Pirfenidone Alleviates Intrauterine Infection-Induced Lung Injuries in Neonates by Targeting Transforming Growth Factor Beta 1/Sma- and Mad-Related Protein Signaling Pathway
Abstract
Objective: To explore the effect and mechanism of pirfenidone (PFD) on lung injuries in newborn rats caused by intrauterine inflammation.
Methods: In vivo, we established the intrauterine inflammation model with lipopolysaccharide (LPS) injection in pregnant rats. The administration of PFD in pregnant rats was performed to evaluate its beneficial effect against intrauterine inflammation-induced lung injuries in neonatal rats. Lung tissues of newborns from three groups of pregnant rats (Saline + DMSO, LPS + DMSO, and LPS + PFD) were collected. Immunohistochemistry (IHC) and Western blot were used to detect the fibrotic-related protein expressions. In vitro, LPS-induced mouse lung epithelial cells (MLE 12) were employed to explore the underlying mechanism of PFD against intrauterine inflammation-induced lung injury in neonates.
Results: In vivo, the radial alveolar count (RAC) was decreased, the mean linear intercept (MLI) was increased, and the lung injury score was high in intrauterine inflammation (LPS + DMSO-treated pregnant rats) induced lung injuries in newborns. The protein level of matrix metallopeptidase 9 (MMP-9), TGF-β1 (transforming growth factor beta 1), phospho-Smad2 (Sma- and mad-related protein 2), and phosphor-Smad3 (Sma- and mad-related protein 3) levels were upregulated in neonatal lungs with intrauterine infection. Lung injuries were alleviated in LPS + PFD groups, and the protein levels of TGF-β1, p-Smad2, p-Smad3, and MMP-9 were reduced. In vitro, PFD attenuated the LPS-induced inflammatory response and reduced the expressions of MMP-9, TIMP-1, and Collagen IV in lung epithelial cells through the TGF-β1/Smad2/3 signaling pathway.
Conclusions: PFD alleviates intrauterine infection-induced lung injuries in neonates by targeting transforming growth factor beta 1/Sma- and mad-related protein signaling pathway. The inhibition of TGF-β1 signaling by PFD prevents the neonatal lung injuries by reducing MMP-9 and Collagen IV protein levels. This study provides theoretical basis and insights for PFD-mediated intervention of lung injury in neonates with intrauterine infection.
1. Introduction
Intrauterine infection is characterized by pathogenic microbial infection in amniotic fluid, placenta, or fetus, which is a risk factor for multiple organ damages in premature infants (the most susceptible organ is the lung) [1]. Intrauterine infection causes airway inflammation, airway destruction, pulmonary fibrosis, and lung vascular/interstitial disorders, culminating in bronchopulmonary dysplasia in infants [2]. A previous study [3] has revealed that elevated levels of inflammatory cytokines such as interleukins (IL-1, IL-6, and IL-8) and tumor necrosis factor-α (TNF-α) in amniotic fluid could contribute to lung injuries in neonates. Intraperitoneal injection of endotoxin is able to produce lung injuries and histological changes similar to bronchopulmonary dysplasia in animals [4, 5]. The animal model of intrauterine infection is a valuable tool to explore the underlying mechanisms by which intrauterine infections induce lung injuries and dysplasia and to evaluate the therapeutic potential of medications.
Matrix metalloproteinases (MMPs) are extracellular endopeptidases involved in the remodeling of the extracellular matrix, which are regulated by tissue inhibitors of metalloproteinases (TIMPs) and cytokines. The relative abundance of MMPs and TIMPs plays an important role in extracellular matrix homeostasis [6]. Accumulating evidence indicates that MMPs play critical roles in lung tissue extracellular matrix remodeling by regulating immune cell infiltration during inflammation and lung disease development [7–11]. The relative expression levels of MMP-9 and TIMP-1 were found to be increased in lung tissues of premature infants with bronchopulmonary dysplasia [11, 12]. An altered ratio of MMP-9/TIMP-1 was reported in neonatal rats with hyperoxia-induced lung damages [13]. However, the regulatory mechanism of MMP expression is complex. Previous evidence suggests that transforming growth factor-beta 1 (TGF-β1) is a key factor in regulating MMP-9 and MMP-2 expression in trophoblasts during early pregnancy [14, 15].
