Access to prescribed medications varied notably between glycemic control groups. In the good control group, 39.49% of participants reported always having access to their medications, while this proportion was markedly lower among those with poor control (17.41%). Conversely, limited access was more common in the poor control group: 35.19% reported “sometimes” having access, and 15.19% reported “rarely” having access, compared to 21.38% and 5.80%, respectively, in the good control group. The distribution of access levels differed significantly between groups (p < 0.001), indicating substantial variation in medication availability among participants (Table 1).
3.3. Lifestyle Patterns and Behavioral Characteristics of Participants
Differences in lifestyle behaviors were observed between participants with good and poor glycemic control (Table 2). Low PA levels were more frequently reported in the poor control group (74.44%) compared to the good control group (58.33%), while high PA was more common among individuals with good control (17.03% vs. 10.37%, p < 0.001). Median MET scores were also lower in the poor control group (156.75) than in the good control group (456.00), reflecting lower overall activity levels (p < 0.001).
| Characteristic | Overall N = 546a | Good control N = 276a | Poor control N = 270a | p valueb |
|---|---|---|---|---|
| Smoker | 0.077 | |||
| Yes | 46 (8.42%) | 29 (10.51%) | 17 (6.30%) | |
| No | 500 (91.58%) | 247 (89.49%) | 253 (93.70%) | |
| Alcoholic | 0.69 | |||
| Yes | 6 (1.10%) | 4 (1.45%) | 2 (0.74%) | |
| No | 540 (98.90%) | 272 (98.55%) | 268 (99.26%) | |
| Physical activity | < 0.001 | |||
| Low activity | 362 (66.30%) | 161 (58.33%) | 201 (74.44%) | |
| Moderate activity | 109 (19.96%) | 68 (24.64%) | 41 (15.19%) | |
| High activity | 75 (13.74%) | 47 (17.03%) | 28 (10.37%) | |
| Perceived stress | 0.11 | |||
| Mean (SD) | 18.66 (5.22) | 18.12 (4.46) | 19.20 (5.85) | |
| Median (Q1, Q3) | 18.00 (16.00, 21.00) | 18.00 (16.00, 20.00) | 18.00 (16.00, 21.00) | |
| MET | < 0.001 | |||
| Mean (SD) | 1174.10 (2099.63) | 1471.23 (2345.38) | 870.37 (1767.74) | |
| Median (Q1, Q3) | 297.00 (0.00, 1188.00) | 456.00 (0.00, 1920.00) | 156.75 (0.00, 600.00) |
- an (%).
- bPearson’s chi-squared test, Fisher’s exact test, Wilcoxon rank sum test.
Smoking was reported by 10.51% of individuals in the good control group and 6.30% in the poor control group (p = 0.077). Alcohol consumption was infrequent overall, with no significant difference between groups (1.45% vs. 0.74%, p = 0.69).
As shown in Table 3, adherence to dietary regimens showed slightly higher frequencies among those with good control, although the overall distribution was not statistically different between groups (p = 0.092). Fruit and vegetable intake patterns were similar in both groups, with no meaningful variation in frequency of consumption (p = 0.23).
| Characteristic | Overall N = 546a | Good control N = 276a | Poor control N = 270a | p valueb |
|---|---|---|---|---|
| Adherence to food regimen | 0.092 | |||
| 0 | 28 (5.13%) | 9 (3.26%) | 19 (7.04%) | |
