Pharmacokinetics/Pharmacodynamics and Therapeutic Drug Monitoring of Ceftazidime/Avibactam in Patients With Gram-Negative Bacterial Infections
Abstract
Objectives: This study aimed to characterize ceftazidime/avibactam trough concentrations (Cmin) in patients infected with resistant Gram-negative bacteria. Additionally, we assessed the risk factor associated with clinical cure, including joint optimal pharmacokinetic/pharmacodynamic (PK/PD) target.
Methods: We conducted a single-center observational retrospective study involving patients receiving ceftazidime/avibactam therapy for Gram-negative infections and undergoing therapeutic drug monitoring of ceftazidime and avibactam. Analysis was performed on the Cmin of ceftazidime and avibactam. The joint PK/PD target of ceftazidime/avibactam was considered as optimal when both the PK/PD targets of ceftazidime (100%fT > 4 × MIC) and avibactam (100%fT > CT) were simultaneously achieved; quasi-optimal if only one was achieved; and suboptimal if neither was achieved. Finally, logistic regression analysis was utilized to detect potential variables associated with clinical cure.
Results: This study analyzed a total of 39 infected patients, with results showing that the Cmin of ceftazidime and avibactam was 24.1 ± 14.3 mg/L (coefficients of variation 59.5%), and 6.0 ± 4.4 mg/L (coefficients of variation 73.5%), respectively. Patients with renal insufficiency exhibited significantly higher Cmin of ceftazidime and avibactam compared to those with normal renal function (p < 0.05). Similarly, Cmin were elevated in patients receiving continuous renal replacement therapy (CRRT) versus non-CRRT recipients (p < 0.05). No significant difference in Cmin of ceftazidime and avibactam was observed between elderly and nonelderly patients. Clinically cured patients accounted for 76.9% (30/39) of the total. For 23 infected patients with minimum inhibitory concentration results, optimal joint PK/PD target was observed in 16 patients (69.6%) and quasi-optimal in 7 patients (30.4%). Independent predictors of clinical cure were found to be optimal joint PK/PD target and co-administered with aztreonam.
Conclusions: The findings indicated that the Cmin of ceftazidime and avibactam exhibit significant variability. With the current regimen of ceftazidime/avibactam, the majority of patients can achieve clinical cure and optimal PK/PD target.
1. Introduction
The global spread of multidrug-resistant organisms, especially carbapenem-resistant Gram-negative bacteria, has resulted in escalating treatment failure and mortality among infected patients, posing a serious threat to public health and safety [1–3].
Infectious Diseases Society of America 2024 Guidance points out that ceftazidime/avibactam is an important agent for the treatment of drug-resistant Gram-negative infections [4]. Ceftazidime/avibactam, a novel β-lactam/β-lactamase inhibitor (BL/BLI), exhibits broad-spectrum antibiotic active against difficult-to-treat pathogens, such as multidrug-resistant Enterobacteriaceae and Pseudomonas aeruginosa [5, 6]. However, the studies on resistance to ceftazidime/avibactam have been published, raising concerns about its long-term clinical utility [7, 8]. Additionally, a study has indicated suboptimal clinical efficacy of ceftazidime/avibactam in patients infected with carbapenem-resistant Enterobacteriaceae [9]. These findings underscore the critical need for therapeutic optimization strategies aimed at both enhancing efficacy and potentially mitigating the emergence of drug-resistant bacteria.
