Examination of the Association Between Bisphenol-Related Genes and Lung Cancer
Abstract
In recent years, the release of substantial amounts of synthetic endocrine-disrupting chemicals (EDCs) into the environment has posed significant threats to human health. Among these EDCs, bisphenol A (BPA) and its substitutes, such as bisphenol S (BPS), bisphenol F (BPF), and bisphenol AF (BPAF), are widely used and have been implicated in disrupting various biological processes. This research aimed to evaluate the potential link between bisphenol-related gene expression and lung cancer prognosis. Using the Comparative Toxicogenomics Database (CTD), we identified genes involved in bisphenol metabolism and their significant associations with key oncogenes and hormone-disrupting pathways, including INS, ESR1, ESR2, AR, MAPK1, MAPK3, PPARG, and CYP19A1. Our Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses indicate that bisphenol-related genes may be associated with a variety of cancers, particularly lung cancer. To develop a risk model, we employed Cox regression and LASSO regression analyses, constructing a prognostic prediction model for lung cancer based on bisphenol-related gene expression (BBPPM). This model demonstrated prognostic significance, with lung cancer patients categorized into high-risk and low-risk groups, revealing significant differences in survival rates and highlighting the model’s accuracy in predicting lung cancer outcomes. In addition to bioinformatics analyses, experimental studies were conducted to evaluate the effect of BPA on lung cancer cell behavior. BPA exposure significantly promoted the proliferation of A549 lung cancer cells, as assessed by the CCK-8 assay, and increased the clonogenic potential of the cells in a colony formation assay.
1. Introduction
In recent decades, numerous artificial endocrine-disrupting chemicals (EDCs) have been released into the environment in significant quantities, posing threats to both animals and humans [1–3]. The annual production of bisphenol A (BPA), the most significant artificial xenoestrogen, amounts to 3.8 million tons [4–6]. Dihydrofolate reductase inhibition decreases the metabolism of dihydrofolate, potentially disrupting the activity of estrogen nuclear hormone receptors, carbohydrate and lipid metabolism, and folate metabolism [7]. A movement is gaining momentum to prohibit BPA use, and various novel substitutes are being developed.
Industrial applications often substitute BPA with bisphenol S (BPS) due to its excellent resistance to heat and sunlight, along with bisphenol F (BPF) and bisphenol AF (BPAF). Urine analyses in Japan and Vietnam found BPS to be the second most prevalent bisphenol congener after BPA [8]. BPS and BPF are present in a wide range of common items, including personal care products and paper products [9]. The increasing use of BPA alternatives has led to greater environmental distribution of non-BPA alternatives. Like BPA, BPAF, BPS, and BPF mimic estrogen effects or function as antiandrogens [10]. Contradictory findings in the scientific literature have raised concerns about the potential genotoxicity of BPS [11]. Given the growing utilization of BPA alternatives, it is imperative to enhance our understanding of their potential negative impacts on physiological functions.
In 2016, approximately 224,390 new cases of lung cancer were diagnosed, resulting in 158,080 deaths [12]. Lung cancer is primarily classified into two types: small-cell lung cancer, accounting for 15% of cases, and non-small-cell lung cancer, accounting for 85% [13]. The main types of lung cancer are adenocarcinoma, squamous cell carcinoma, and large cell carcinoma [14]. Early intervention is crucial for patients with stage IV lung cancer, as the survival rate declines to four months over time [15]. Despite increased exposure to environmental endocrine disruptors, lung cancer has not been definitively linked to a specific cause [16]. Several cancers, such as colorectal and gastric cancer, have been associated with environmental endocrine disruptors, but there is limited research on lung cancer and these disruptors [17]. Studies have shown a connection between BPA exposure and lung cancer development, influenced by variations in the estrogen receptor 1 (ESR1) gene [18, 19]. Another study found that BPA upregulated signaling pathways that promote lung cancer cell invasion, including MMP, GPER, EGFR, and ERK1/2. Examining the relationship between lung cancer and environmental endocrine disruptors is crucial [18].