| 1 | 25 (4.58%) | 7 (2.54%) | 18 (6.67%) | |
| 2 | 87 (15.93%) | 46 (16.67%) | 41 (15.19%) | |
| 3 | 193 (35.35%) | 101 (36.59%) | 92 (34.07%) | |
| 4 | 108 (19.78%) | 61 (22.10%) | 47 (17.41%) | |
| 5 | 53 (9.71%) | 28 (10.14%) | 25 (9.26%) | |
| 6 | 21 (3.85%) | 8 (2.90%) | 13 (4.81%) | |
| 7 | 31 (5.68%) | 16 (5.80%) | 15 (5.56%) | |
| Fruit and vegetable intake | 0.23 | |||
| 0 | 24 (4.40%) | 12 (4.35%) | 12 (4.44%) | |
| 1 | 62 (11.36%) | 39 (14.13%) | 23 (8.52%) | |
| 2 | 167 (30.59%) | 80 (28.99%) | 87 (32.22%) | |
| 3 | 156 (28.57%) | 72 (26.09%) | 84 (31.11%) | |
| 4 | 50 (9.16%) | 30 (10.87%) | 20 (7.41%) | |
| 5 | 30 (5.49%) | 18 (6.52%) | 12 (4.44%) | |
| 6 | 19 (3.48%) | 8 (2.90%) | 11 (4.07%) | |
| 7 | 38 (6.96%) | 17 (6.16%) | 21 (7.78%) | |
| Fatty food intake | 0.001 | |||
| 0 | 204 (37.36%) | 107 (38.77%) | 97 (35.93%) | |
| 1 | 127 (23.26%) | 73 (26.45%) | 54 (20.00%) | |
| 2 | 109 (19.96%) | 63 (22.83%) | 46 (17.04%) | |
| 3 | 57 (10.44%) | 21 (7.61%) | 36 (13.33%) | |
| 4 | 25 (4.58%) | 7 (2.54%) | 18 (6.67%) | |
| 5 | 14 (2.56%) | 3 (1.09%) | 11 (4.07%) | |
| 6 | 4 (0.73%) | 0 (0.00%) | 4 (1.48%) | |
| 7 | 6 (1.10%) | 2 (0.72%) | 4 (1.48%) | |
| Carbohydrate intake | 0.36 | |||
| 0 | 276 (50.55%) | 149 (53.99%) | 127 (47.04%) | |
| 1 | 152 (27.84%) | 72 (26.09%) | 80 (29.63%) | |
| 2 | 70 (12.82%) | 36 (13.04%) | 34 (12.59%) | |
| 3 | 29 (5.31%) | 13 (4.71%) | 16 (5.93%) | |
| 4 | 14 (2.56%) | 5 (1.81%) | 9 (3.33%) | |
| 6 | 3 (0.55%) | 0 (0.00%) | 3 (1.11%) | |
| 7 | 2 (0.37%) | 1 (0.36%) | 1 (0.37%) | |
| Self-monitoring blood glucose | 0.36 | |||
| 0 | 400 (73.26%) | 213 (77.17%) | 187 (69.26%) | |
| 1 | 65 (11.90%) | 26 (9.42%) | 39 (14.44%) | |
| 2 | 26 (4.76%) | 11 (3.99%) | 15 (5.56%) | |
| 3 | 26 (4.76%) | 13 (4.71%) | 13 (4.81%) | |
| 4 | 9 (1.65%) | 5 (1.81%) | 4 (1.48%) | |
| 5 | 12 (2.20%) | 5 (1.81%) | 7 (2.59%) | |
| 6 | 1 (0.18%) | 1 (0.36%) | 0 (0.00%) | |
| 7 | 7 (1.28%) | 2 (0.72%) | 5 (1.85%) |
- an (%).
- bPearson’s chi-squared test, Fisher’s exact test.
Fatty food intake was reported more frequently among participants with poor glycemic control. A greater proportion of this group reported consuming fatty foods three or more times per week, while those with good control were more likely to report no consumption (p = 0.001).
Carbohydrate intake frequency did not differ significantly between groups. About half of the participants in each group reported no carbohydrate consumption in the prior week (p = 0.36). Self-monitoring of blood glucose was also not significantly different, with the majority in both groups reporting no monitoring at all, though slightly more individuals in the poor control group reported occasional monitoring (1–2 times per week, p = 0.36).
3.4. Clinical and Laboratory Findings
Clinical and laboratory characteristics varied across glycemic control groups (Table 4). Participants with poor glycemic control had a significantly higher mean body weight (75.27 kg) compared to those with good control (71.51 kg, p < 0.001), while no difference was observed in height (p = 0.75).