Pharmacokinetics/pharmacodynamics (PK/PD) can elucidate the correlation between drug concentration and antibacterial activity, providing a basis for optimizing regimen [4]. For ceftazidime/avibactam, the joint PK/PD target must be considered [10]. Ceftazidime exhibits time-dependent activity, with the PK/PD target being the percentage of time during the dosing interval that free drug concentrations remained above minimum inhibitory concentrations (%fT > MIC) [11]. The PK/PD target of avibactam is the percentage of time during the dosing interval that free drug concentrations remain above the threshold concentration (%fT > CT) [12, 13]. The recommended PK/PD targets of ceftazidime and avibactam are 50% fT > MIC and 50% fT > CT at a concentration of 1.0 mg/L [14]. However, recent research indicates that the PK/PD target of β-lactam may be insufficient in critically ill patients. For β-lactam, it has been proposed to use 100%fT > 4 × MIC as the PK/PD target to maximize clinical outcome and prevent drug resistance [15]. Therapeutic drug monitoring (TDM) is increasingly recommended for individualized antibiotic therapy, as it enables clinical to adjust the antibiotics regimen based on the patient’s individual PK parameters [16]. The guideline recommended to conduct TDM of β-lactam antibiotics in patients with significant PK variability, such as critically ill patients [15]. The study conducted by Gatti revealed that the concentration ratio of ceftazidime and avibactam exhibited variability during administration, necessitating simultaneous determination of their concentrations for TDM [17].
This retrospective study was conducted to characterize the distribution of ceftazidime and avibactam trough concentrations (Cmin), and to evaluate the risk factors associated with clinical cure, included the joint PK/PD target of ceftazidime/avibactam.
2. Materials and Methods
2.1. Study Design and Population
We conducted a single-center observational retrospective study at the First Affiliated Hospital of Xi’an Jiaotong University, focusing on hospitalized adult patients, from January 2020 to December 2021. The study specifically included patients who were treated with ceftazidime/avibactam and underwent TDM for both agents. Approval for this study was obtained from the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF2021LSK-345).
2.2. Data Collection
The clinical data of the patients were extracted through the electronic medical record system, encompassing demographic information (age, gender, and weight), clinical characteristics (clinical diagnosis, hospitalization ward, comorbidities, infection site/type and ceftazidime/avibactam regimen), microbiological data (pathogen identification, MIC and carbapenemase genotype), as well as laboratory outcomes (creatinine, bilirubin and cystatin C, etc.).
2.3. Ceftazidime/Avibactam Administration and Monitoring
The standard regimens consisted of ceftazidime 2 g/avibactam 0.5 g administered via a 2-h intravenous infusion every 8 h, with a dose adjustment based on the renal function of patients (https://www.ema.europa.eu/documents/product-information/zavicefta-epar-product-information_en.pdf). In this study, residual blood samples were collected from patients after determining liver and kidney functions.
The established high performance liquid chromatography-tandem mass spectrometry method was utilized to determine the plasma concentrations. The calibration curve demonstrated linearity within 0.5–100 mg/L for ceftazidime and 0.1–50 mg/L for avibactam. The intraday and interday precision, accuracy matrix effects and recovery rates were within a range of 15% for ceftazidime and avibactam. Total concentrations of ceftazidime and avibactam were measured with free fractions of ceftazidime and avibactam were 85% and 92% [18].
2.4. Outcomes and Definitions
Optimal PK/PD targets for both ceftazidime (100% fT > 4 × MIC, i.e., f Cmin/MIC > 4) and avibactam (100%fT > CT, i.e., f Cmin/CT > 1) simultaneously achieved was considered joint PK/PD target of ceftazidime/avibactam [17]. If only one of the two targets was met, it would be deemed quasi-optimal; if neither was achieved, it would be classified as suboptimal [10]. Additionally, clinical efficacy was evaluated based on clinical cure, defined as the complete resolution of infection-related signs and symptoms, coupled with documented microbiological eradication at the end of treatment [19].
2.5. Statistical Analysis
SPSS (version 19.0), and GraphPad Prism (version 8.0) were used for statistical analysis and graphing. In this study, the internationally recognized two-stage normality testing strategy was adopted: for groups with sample size ≥ 50, the Kolmogorov–Smirnov test was used; for subgroups with sample size < 50, the Shapiro–Wilk test was used [20]. We selected appropriate test method based on the sample size to assess the normality of the concentration distribution characteristics of ceftazidime and avibactam. If the data followed a normal distribution, they were presented as mean ± standard deviation (SD), and independent-samples t-tests were conducted. Conversely, if the data did not adhere to a normal distribution, they were reported as median (interquartile range), and Mann–Whitney U test was performed. While categorical variables were expressed as frequencies and percentages. We used the coefficient of variation to characterize interindividual variability of ceftazidime and avibactam concentration. Furthermore, univariate and multivariate logistic regression analysis was conducted in patients with MIC results to evaluate the potential factors affecting the clinical cure. Variables with p < 0.1 in the univariate analysis were combined in the multivariate analysis. Statistical significance was defined as a p value < 0.05.