The pineal gland releases melatonin, a neuroendocrine hormone with powerful antioxidant effects [20]. Recent studies suggest that melatonin can directly eliminate harmful free radicals and stimulate the body’s antioxidant enzymes, providing protection against reactive oxygen species [21]. Additionally, melatonin may have radioprotective effects and reduce the likelihood of developing cancer [22]. Multiple studies have shown that melatonin decreases lung cancer growth by over 50% [23]. Melatonin is effective in treating early-stage lung cancer, either alone or in combination with other medications [24]. Various studies have found a negative correlation between melatonin levels in the bloodstream and the incidence of lung cancer [25]. Patients with elevated melatonin levels in their urine had a reduced risk of developing advanced disease [26]. Oral supplementation also slowed the biochemical recurrence of lung cancer [27]. Additionally, melatonin has demonstrated protective effects against ovarian and colorectal cancer, as well as glioblastoma [28]. One study elucidates the intricate relationship between BPS exposure and gastric cancer, highlighting the critical role of interactive genes, particularly through the modulation of ESR1 [29–33]. This study presents a pioneering bisphenol-based prognostic model that significantly advances our understanding of the impact of environmental toxicants on cancer progression and patient prognosis. This study aims to investigate whether alternative compounds to BPA can cause lung cancer and to explore the preventative benefits of melatonin in relation to lung cancer.
2. Method
2.1. Data Sources
We utilized data from the Cancer Genome Atlas (TCGA) to obtain gene expression profiles and clinical features associated with renal clear cell carcinoma (ccRCC). mRNA data from TCGA were used for gene expression analysis. Additionally, we accessed the Comparative Toxicogenomics Database (CTD) (https://ctdbase.org/) to retrieve genes related to alternative bisphenol compounds such as BPS, BPF, and BPAF. The 2D and 3D structures of these compounds were downloaded from PubChem, an NIH database that provides information on chemical composition, biological characteristics, toxicity, and more.
2.2. Enrichment Pathway Analysis of Bisphenols Using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
We identified genes associated with BPA substitutes in the CTD database and explored their most relevant connections. GO enrichment analysis included biological process (BP), molecular function (MF), and cellular component (CC) categories. KEGG pathway enrichment analysis was also conducted, with pathways considered statistically significant if the p value was below 0.05.
2.3. Protein–Protein Interaction (PPI) Network Analysis
To investigate the interactions among proteins encoded by genes associated with bisphenols, we performed a PPI network analysis. Interaction data for the selected genes were retrieved from the STRING database (version 11.0), which provides known and predicted PPIs. We used a high confidence score (interaction score > 0.9) to ensure the reliability of the interactions included in the analysis. The retrieved interaction data were used to construct a PPI network and visualized and analyzed using Cytoscape (version 3.8.2), an open-source software platform for visualizing complex networks. Each node in the network represents a protein, and each edge represents an interaction between two proteins.
2.4. Forecasting the Impact of Bisphenols and Melatonin
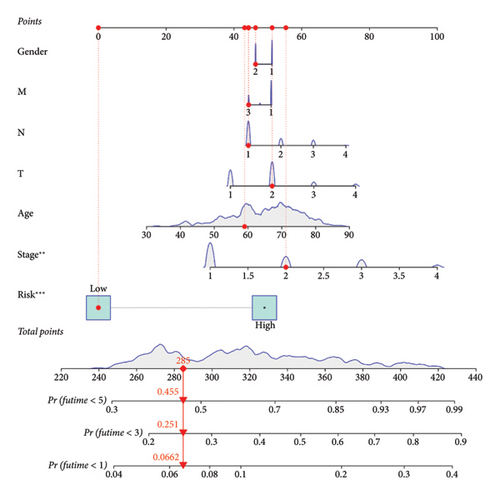
To evaluate the impact of bisphenol-related genes on the prognosis of lung cancer, we developed a forecast prediction model using the TCGA-LUAD cohort. Initially, univariate Cox proportional hazards regression was performed for each bisphenol-related gene to identify those significantly associated with overall survival (OS), using a significance threshold of p < 0.05. Genes meeting this criterion were selected for further analysis. Subsequently, the Least Absolute Shrinkage and Selection Operator (LASSO) regression was applied to identify the most significant prognostic markers and reduce overfitting. Using the “glmnet” package in R, ten-fold cross-validation determined the optimal lambda value, and genes with nonzero coefficients at this lambda were deemed significant. These significant genes were then included in a multivariate Cox proportional hazards regression to develop a robust prognostic model. This analysis adjusted for potential confounders, providing an independent assessment of each gene’s contribution to patient survival. The risk score for each patient was calculated using the formula ∑(coef ∗ Exp(genes)), where “coef” represents the regression coefficient and “Exp(genes)” the expression level of each gene. The prognostic accuracy of the bisphenol-based prognostic prediction model (BBPPM) was validated using Kaplan–Meier survival analysis and ROC curve analysis to stratify patients into high-risk and low-risk groups based on their risk scores. A nomogram was created using the “rms” package in R. The nomogram integrated gene expression data with clinical variables to predict OS. Calibration plots were generated to evaluate the performance of the nomogram by comparing predicted and observed survival outcomes.