PFD is a small molecule inhibitor for the inflammatory and fibrotic response. Specifically, PFD can reduce collagen secretion and lung fibrosis [16, 17]. A previous report showed that PFD was able to suppress inflammation and MMP-9 expression in the mouse model of lung injury [18], with significant alleviation on lung fibrosis, edema, and inflammatory cell infiltration [19]. The mechanisms by which intrauterine infections and inflammations trigger lung injury and developmental abnormalities are unclear. Proper medical interventions for mothers with chorioamnionitis and intrauterine infections are crucial for mitigating the potential damages in the newborns.
In this study, we established an intrauterine infection model using pregnant rats in vivo to evaluate the histological changes in newborn neonatal lung tissues and investigate associated molecular changes. The protective effects of PFD on lung injuries of neonatal rats were evaluated. In vitro, lipopolysaccharide (LPS)-treated mouse lung epithelial cells were employed to investigate the underlying molecular mechanisms of PFD efficacy against intrauterine infection-induced lung injury.
2. Materials and Methods
2.1. Animal Model of Intrauterine Infection
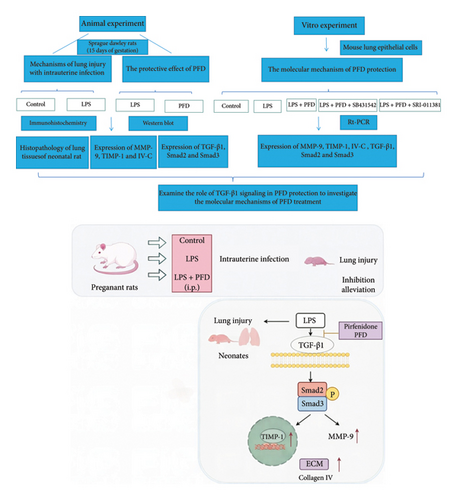
The experimental procedures in the animal model were approved by the Animal Use and Care Committee of Qingdao University (Qingdao, China). Eighteen pregnant Sprague Dawley rats (15 days of gestation; weighing 300–350 g) were acquired from the Experimental Animal Center of Qingdao University, housed in a pathogen-free animal house at controlled temperature (22 ± 2°C), with a 12-h light/dark cycle. After 3 days of acclimation, the rats were randomly assigned into three groups (n = 6 each): control, intraperitoneal (i.p.) LPS injection, and i.p. LPS + PFD injection. Specifically, rats in the LPS group were i.p.-injected with 0.6 mg/kg of LPS (E. coli; Sigma Chemicals, St. Louis, MO, USA), and the control group rats were administrated with an equal volume of sterile saline. Rats in the PFD group were injected with PFD (200 mg/kg [20]) 2 h before and 12 h after the LPS injection. The other two groups of rats were injected with an equal volume of the vehicle (dimethyl sulfoxide, Sigma). One rat from each group was anesthetized using 4% isoflurane and underwent a cesarean section at gestational day 21, while all other rats underwent natural labor. There were 41 newborns in the control group, 36 newborns in the LPS-treated group, and 37 newborns in the PFD intervention group. The survival rate and survival period of each newborn were recorded, and the neonatal rats in each group were separated into two subgroups for tissue collection at 1 and 3 days after birth, respectively.
2.2. Tissue Processing, Histopathology, and Immunohistochemistry (IHC)
After the cesarean section, the rat placenta was placed on a filter paper, weighed using an electronic balance, and then fixed in 4% neutral formaldehyde before tissue processing and hematoxylin & eosin (H&E) staining. Lung tissues were also dissected from each group of newborn rats at 24 h or 72 h after birth after CO2 euthanization. The weight of the neonatal lung, neonatal body, and placenta was recorded. The left half of lung tissues was fixed in 4% neutral formaldehyde for 48 h and then subjected to IHC and H&E staining. The right half of lung tissues was immediately stored in an ultralow-temperature freezer for cryopreservation and used for Western blot analysis.
The tissue blocks of lungs and placentas were cut into 4 μm thick sections and processed by an H & E staining kit (Beyotime, Qingdao China). The stained sections were photographed and examined under a light microscope (Beyotime, Qingdao, China) for histological examination. Parameters including leukocyte infiltration in alveolar space and interstitial space, the presence of hyaline membrane, and alveolar septal thickening were assessed. The following histological scoring system was used: 0, no lung injury; l, mild level of damage; 2, moderate degree of damage; 3, severe degree of damage; and 4, very severe histological damage [21]. Alveolization was estimated by the radial alveolar count (RAC) method proposed by Emery and Mithal. The mean linear intercept (MLI) was calculated from the H&E-stained lung sections by dividing the total length of a line (μm) drawn across the entire field by the total number of alveolar intercepts encountered along the length of the line. Septal thickness was assessed in elastin-stained lung sections.