3. Results
3.1. Baseline Patient Characteristics
Overall, a total of 39 infected patients were included in this retrospective study. The demographic characteristics of the patients are presented in Table 1, indicating median (interquartile range) age of 55 (39, 68) years and 71.8% of the patients being male. Of these patients, the 19 (48.7%) patients were admitted to the intensive care unit. The predominant comorbidities observed were hypertension (38.5%) and solid organ transplantation (28.2%). There were fifteen infected patients were treated with continuous renal replacement therapy (CRRT). Among the 39 patients, pulmonary infections accounted for 53.9%, while intra-abdominal infections accounted for 15.4%. The clinical cure rate of infected patients was 76.9% and clinical failure rate was 23.1%.
| Baseline characteristics | Patients |
|---|---|
| Number of patients, n | 39 |
| Demographic data | |
| Age, years, median (IQR) | 55 (39, 68) |
| Male sex, n (%) | 28 (71.8) |
| Weight, kg, median (IQR) | 54 (60, 70) |
| Ward of hospitalization, n (%) | |
| Intensive care unit | 19 (48.7) |
| Kidney transplantation department | 9 (23.1) |
| Other | 11 (28.2) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 8 (20.5) |
| Hypertension | 15 (38.5) |
| Solid organ transplantation | 11 (28.2) |
| Other | 5 (12.8) |
| Continuous renal replacement therapy | |
| Yes | 15 (38.5) |
| No | 24 (61.5) |
| Source of infection, n (%) | |
| Pulmonary infection | 21 (53.9) |
| Intra-abdominal infection | 6 (15.4) |
| Urinary-tract infection | 3 (7.7) |
| Other infection | 9 (23.0) |
| Baseline laboratory data | |
| Cystatin C, mg/L, median (IQR) | 2.19 (1.3, 3.0) |
| Creatinine, μmol/L, median (IQR) | 87 (47.5, 173.0) |
| Bilirubin, μmol/L, median (IQR) | 13.9 (7.3, 31.9) |
| Outcomes, n (%) | |
| Clinical cure | 30 (76.9) |
| Clinical failure | 9 (23.1) |
- Abbreviation: IQR, interquartile range.
3.2. Ceftazidime/Avibactam Cmin Distribution
A total of 82 blood samples were analyzed, with normality testing confirming normal distribution for both ceftazidime (Shapiro–Wilk p = 0.168) and avibactam (p = 0.188) concentrations (p > 0.05 for both). Therefore, the concentration data was consequently expressed as mean ± SD, revealing Cmin of 24.1 ± 14.3 mg/L for ceftazidime and 6.0 ± 4.4 mg/L for avibactam (Figure 1), accompanied by substantial interindividual variability (coefficients of variation: 59.5% and 73.5%, respectively).
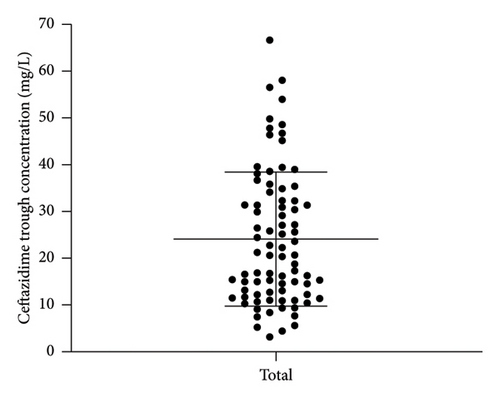
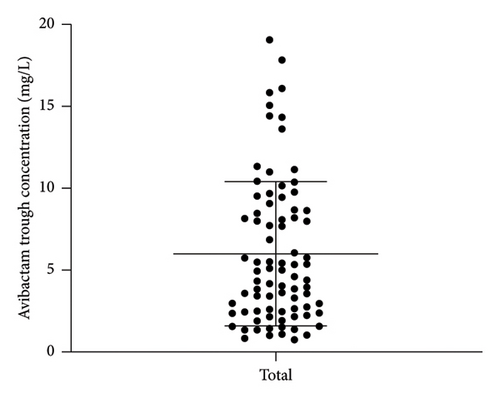
Figure 2 illustrated the Cmin distribution of ceftazidime and avibactam across different clinical subgroups (renal insufficiency, CRRT recipients, and elderly patients). Non-normal distributions in some subgroups (e.g., elderly cohort, p < 0.05) prompted nonparametric analysis using median (interquartile range) and Mann–Whitney U tests.