2.5. Assessment of the Lung Cancer Predictive Model Using Bisphenols
The stability of the BBPPM was evaluated using Kaplan–Meier survival analysis. ROC curves were created to assess the model’s ability to diagnose lung cancer and predict patient outcomes, with AUC values calculated to determine model accuracy. The “pROC” package in R was used to generate ROC curves for OS.
2.6. Compounds and Receptor Data
We used the following PubChem compound identifier (CID) numbers for our compounds of interest: BPA—CID 6623, bisphenol B (BPB)—CID 66166, BPAF—CID 73864, and melatonin—CID 896. The target receptor, ESR1, was represented by the Protein Data Bank (PDB) structure with a specific identifier.
2.7. Docking Procedure
Docking simulations were performed to accurately target the known ligand-binding domain of ESR1. The center coordinates were set at [X = 30, Y = 50, Z = 40] angstroms, with a grid box dimension of [60 × 60 × 60] angstroms to comprehensively cover the active site. Molecular interactions and bonding conformations were visualized using PyMOL (version 2.3) and Biovia Discovery Studio Visualizer (version 2020). These tools provided qualitative and quantitative insights into the binding affinities and orientations of the molecules within the active site of ESR1.
2.8. Cell Culture
The A549 human lung cancer cell line was cultured in DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin in a humidified incubator at 37°C with 5% CO2. Cells were monitored regularly, and the medium was replaced every 2-3 days. When cells reached approximately 80% confluence, they were passaged for further experimentation. All assays were performed on cells in the logarithmic growth phase.
2.9. Cell Counting Kit-8 (CCK-8) Assay
The effect of BPA on the proliferation of A549 cells was evaluated using CCK-8 (Vazyme). A549 cells were seeded in 96-well plates at a density of [20000] cells per well in 100 μL of medium. After 24 h, cells were treated with BPA or a negative control (NC) and incubated for 0, 2, and 4 days. At each time point, 10 μL of CCK-8 solution was added to each well, and the plates were incubated for 2 h at 37°C. Absorbance was measured at 450 nm using a microplate reader to assess cell viability. All experiments were conducted in triplicate, and data are presented as mean ± standard deviation.
2.10. Colony Formation Assay
The colony formation assay was used to evaluate the long-term proliferative capacity of A549 cells treated with BPA. Cells were seeded in 6-well plates at a density of [specific number] cells per well and allowed to attach overnight. Cells were then treated with BPA or NC and incubated for 14 days. The medium was refreshed every 3 days. After 14 days, the cells were fixed with 4% paraformaldehyde for 15 min and stained with 0.5% crystal violet for 30 min to visualize colonies. Colonies containing more than 50 cells were counted under a microscope. Each experiment was repeated in triplicate.
2.11. Statistical Analysis
Survival curves were generated using the Kaplan–Meier technique and assessed with the log-rank test. Cox proportional hazards regression models were used for univariate and multivariate analyses to evaluate the prognostic significance of bisphenol-related genes. Variables included in the multivariate analysis were selected based on their significance in univariate analysis and included age, sex, stage, and risk score. Statistical analyses were performed using R software version 3.6. Significance was attributed to findings with p values below 0.05.
3. Results
3.1. Analyzing Pathway Enrichment by Identifying Genes Associated With Bisphenols
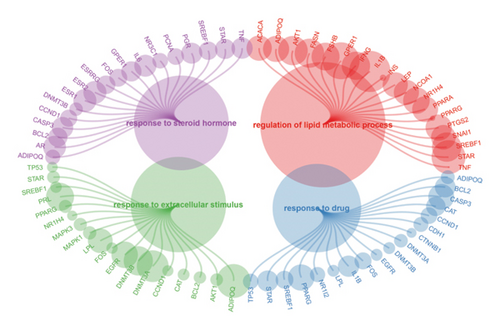
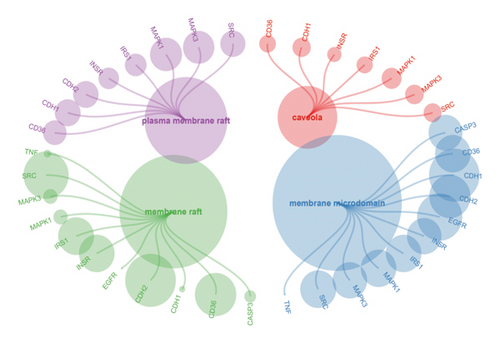