| Characteristic | Overall N = 546a | Good control N = 276a | Poor control N = 270a | p valueb |
|---|---|---|---|---|
| Diagnosis duration | 0.025 | |||
| Mean (SD) | 8.68 (5.66) | 8.34 (6.00) | 9.04 (5.27) | |
| Median (Q1, Q3) | 7.00 (5.00, 12.00) | 7.00 (4.00, 12.00) | 8.00 (5.00, 12.00) | |
| Insulin duration (year) | 0.007 | |||
| Mean (SD) | 1.19 (3.60) | 1.60 (4.36) | 0.77 (2.56) | |
| Median (Q1, Q3) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | |
| Insulin dose | 0.055 | |||
| Mean (SD) | 3.23 (11.25) | 2.01 (6.25) | 4.47 (14.60) | |
| Median (Q1, Q3) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | |
| Oral medication duration | < 0.001 | |||
| Mean (SD) | 6.97 (5.59) | 6.13 (5.81) | 7.83 (5.23) | |
| Median (Q1, Q3) | 6.00 (2.00, 10.00) | 5.00 (1.00, 10.00) | 7.00 (4.00, 11.00) | |
| HDL | 0.004 | |||
| Mean (SD) | 47.87 (13.75) | 49.41 (13.90) | 46.30 (13.44) | |
| Median (Q1, Q3) | 46.00 (39.00, 55.00) | 47.00 (40.00, 58.00) | 44.00 (37.00, 54.00) | |
| LDL | 0.75 | |||
| Mean (SD) | 106.14 (34.75) | 106.38 (34.57) | 105.88 (35.01) | |
| Median (Q1, Q3) | 101.00 (84.80, 129.00) | 102.00 (85.50, 130.00) | 101.00 (83.00, 127.00) | |
| Systolic blood pressure | 0.001 | |||
| Mean (SD) | 121.32 (10.40) | 119.93 (10.23) | 122.75 (10.40) | |
| Median (Q1, Q3) | 120.00 (110.00, 130.00) | 120.00 (110.00, 130.00) | 120.00 (120.00, 130.00) | |
| Diastolic blood pressure | < 0.001 | |||
| Mean (SD) | 74.43 (8.03) | 72.17 (7.86) | 76.74 (7.55) | |
| Median (Q1, Q3) | 70.00 (70.00, 80.00) | 70.00 (70.00, 80.00) | 80.00 (70.00, 80.00) | |
| Diabetic complications | 0.17 | |||
| Yes | 269 (49.27%) | 144 (52.17%) | 125 (46.30%) | |
| No | 277 (50.73%) | 132 (47.83%) | 145 (53.70%) | |
| Nephropathy | 0.002 | |||
| Yes | 69 (12.64%) | 47 (17.03%) | 22 (8.15%) | |
| No | 477 (87.36%) | 229 (82.97%) | 248 (91.85%) | |
| Retinopathy | 0.077 | |||
| Yes | 157 (28.75%) | 70 (25.36%) | 87 (32.22%) | |
| No | 389 (71.25%) | 206 (74.64%) | 183 (67.78%) | |
| Neuropathy | 0.31 | |||
| Yes | 144 (26.37%) | 78 (28.26%) | 66 (24.44%) | |
| No | 402 (73.63%) | 198 (71.74%) | 204 (75.56%) | |
| Cardiovascular complications | 0.72 | |||
| Yes | 68 (12.45%) | 33 (11.96%) | 35 (12.96%) | |
| No | 478 (87.55%) | 243 (88.04%) | 235 (87.04%) | |
| Hypertension | 0.005 | |||
| Yes | 228 (41.76%) | 99 (35.87%) | 129 (47.78%) | |
| No | 318 (58.24%) | 177 (64.13%) | 141 (52.22%) | |
| Medication types | < 0.001 | |||
| Oral only | 445 (81.50%) | 217 (78.62%) | 228 (84.44%) | |
| Insulin only | 71 (13.00%) | 49 (17.75%) | 22 (8.15%) | |
| Both | 29 (5.31%) | 9 (3.26%) | 20 (7.41%) | |
| None | 1 (0.18%) | 1 (0.36%) | 0 (0.00%) | |
| Diabetic foot | 0.86 | |||
| Yes | 21 (3.85%) | 11 (3.99%) | 10 (3.70%) | |
| No | 525 (96.15%) | 265 (96.01%) | 260 (96.30%) |
- an (%).
- bWilcoxon rank sum test, Pearson’s chi-squared test, Fisher’s exact test.
Blood pressure measurements showed statistically significant differences. Mean systolic blood pressure was higher in the poor control group (122.75 mmHg) than in the good control group (119.93 mmHg, p = 0.001). Similarly, mean diastolic blood pressure was higher in the poor control group (76.74 mmHg) compared to the good control group (72.17 mmHg, p < 0.001). Hypertension was more prevalent among individuals with poor glycemic control (47.78% vs. 35.87%, p = 0.005).
In terms of lipid profiles, HDL cholesterol levels were lower in the poor control group (mean = 46.30 mg/dL) than in the good control group (mean = 49.41 mg/dL, p = 0.004). LDL cholesterol levels did not differ significantly between groups (p = 0.75).