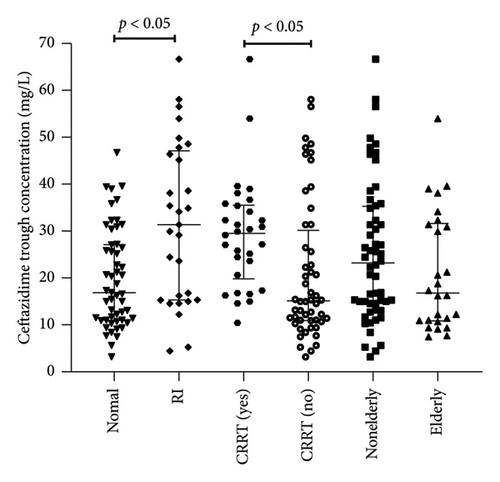
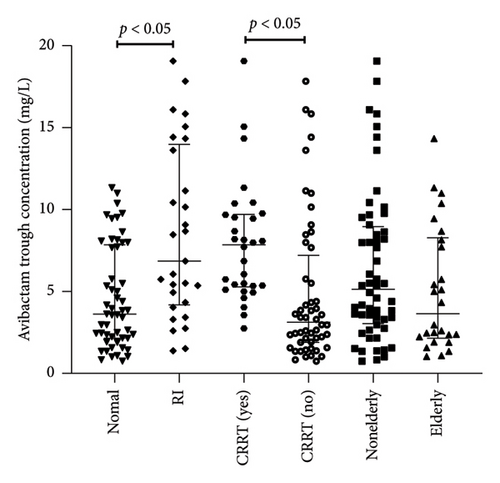
The Cmin of ceftazidime in patients with renal insufficiency (n = 29) was significantly higher than that in patients with normal renal (n = 53) (32.7 [15.3–48.0] vs. 16.9 [10.0–25.3] mg/L, p < 0.05) (Figure 2). The Cmin in patients receiving CRRT (n = 30) was significantly higher than that in patients without CRRT (n = 52) (30.6 [19.8–36.1] vs. 15.4 [8.9–45.5] mg/L, p < 0.05). There was no significant difference in Cmin of ceftazidime between elderly patients (n = 26) and nonelderly patients (n = 56) 16.8 [10.8–31.6] mg/L vs. 23.2 [15.1–46.7] mg/L, p > 0.05). The Cmin of avibactam in patients with renal insufficiency (n = 29) was significantly higher than that in patients with normal renal (n = 53) (6.8 [4.3–14.3] vs. 3.5 [2.1–8.0] mg/L, p < 0.05) (Figure 2). The trough concentration of Cmin in patients receiving CRRT (n = 30) was significantly higher than that in patients without CRRT (n = 52) (7.8 [5.3–9.9] vs. 3.1 [2.3–10.4] mg/L, p < 0.05). There was no significant difference between elderly patients (n = 26) and nonelderly patients (n = 56) (3.63 [2.1–8.3] vs. 6.5 [3.5–13.8] mg/L, p > 0.05).