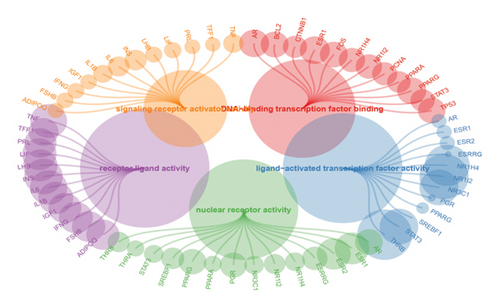
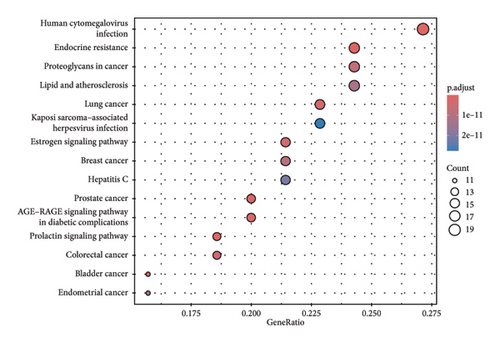
We obtained genes and proteins associated with bisphenols (BPA, BPS, BPF, and BPAF) from the CTD. Through data preprocessing, we successfully identified the genes involved in bisphenol degradation. The findings indicated a strong association between bisphenols and INS, ESR1, ESR2, AR, MAPK1, MAPK3, PPARG, and CYP19A1. By examining the key pathways associated with bisphenols, we can effectively suggest optimal courses of action. Following this, we conducted an enrichment analysis using GO and KEGG, focusing on genes associated with bisphenols. According to the KEGG analysis, bisphenols may be linked to various cancers, including small-cell lung cancer, non-small-cell lung cancer, pancreatic cancer, and gastric cancer. Bisphenols may also be responsible for the prevalence of lung cancer and are associated with hepatitis B as well. In the GO enrichment analysis, the BP, CC, and MF categories were considered. Figure 1(a) illustrates four elements playing a role in GO BP drug reactions: control of lipid metabolism, reaction to a hormone derived from steroids, and reaction to an external stimulus. Estrogens and estriol are primarily produced by the liver through the conversion of estradiol. The expression of beta in lung cancer may potentially correlate with survival rates. Research has shown that both external estrogen (like hormone therapy and birth control pills) and internal hormone levels (such as the timing of menopause) can influence the development of lung cancer. The probability of developing lung adenocarcinoma decreases after menopause, while the use of estrogen replacement therapy has been associated with an increased likelihood of lung cancer. Furthermore, the potential of estrogen as a treatment for lung cancer has been explored in studies. Research has shown that estrogen blockers can reduce cell growth and tumor development in both lab experiments and living organisms. The findings revealed a significant connection between bisphenol-related genes and the enrichment pathways of BP CC, including plasma membrane raft and protein kinase complex (Figure 1(b)). In the pathways for enhancing BPs and MFs, genes associated with bisphenols showed significant enrichment in various activities, including nuclear receptor function, transcription factor activity triggered by ligands, receptor–ligand interaction, DNA-binding transcription factor binding, signaling receptor activation, and steroid binding (Figure 1(c)).
3.2. Network of Interactions Between Proteins Involving Bisphenols
We performed a PPI study on the genes linked to bisphenols, achieving an overall rating of 0.9. Several genes, such as JUN, STAT3, SRC, ESR1, RELA, NCOA1, MAPK3, TP53, and CTNNB1, had more than 50 interactive counts. The findings suggest that proteins associated with bisphenols exhibited robust internal connections. The strong internal connection between bisphenols and these proteins (Figure 2(a)) could potentially have a notable impact on the progression of lung cancer.
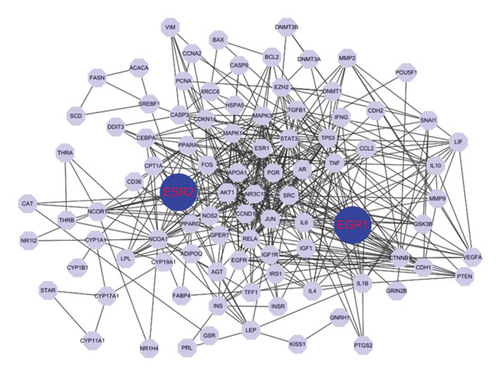
3.3. Examining the Expression of Genes Associated With Bisphenols in the TCGA-LUAD Group
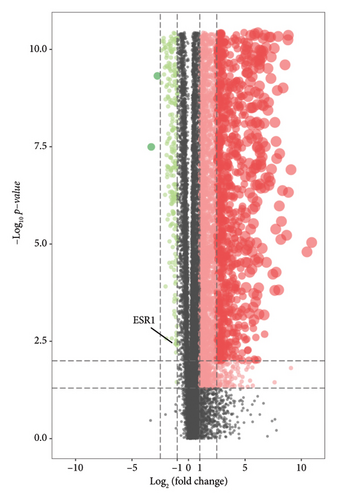
We performed a differential expression analysis within the TCGA-LUAD cohort to identify genes linked to bisphenols that contribute to the progression of lung cancer. Compared to the normal population, the TCGA-LUAD cohort exhibited a distinct expression pattern in 29 out of 100 genes associated with bisphenols, with p values below 0.05 and Log2FC exceeding 1. In the TCGA-LUAD dataset, 16 genes exhibited increased expression, while 13 genes showed upregulation in the control group (Figure 2(b)).