The duration of diabetes was slightly longer among participants with poor glycemic control (mean = 9.04 years) compared to those with good control (mean = 8.34 years, p = 0.025). Duration of oral medication use was also longer in the poor control group (7.83 vs. 6.13 years, p < 0.001), while the duration of insulin use was shorter (0.77 vs. 1.60 years, p = 0.007).
Regarding complications, nephropathy was observed more frequently among those with good control (17.03%) than in the poor control group (8.15%, p = 0.002). Other complications such as retinopathy (p = 0.077), neuropathy (p = 0.31), and cardiovascular conditions (p = 0.72) did not differ significantly. The prevalence of diabetic foot was low and similar in both groups (p = 0.86).
Medication use patterns varied significantly. Participants in the poor control group were more likely to be treated with oral medications only (84.44%) compared to those in the good control group (78.62%). Insulin-only regimens were more common among those with good glycemic control (17.75% vs. 8.15%, p < 0.001). Access to medications also differed: only 17.41% of the poor control group reported “always” having access, compared to 39.49% in the good control group (p < 0.001).
3.5. Predictors of Poor Glycemic Control
The results of the multivariate logistic regression analysis, presented in Table 5, provide a comprehensive understanding of factors associated with glycemic control. Among demographic factors, residence area plays a significant role; individuals living in rural areas are three times more likely to experience poor glycemic control compared to their urban counterparts (OR = 3.04, 95% CI: 1.98–4.65, p < 0.001). Financial access to medication was a significant determinant of glycemic control. Participants who “rarely” or “sometimes” had access to medications had substantially higher odds of poor glycemic control compared to those with “always” reliable access (OR = 5.16, 95% CI: 2.41–11.06, p < 0.001; OR = 3.75, 95% CI: 2.20–6.40, p < 0.001, respectively). Those who reported “often” having access to medications also faced elevated odds (OR = 1.89, 95% CI: 1.13–3.16, p = 0.01). Although participants with “never” having access to medications showed a high OR = 6.99, this result was not statistically significant (p = 0.09), likely due to the small sample size in this category.
| Odds ratio | Standard error | t value | p value | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|---|---|
| Residence area | ||||||
| Rural | 3.04 | 0.66 | 5.11 | < 0.001 | 1.98 | 4.65 |
| Urban (reference) | ||||||
| Medication access | ||||||
| Never | 6.99 | 8.22 | 1.65 | 0.09 | 0.69 | 70.17 |
| Rarely | 5.16 | 2.00 | 4.23 | < 0.001 | 2.41 | 11.06 |
| Sometimes | 3.75 | 1.02 | 4.85 | < 0.001 | 2.2 | 6.4 |
| Often | 1.89 | 0.49 | 2.44 | 0.01 | 1.13 | 3.16 |
| Always (reference) | ||||||
| Physical activity | ||||||
| Moderate activity | 0.49 | 0.13 | −2.59 | 0.01 | 0.29 | 0.84 |
| High activity | 0.40 | 0.13 | −2.89 | 0.004 | 0.21 | 0.74 |
| Low (reference) | ||||||
| Medication type | ||||||
| Insulin only | 0.28 | 0.14 | −2.50 | 0.01 | 0.10 | 0.76 |
| Both | 1.02 | 0.52 | 0.04 | 0.97 | 0.37 | 2.79 |
| None | 1 | (Omitted) | ||||
| Oral medications only (reference) | ||||||
| HDL (per 10 mg/dL) | 0.86 | 0.06 | −2.03 | 0.04 | 0.75 | 0.99 |
| Fatty food consumption (days/week) | 1.26 | 0.09 | 3.17 | 0.002 | 1.09 | 1.46 |
| Insulin dose (units/day) | 1.04 | 0.02 | 2.57 | 0.01 | 1.01 | 1.08 |
| Oral medication duration (years) | 1.05 | 0.02 | 2.28 | 0.02 | 1.01 | 1.09 |
| Constant | 0.31 | 0.14 | −2.50 | 0.01 | 0.12 | 0.77 |
Lifestyle factors were also significantly associated with glycemic control. PA levels were inversely related to poor glycemic control (Figure 2). Participants engaging in moderate or high PA had reduced odds (OR = 0.49 and 0.40, respectively, p = 0.01) compared to those with low activity. In terms of dietary habits, fatty food consumption was positively associated with poor glycemic control, with each additional day of fatty food intake per week increasing the odds by 26% (OR = 1.26, 95% CI: 1.09–1.46).