3.3. Microbiological and PK/PD Result
This subsequent analysis involved 23 infected patients with MIC results, revealed that 43.5% (10/23) of strains had a MIC of 2 mg/L (Table 2). Infections were predominantly caused by carbapenem-resistant Klebsiella pneumonia (18/23, 78.3%) and carbapenem-resistant Pseudomonas aeruginosa (5/23, 21.7%). Furthermore, the strains isolated from 19 infected patients exclusively produced serine enzyme, while those isolated from the remaining 4 infected patients produced serine enzyme and metalloenzyme. And resistance to ceftazidime/avibactam occurred in 4 out of the 23 cases due to the production of metalloenzyme. Among the 23 patients with MIC results, optimal joint PK/PD targets for ceftazidime/avibactam were achieved in 16 patients (69.6%), while quasi-optimal achievement was observed in 7 patients (30.4%) (Table 2). In the 7 patients who reached quasi-optimal PK/PD target, it was noted that the pathogens of 4 patients produced serine enzyme and metalloenzyme. Then among the 4 cases, successfully treatment was achieved in 2 who were successfully treated were co-administered with aztreonam.
| Patient ID | Site of infection | Bacterial | MIC (mg/L) | Aetiology | PK/PD target | Outcome |
|---|---|---|---|---|---|---|
| 1 | Pulmonary infection | Pseudomonas aeruginosa | 4 | Serine enzyme | Optimal | Clinical cure |
| 2 | Pulmonary infection and intra-abdominal infection | Klebsiella pneumoniae | 2 | Serine enzyme | Optimal | Clinical cure |
| 3 | Pulmonary infection and urinary-tract infection | Klebsiella pneumoniae | 4 | Serine enzyme | Optimal | Clinical cure |
| 4 | Intra-abdominal infection | Klebsiella pneumoniae | 2 | Serine enzyme | Quasi-optimal | Clinical cure |
| 5 | Pulmonary infection | Klebsiella pneumoniae | 4 | Serine enzyme | Optimal | Clinical cure |
| 6 | Intra-abdominal infection | Klebsiella pneumoniae | 2 | Serine enzyme | Optimal | Clinical cure |
| 7 | Intra-abdominal infection | Klebsiella pneumoniae | 2 | Serine enzyme | Optimal | Clinical cure |
| 8 | Pulmonary infection | Klebsiella pneumoniae | 0.25 | Serine enzyme | Optimal | Clinical failure |
| 9 | Urinary-tract infection | Klebsiella pneumoniae | 128 | Serine enzyme and metalloenzyme | Quasi-optimal | Clinical cure |
| 10 | Intra-abdominal infection | Klebsiella pneumoniae | 1 | Serine enzyme | Optimal | Clinical cure |
| 11 | Intra-abdominal infection | Klebsiella pneumoniae | 1 | Serine enzyme | Optimal | Clinical cure |
| 12 | Pulmonary infection | Klebsiella pneumoniae | 128 | Serine enzyme and metalloenzyme | Quasi-optimal | Clinical cure |
| 13 | Intra-abdominal infection | Klebsiella pneumoniae | 4 | Serine enzyme | Quasi-optimal | Clinical cure |
| 14 | Biliary duct infection | Klebsiella pneumoniae | 2 | Serine enzyme | Optimal | Clinical cure |
| 15 | Intra-abdominal infection | Klebsiella pneumoniae | 128 | Serine enzyme and metalloenzyme | Quasi-optimal | Clinical failure |
| 16 | Pulmonary infection | Pseudomonas aeruginosa | 4 | Serine enzyme | Quasi-optimal | Clinical failure |
| 17 | Pulmonary infection | Klebsiella pneumoniae | 2 | Serine enzyme | Optimal | Clinical cure |
| 18 | Pulmonary infection | Klebsiella pneumoniae | 2 | Serine enzyme | Optimal | Clinical failure |
| 19 | Biliary duct infection | Pseudomonas aeruginosa | 2 | Serine enzyme | Optimal | Clinical cure |
| 20 | Pulmonary infection | Pseudomonas aeruginosa | 2 | Serine enzyme | Optimal | Clinical failure |
| 21 | Pulmonary infection | Pseudomonas aeruginosa | 2 | Serine enzyme | Optimal | Died |
| 22 | Pulmonary infection and urinary-tract infection | Klebsiella pneumoniae | 0.5 | Serine enzyme | Optimal | Died |
| 23 | Pulmonary infection | Klebsiella pneumoniae | 128 | Serine enzyme and metalloenzyme | Quasi-optimal | Died |
- Abbreviations: MIC, minimal inhibitory concentrations; PK/PD, pharmacokinetic/pharmacodynamic.