3.4. Construction of a BBPPM Cohort for Lung Carcinoma
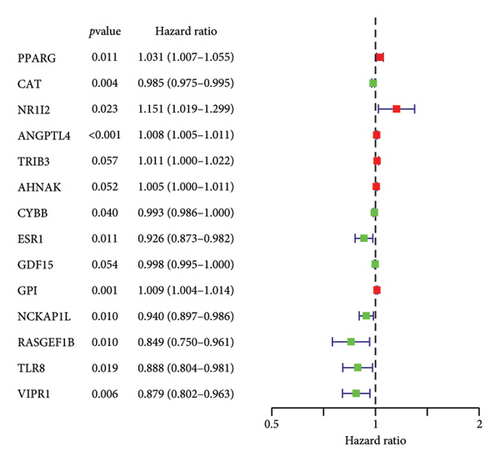
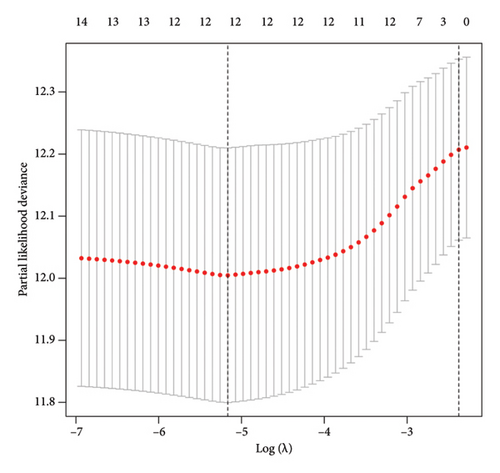
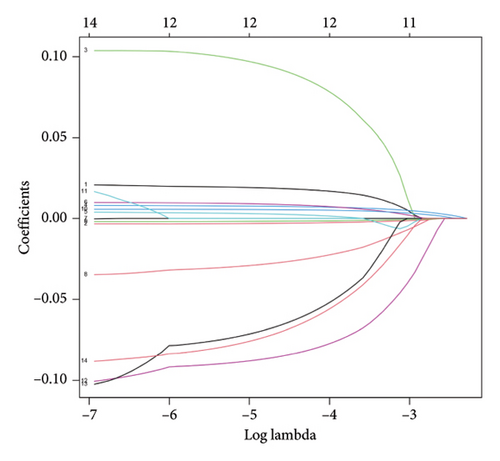
To examine the associations between bisphenols and lung cancer patient prognoses, we investigated the differentially expressed genes related to bisphenols. After conducting a univariate Cox regression analysis, 14 genes exhibited a significant association (p < 0.05), as shown in Figure 3(a). Additionally, the LASSO regression method was utilized to uncover possible risk factors for lung cancer through the analysis of multivariate Cox regression results (Figures 3(b) and 3(c)). The conclusion from the multivariate Cox regression analysis indicated that the prognostic forecast included eight genes connected to bisphenols: PPARG, ANGPTL4, AHNAK, ESR1, GDF15, GPI, TLR8, and VIPR1. The risk profile for these eight bisphenol-related genes was calculated using the formula “∑(coef ∗ Exp(genes))” as follows: risk score = PPARG ∗ 0.0320117573570723 + ANGPTL4 ∗ 0.00746135606875013 + AHNAK ∗ 0.00981315191611238 + FCN1 ∗ −0.0468893338353989 + GDF15 ∗ −0.00200527501598102 + GPI ∗ 0.00673907314256532 + TLR8 ∗ −0.12103605643474 + VIPR1 ∗ −0.112128647771262.