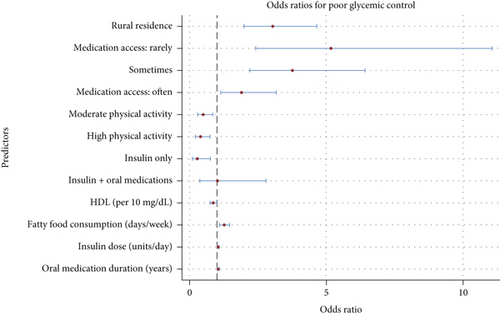
Medical factors further delineated the predictors of glycemic control. Insulin usage alone was associated with significantly reduced odds of poor glycemic control compared to oral medication use (OR = 0.28, p = 0.01), whereas combining insulin with oral medications showed no additional benefit. Moreover, each 10 mg/dL increase in HDL levels was associated with a 14% reduction in the odds of poor glycemic control (OR = 0.86, p = 0.04), whereas each additional year of oral medication use was linked to a 5% increase in the odds of poor control (OR = 1.05, p = 0.02). Conversely, increasing insulin doses (per unit/day) modestly raised the odds of poor glycemic control (OR = 1.04, p = 0.01), suggesting that higher doses may reflect more severe disease states (Figure 2).
4. Discussion
The findings of this study illuminate the complex interplay of demographic, financial, lifestyle, and medical factors that influence glycemic control in diabetes patients. Participants living in rural areas were more likely to have poor glycemic control compared to those in urban settings, emphasizing the role of geographic disparities. Limited access to medication emerged as a significant barrier, with inconsistent availability associated with worse control. Lifestyle factors also played a crucial role; low PA and frequent fatty food consumption were strongly linked to poorer outcomes, while moderate to high PA showed a protective effect. Additionally, individuals with better lipid profiles, particularly higher HDL levels, tended to have better glycemic control.
Individuals living in rural areas were found to be three times as likely to experience poor glycemic control compared to their urban counterparts. This suggests that rural residents face significant challenges, likely stemming from reduced access to healthcare services, limited educational opportunities, and socioeconomic disadvantages. Consistently, urban populations were observed to have better glycemic control, which aligns with evidence linking higher education levels and better awareness of diabetes to improved health outcomes, as reported by Nigussie et al. and Xing et al. [17, 18].
The findings of this study, alongside existing evidence, emphasize the profound impact of socioeconomic factors on glycemic control, particularly access to medications. In this study, individuals with inconsistent access to medications—whether rarely or occasionally—were substantially more likely to experience poor glycemic control compared to those with consistent access. This reinforces the results of research from the Health and Retirement Study (HRS), which found that financial hardships such as cost-related medication nonadherence and difficulty paying bills were significantly associated with higher HbA1c levels. Specifically, older adults who reduced their medication intake due to cost exhibited an increase in HbA1c, while each additional financial hardship raised HbA1c by 0.09% [19].
The findings of this study corroborate existing evidence on the critical role of PA in improving glycemic control among individuals with T2DM. Our study revealed that individuals who engaged in regular PA were significantly less likely to experience poor glycemic control compared to those with sedentary lifestyles. This is consistent with a systematic review and meta-analysis conducted on Ethiopian diabetes patients, which found that physically active individuals were 2.4 times more likely to achieve better glycemic control than their inactive counterparts (OR = 2.40, 95%CI = 1.57–3.69). This reinforces the understanding that PA serves as a simple, cost-effective intervention to manage blood glucose levels effectively [20].
Similarly, another systematic review demonstrated that various physical exercise (PE) modalities—such as aerobic exercise (AE), interval exercise (IE), resistance exercise (RE), and combined exercise (COM)—significantly improve glycemic control and body composition in adults with T2DM. These results emphasize that both short- and long-term structured exercise programs can yield substantial benefits, particularly when components like frequency, intensity, duration, and volume are carefully tailored to individual needs [21].
In our study, both HDL levels and fatty food consumption were significantly associated with glycemic control, underscoring the critical relationship between lipid metabolism and glucose regulation in diabetes. A 10 mg/dL increase in HDL was associated with a 14% reduction in the odds of poor glycemic control, while each additional day of fatty food consumption per week increased the likelihood of poor glycemic control by 26%. These results align with findings from Huang et al., who reported an inverse relationship between HDL-C and glycated hemoglobin levels, demonstrating HDL’s protective role in glycemic control. Huang’s study also highlighted that the relationship may vary by age and ethnicity, with U-shaped and inverted U-shaped patterns observed, suggesting that excessively high HDL levels may not always confer additional benefits for certain populations [22].