3.4. Clinical Cure Evaluation
The risk factor analysis testing the variables possibly associated with the clinical cure is shown in Table 3. Our findings of univariate logistic regression analysis indicated that achieving optimal joint PK/PD target (p < 0.001) and co-administered with aztreonam (p = 0.002) were independent factor of clinical cure in univariate logistic regression analysis. Then the results demonstrated that the site of infection, such as pulmonary or intra-abdominal infection, did not have a significant impact on the clinical efficacy of patients. Furthermore, the co-administration with carbapenems, cephalosporins, and other antibiotic did not significantly impact the clinical cure of patients. However, no influences were found in the multivariate logistic regression.
| Clinical cure | Clinical failure | p value | |
|---|---|---|---|
| (n = 15) | (n = 8) | ||
| Demographics | |||
| Age, years, median (IQR) | 46 (38, 58) | 56.5 (28.8, 77) | 0.745 |
| ICU admission, n (%) | 5 (33.3) | 4 (50.0) | 0.657 |
| CRRT, n (%) | 6 (40.0) | 3 (37.5) | 1 |
| Site of infection, n (%) | |||
| Pulmonary infection | 8 (53.3) | 8 (100.0) | 0.052 |
| Intra-abdominal infection | 4 (26.7) | 0 (0) | 0.257 |
| Drug combination, n (%) | |||
| Carbapenem | 13 (86.7) | 6 (75.00) | 0.589 |
| Cephalosporin | 2 (13.33) | 0 (0) | 0.526 |
| β-lactam/β-lactamase inhibitora | 8 (53.3) | 7 (87.5) | 0.176 |
| Glycopeptides | 10 (66.7) | 6 (75.0) | 1 |
| Macrolide | 1 (6.7) | 1 (12.5) | 1 |
| Quinolone | 5 (33.3) | 2 (25.0) | 1 |
| Aztreonam | 6 (40.0) | 1 (12.5) | 0.002 |
| PK/PD joint target, n (%) | |||
| Optimal joint PK/PD | 15 (100.00) | 1 (12.5) | < 0.001 |
- Abbreviation: PK/PD, pharmacokinetic/pharmacodynamic.
- aContaining cefoperazone/sulbactam and piperacillin/tazobactam.
4. Discussion
We conducted TDM in patients with Gram-negative bacteria infections receiving ceftazidime/avibactam. This study revealed significant variability in the Cmin of ceftazidime and avibactam. Furthermore, achieving optimal joint PK/PD target and co-administration with aztreonam were identified as risk factors associated with clinical cure.
Ceftazidime/avibactam, a novel BL/BLI, was the first-line therapy for Gram-negative bacteria infections [21]. The clinical cure observed in this study was 76.9% consistent with a comprehensive phase III study conducted by Torres, which evaluated the efficacy of ceftazidime/avibactam in pulmonary infections and demonstrated a clinical cure rate of 78.1% [22].
Our study found that the Cmin of ceftazidime and avibactam in patients with renal insufficiency and receiving CRRT treatment were significantly higher than those in patients with normal renal function and without CRRT treatment, which may be related to the fact that patients receiving CRRT are often accompanied by renal insufficiency and the drug clearance is reduced. The utilization of CRRT is indispensable in the treatment of critically ill patients and currently there is limited evidence regarding the regimen of ceftazidime/avibactam in patients undergoing CRRT [23–25]. Both ceftazidime and avibactam are metabolized by kidney. Then the antibiotic dosage for infected patients undergoing CRRT should be adjusted based on the status of organ function and taking into account the MIC of pathogenic bacteria.
We also found that there was no significant difference between the Cmin of ceftazidime and avibactam in elderly patients and nonelderly patients. The findings from a phase I study indicated no significant differences in the PK parameters of ceftazidime and avibactam between elderly and young patients receiving ceftazidime/avibactam [26]. Considering that elderly patients often associate with renal insufficiency and other underlying disease, personalized regimens adjustments are necessary.