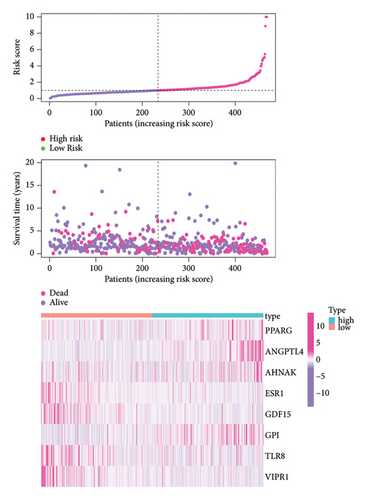
3.5. Investigating the Relationship Between BBPPM and Clinical Features in Lung Cancer Patients
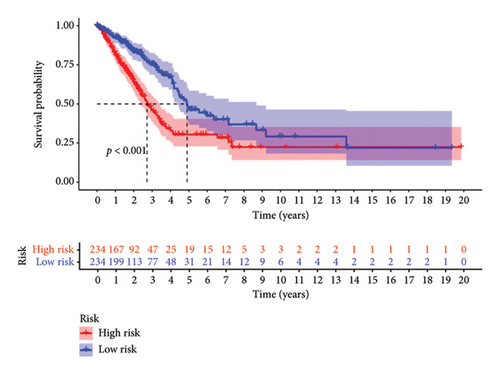
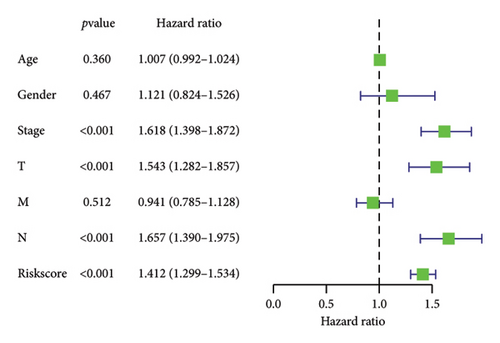
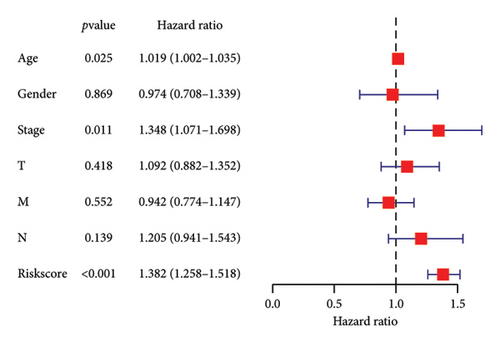
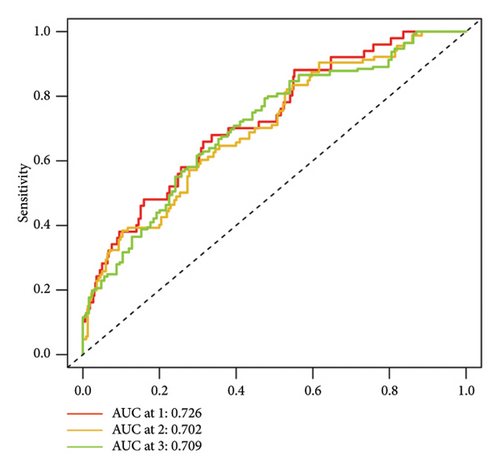
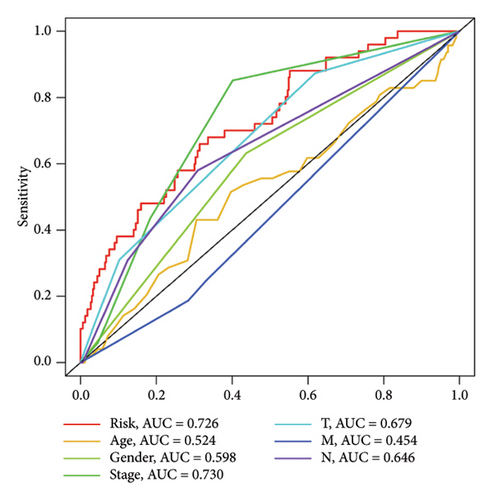
To determine the connection between bisphenol-related genes and lung cancer prognosis, mRNA expression data and clinical information from lung cancer patients were randomly divided into low-risk and high-risk groups, as illustrated in Figure 3(e). Patients diagnosed with high-risk lung cancer had a greater mortality rate compared to those with low-risk lung cancer (Figure 3(d)). Prognostic studies were performed to evaluate the effectiveness of separate predictive models related to bisphenols. Figure 4(a) reveals a correlation between the survival rate of individuals with lung cancer and both stage and risk score in a univariate independent prognostic assessment. Studies focusing on genes linked to bisphenols have shown that multivariate independent prognostic models are effective in predicting the prognosis of lung cancer. Furthermore, the prognostic potential of bisphenol-related genes in lung cancer patients has been illustrated by ROC curves (Figures 4(c), 4(d), and 4(e)).