Similarly, Harding et al. showed that higher total fat intake was associated with increased HbA1c levels, whereas a higher polyunsaturated-to-saturated fat ratio was linked to better glycemic control. This emphasizes the dual importance of limiting overall fat intake and improving the quality of dietary fats [23].
Typically, epidemiologic studies show a strong connection between poor glycemic control and diabetic nephropathy [24, 25]. However, our cross-sectional analysis found a higher prevalence of nephropathy among participants with HbA1c below 7%. This unexpected finding likely reflects reverse causation and confounding by indication. Once nephropathy is detected, clinicians often intensify therapy, transitioning patients to insulin regimens to achieve tighter glycemic targets. Our results support this, showing that insulin-only regimens were more common among those with good glycemic control (17.75% compared to 8.15%). As a result, HbA1c improves after kidney injury occurs, which may have led to a spurious association between lower HbA1c and nephropathy.
Insulin monotherapy is often required when β-cell function declines significantly, a process that typically occurs in long-standing diabetes and contributes to an increased risk of microvascular complications [26]. Additionally, insulin monotherapy serves as a marker of severe disease that necessitates polypharmacy, yet many medications used for comorbid conditions independently contribute to kidney damage, creating a vicious cycle that accelerates renal decline [27, 28]. Together, these factors illustrate how treatment timing, disease severity, and the complex nature of renal pathophysiology can distort cross-sectional associations between HbA1c and nephropathy. This underscores the importance of longitudinal studies to better understand causal relationships.
This study has several limitations. First, its cross-sectional design prevents establishing causal relationships between the identified factors and glycemic control. While significant associations were observed, the directionality of these relationships remains uncertain. Second, the reliance on self-reported data for lifestyle factors such as dietary intake and PA introduces the potential for recall bias, which may have affected the accuracy of the findings.
A notable limitation of our study is the absence of genetic determinants as variables in the analysis. Although this was a cross-sectional study primarily focused on sociodemographic, lifestyle, and clinical factors influencing glycemic control, genetic factors can contribute significantly to the variability observed in diabetes complications, including nephropathy. Genetic predispositions may influence individual susceptibility to microvascular damage and response to treatment, potentially confounding the associations observed with clinical and behavioral factors. Future research incorporating genetic data could provide a more comprehensive understanding of the determinants of poor glycemic control and related complications, especially in diverse populations where genetic variability may play a critical role. Including genetic factors would enhance the precision of risk stratification and help tailor personalized interventions for diabetes management.
Additionally, the study population’s specific geographic and socioeconomic context may limit the generalizability of the results to other populations with different healthcare systems or cultural practices. Lastly, while the associations between HDL, fatty food consumption, and glycemic control are notable, interactions with other lipid parameters or dietary components not fully captured in this study could influence the results. Further research, especially longitudinal or interventional studies, is needed to validate these findings.
5. Conclusion
This study highlights the multifaceted factors influencing glycemic control in individuals with diabetes, emphasizing the roles of demographic, lifestyle, and medical variables. Rural residence, limited access to medications, low PA, and frequent fatty food consumption were strongly associated with poor glycemic control. Additionally, higher HDL levels emerged as a protective factor, underscoring the importance of lipid management in diabetes care. These findings reinforce the need for targeted interventions, such as improving healthcare access in rural areas, promoting PA, and advocating for healthier dietary patterns. While the cross-sectional nature of this study limits causal inferences, it provides valuable insights into the complex interplay of factors affecting glycemic control and underscores the necessity for comprehensive, individualized approaches to diabetes management.
Ethics Statement
The study protocol was approved by the Research Ethics Committee of Shahid Beheshti University of Medical Sciences (Approval ID: IR.SBMU.PHNS.REC.1402.036). This study is not a clinical trial and did not require registration. This study does not involve any animal research.
Consent
Written informed consent was obtained from all participants prior to their inclusion in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Acknowledgments
The authors express their gratitude to the staff of the diabetes clinic at Imam Hossein Hospital, Tehran, for their support during the study. Special thanks go to the participants who contributed their time and effort to this research.
Open Research
Data Availability Statement
Due to the confidentiality of the data, researchers are not permitted to publicly share the dataset(s) used in this study. However, anonymized versions of the dataset(s) used and/or analyzed during the study can be made available from the corresponding author on reasonable request.