In our study, 69.6% and 30.4% of patients achieved the optimal and quasi-optimal joint PK/PD target, respectively. Fresan conducted a study on continuous infusion of ceftazidime/avibactam in patients with resistant Gram-negative bacterial infections and 83.9% of patients achieved a 100% fT > 4 × MIC of ceftazidime [27]. The discrepancy between studies may be attributed to the differences in the infusion time of ceftazidime/avibactam, with 2 h infusion in our study and continuous infusion in this study. The utilization of continuous infusion is a crucial approach to enhance the rate of achieving the PK/PD target for time-dependent antibiotics [15]. Furthermore, the published PK/PD study on BL/BLI also demonstrated the benefits of continuous infusion in enhancing the PK/PD target of ceftazidime/avibactam [11]. Tumbarello discovered that prolonged infusion was associated with reduced mortality among patients [28]. These findings underscore the importance of continuous infusion to enhance clinical outcomes for individuals battling bacterial infections.
The results demonstrated that achieving optimal joint PK/PD target in combination with aztreonam was the independent predictor of clinical cure in univariate logistic regression analysis. However, no influences were found in the multivariate logistic regression and a discrepancy may be due to sample size limitations. However, other clinical results and microbial results confirmed this conclusion from the side. A study conducted by Gatti involved TDM analysis on patients with drug-resistant infections who were treated with ceftazidime/avibactam of continuous infusion [17]. This study revealed that suboptimal/quasi-optimal joint PK/PD target of continuous infusion ceftazidime/avibactam was the only risk factor influencing of microbiological failure. The above findings indicate that optimizing the joint PK/PD target of ceftazidime/avibactam could present a promising strategy for achieving clinical cure. Ceftazidime/avibactam is effective against Gram-negative bacteria producing class A, C, and some D enzymes. When combined with aztreonam, it has demonstrated superior clinical efficacy in treating isolated strains that produce metalloprotease. Another study utilizing the checkerboard method found a synergistic effect of aztreonam in combination with ceftazidime/avibactam against 81.3% of pathogenic bacteria producing metalloproteinases-producing Enterobacteriaceae [29]. These highlighted the significance of this combination for treating infections caused by such Gram-negative bacteria.
For different infection sites, it is crucial to achieve the effective concentration of antibiotic in the interstitial fluid. Our study revealed that the site of infection did not exert a significant impact on clinical cure. However, Shields demonstrated a low clinical cure rate for ceftazidime/avibactam in pulmonary infections [9]. Furthermore, Tumbarello indicated an association between lower respiratory tract infections and increased mortality among infected patients [28]. Based on the pathogenic bacteria, infection site, and the PK/PD of antibiotics, the antimicrobial treatment plan was formulated.
We acknowledge the limitations of our study. The sample size of the study was small, so no influencing factors were found in multivariate logistic regression analysis. Additionally, we measured the total concentrations of ceftazidime and avibactam in this study, and subsequently determined the free drug concentrations based on reported fractions of unbound drug, while disregarding inter-individual variations in protein levels among patients. Furthermore, the regimen was not adjusted according to the PK/PD results for ceftazidime/avibactam. Finally, we did not perform TDM and dose adjustment on aztreonam.
5. Conclusion
In this study, we observed that the clinical cure rate in this study was 76.9%, with 69.6% of patients achieving joint optimal PK/PD targets for ceftazidime/avibactam. Furthermore, there is significant variability in the Cmin of ceftazidime and avibactam. The risk factors of clinical cure were identified as optimal joint PK/PD targets and co-administration with aztreonam. However, due to the limitations of our sample size, further validation through large-scale and high-quality clinical studies is necessary.
Ethics Statement
The Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University approved the study protocol. The abandonment of informed consent is due to retrospective and nonintrusive research design. All patient data are collected anonymously. This study is based on the Helsinki Declaration.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The work was supported by the Key Research and Development Program of Shaanxi, grant number 2019ZDLSF01-05 and 2023-YBSF-066.
Acknowledgments
All authors would like to thank all patients for their cooperation. The authors would also like to thank Prof. Yalin Dong from the First Affiliated Hospital of Xi’an Jiaotong University for her help in the completion of this study.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