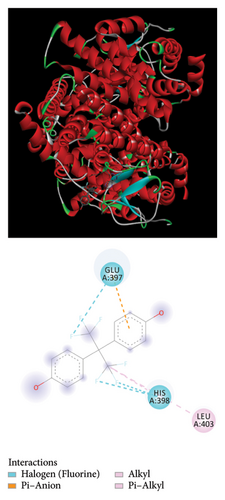
3.6. Molecular Docking of Bisphenols With the Estrogen Receptor (ER) Using Biochemical Methods
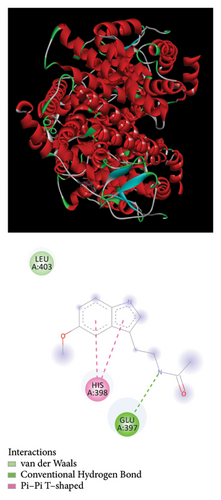
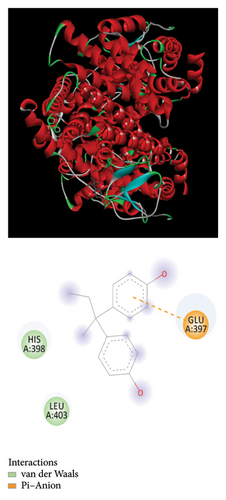
The Glide module in the Schrodinger Suite software was utilized to calculate docking XPG scores for BPA, BPB, BPAF, and melatonin against the active site of the gene ESR1. The 3D structures of BPA, BPB, BPAF, and melatonin are shown in Figure 5. The results indicate that BPA, BPF, BPS, and BPAF all exhibit considerable binding affinities with ESR1, as depicted in Figure 6 and detailed in Table 1.
| Compound | Best mode | Best affinity (kcal/mol) | RMSD l.b. | RMSD u.b. |
|---|---|---|---|---|
| BPA | 1 | −8.2 | 0.000 | 0.000 |
| BPB | 1 | −7.8 | 0.000 | 0.000 |
| BPAF | 1 | −9.1 | 0.000 | 0.000 |
| Melatonin | 1 | −7.1 | 0.000 | 0.000 |
3.7. BPA Promotes Lung Cancer Cell Proliferation and Colony Formation
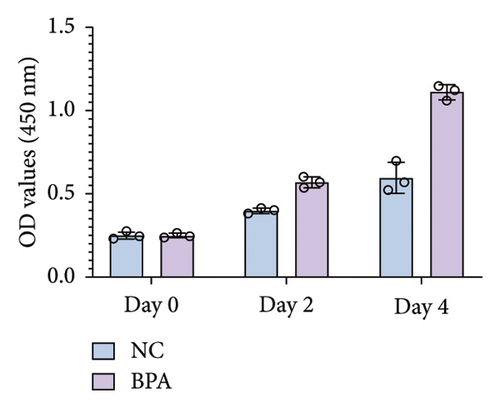
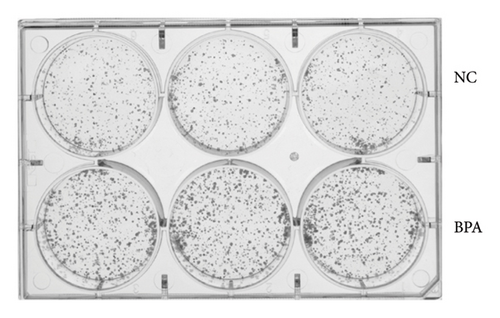
To investigate the effect of BPA on lung cancer cell proliferation, we treated A549 cells with BPA and assessed cell viability using the CCK-8 assay. As shown in Figure 7(a), BPA exposure significantly increased the proliferation of A549 cells over time compared to the NC. At Day 4, the optical density (OD) values of BPA-treated cells were markedly higher than those of the NC group (p < 0.05), indicating enhanced cell proliferation. In addition, we performed a colony formation assay to assess the long-term effects of BPA on the proliferative capacity of A549 cells. As shown in Figure 7(b), BPA treatment led to a substantial increase in the number of colonies formed compared to the NC group. This result suggests that BPA not only promotes short-term cell proliferation but also enhances the clonogenic potential of lung cancer cells.
4. Discussion
Using bioinformatics analysis tools, we discovered numerous pathways in which genes associated with bisphenols are prominently present. These pathways involve various forms of lung cancer, including small-cell lung cancer and non-small-cell lung cancer, as well as the regulation of lipid metabolism, response to steroid hormones, and reaction to external stimuli [34]. Previous research indicates a growing body of evidence linking ER expression with lung cancer prognosis. Furthermore, antiestrogen treatment is extensively employed for lung cancer treatment. ESR1 shows increased expression in the TCGA-LUAD cohort compared to healthy individuals, as indicated by differential gene expression analysis [35].
We constructed a prognostic forecast model using transcriptional expression data and clinical data from individuals diagnosed with lung cancer. Bioinformatics analysis revealed that ESR1 significantly impacts the prognostic prediction model utilizing genes associated with both lung cancer and bisphenols. Our study utilized bioinformatics analysis to predict the presence of environmental substances that disrupt the endocrine system. To investigate the potential involvement of ESR1 in lung cancer, molecular docking studies were conducted to assess its interaction with BPA, BPB, BPAF, and melatonin. Strong hydrogen bonding and hydrophobic interactions were observed. In our GSEA and GSVA analyses, we found that ESR1 is linked to various pathways related to lung cancer, including mRNA metabolism, mitochondrial respiratory chain complex assembly, mRNA degradation suppression, and ribosomal small subunit assembly. Analyzing these results may provide deeper insights into how bisphenol substitutes may impact the onset of lung cancer.
In addition to the bioinformatics findings, we performed in vitro experiments to assess the direct effects of BPA on lung cancer cell behavior. BPA exposure significantly increased the proliferation and colony formation ability of A549 lung cancer cells, suggesting that bisphenol may play a direct role in promoting lung cancer cell growth. These experimental results align with our bioinformatics analyses, reinforcing the hypothesis that BPA contributes to lung cancer progression by affecting both gene expression and cellular behavior.
Recent studies have underscored the significance of environmental pollutants in the etiology of various cancers, providing a crucial context for our investigation into the effects of bisphenols. For instance, Zhang et al. conducted a comprehensive genetic analysis to elucidate the potential threat posed by organophosphate flame retardants (OPFRs) to prostate cancer [36]. Their findings highlight the intricate genetic interactions and pathways through which OPFRs may influence prostate cancer progression, underscoring the importance of genetic understanding in assessing the carcinogenic potential of environmental pollutants. Similarly, Hong et al. demonstrated how comprehensive analysis of environmental chemicals can reveal their roles in cancer progression [37]. Their investigation into triphenyl phosphate, an environmental pollutant, and its association with colorectal cancer progression employs a multidimensional analysis that combines environmental science and oncology to uncover potential mechanisms of action. By examining the environmental explanations behind colorectal cancer progression, Hong et al. contributed to a growing body of literature that seeks to elucidate the complex relationships between environmental exposures and cancer risk [37]. Drawing parallels to our study, their research methodology and findings offer insight into the potential mechanisms through which bisphenols may exert similar effects on lung cancer, reinforcing the critical need for comprehensive analyses in environmental health studies.
Our study provides significant insights into the association between bisphenol-related genes and the progression of lung cancer. However, there are several limitations to consider. First, the study primarily relies on bioinformatics analyses using publicly available data from the TCGA and CTD databases. While these databases are comprehensive, they may contain inherent biases, and the results derived from them require further validation through experimental and clinical studies. Second, our analysis focuses on gene expression profiles and does not account for potential epigenetic modifications or post-translational changes that could influence gene function and disease progression. Third, a key limitation of this study is the lack of smoking data in the databases used, which prevents us from controlling for this major confounding factor. As smoking is a well-established risk factor for lung cancer, it is possible that the observed association between bisphenol-related gene expression and lung cancer prognosis may be influenced by smoking. Without the ability to assess the smoking status of patients, we cannot determine whether the correlation between bisphenol-related gene expression and worse prognosis is causal or potentially confounded by smoking-related gene expression changes. Future studies should include detailed patient data, including smoking history, to better understand the relationship between bisphenol exposure, gene expression, and lung cancer outcomes.
5. Conclusion
In conclusion, our investigation into bisphenol-related gene expression has identified an association with worse prognosis in lung cancer patients. Through a combination of gene association studies, pathway enrichment, and PPI analyses, we observed that bisphenol-related genes may play a role in influencing cancer progression. However, it is important to emphasize that our study did not measure bisphenol levels in the cases, and as a result, we cannot confirm whether the observed associations are due to bisphenol exposure or potentially confounded by smoking, a well-known risk factor for lung cancer. It is possible that smoking could be driving the gene expression changes, with smokers having higher bisphenol exposure. Future studies incorporating direct measurements of bisphenol levels and smoking data are needed to clarify this relationship and its implications for lung cancer prognosis.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors made substantial contributions to the study and manuscript preparation, and their roles can be described as follows according to CRediT guidelines.
Data curation and analysis: Lin Chen.
Writing–original draft: Min Zhou.
Writing–review and editing: Dingliang Lv.
Supervision and experiment guide: Shuiwei Qiu.
Additionally, all authors have read and approved the final manuscript and agree to be accountable for the content and conclusions of this article.
Funding
This work was sponsored by Competitive Project of Quzhou Municipal Science and Technology Bureau, 2023K153.
Open Research
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.