For IHC analysis, tissue sections were incubated in a 67°C oven for 2 h, and deparaffinization was performed in xylene for 1 h. The sections were further dehydrated in a series of ethanol solutions and washed in phosphate-buffered saline (PBS). Antigen retrieval was achieved using the citrate buffer in a microwave oven for 10 min. The peroxidase activity in the samples was quenched by incubation with 3% H2O2 treatment for 10 min. Next, the tissue sections were incubated with 20% normal horse serum in PBS for 30 min and then stained with primary antibody for 2 h at ambient temperature. After washing with PBS, the sections were labeled with the polymer enhancer (Sanying, Wuhan, China) for 20 min at ambient temperature. Then, the sections were incubated with enzyme-labeled antimouse/rabbit polymer for 30 min at ambient temperature. The following antibodies were used in the study: anti-MMP-9 (1:600; Cat. no.: 10375-2-AP), anti-TIMP-1 (1:500; Cat. no.: bs-0415R), and anticollagen IV (1:300; Cat. no.: 55131-1-AP). Colorimetric development was performed using 3, 3′-diaminobenzidine solution (Sanying Wuhan, China) for 5 min, which was monitored under a light microscope for color change. The sections were then counterstained with hematoxylin, differentiated with 0.1% HCl, rinsed in warm tap water, dehydrated in a gradient of alcohol solutions, and sealed using neutral gel. Sealed sections were reviewed and scored by two experienced pathologists under the Olympus BX600 microscope equipped with a SPOT Flex camera (Tokyo, Japan). The semiquantitative scoring system was applied to assess the staining intensity with a scale of 0–3 (0, no signal; 1, moderate staining signal; 2, strong signals; and 3, very strong staining intensity). Their areas (alveolar epithelium, bronchial epithelium, and vascular endothelium) in each sample were evaluated to calculate the average staining intensity of each section. For each time point, six samples were randomly selected for analysis.
2.3. Western Blot
Right lung tissues of neonatal rats were subjected to protein extraction using radioimmunoprecipitation assay buffer containing a tablet of the phosphatase inhibitor (Beyotime, Qingdao, China) and 1 μM dithiothreitol. The lysate was centrifuged at 12,000 rpm for 15 min at 4°C for supernatant collection. Protein concentration quantification was performed using Coomassie Brilliant Blue reagent (Beyotime, Qingdao, China). Protein samples were mixed with 6x loading buffer and boiled at 95°C for 5 min. 15 μg of protein samples were separated in polyacrylamide gel electrophoresis (PAGE) and then transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking in 5% skimmed milk for 1 h at ambient temperature, the membrane was incubated with corresponding antibodies at 4°C overnight. After washing, the membrane was further labeled with secondary antibody for 2 h. Primary antibodies used in this study are as follows: anti-TGF-β1 (1:1000; Cat. no.: bs-0086R; Bioss), anti-p-smad3 (1:1000; Cat. no.: 9520T; Cell Signaling Technologies), anti-p-smad2 (1:1000; Cat. no.: cst18338T; Cell Signaling Technologies), anti-MMP-9 (1:600; Cat. no.: 10,375-2; Sanying Wuhan, China), anti-TIMP-1 (1:500; Cat. no.: bs-0415R; Bioss), and anticollagen IV (1:300; Cat. no.: 55131-1-AP; Sanying Wuhan, China). Protein bands were developed by incubating the membrane with enhanced ECL reagent (Thermo Fisher Scientific). β-actin was used as the loading control for normalization.
2.4. In vitro Experiments
LPS-induced mouse lung epithelial cells were used as the in vitro model to investigate the mechanisms of PFD treatment. Cells were treated with PFD in the presence or absence of the TGF-β signaling agonist and inhibitor. Mouse lung epithelial cells (MLE-12) were acquired from the Shanghai Cell Bank (Chinese Academy of Sciences, Shanghai, China). Three experimental conditions were set up: control group (treated with PBS); LPS group (treated with 4 μg/mL of LPS), and LPS + PFD group (4 μg/mL of LPS and 200 μg/mL of PFD). Cells were treated with indicated drugs for 24 h. In order to examine the role of TGF-β1 signaling, the TGF-β1/Smad3 signaling inhibitor (SB431542) and agonist (SRI-011381) were used together with LPS and PFD in mouse lung epithelial cells. MLE-12 cells were treated under 5 conditions: control, LPS treatment, LPS + PFD treatment, LPS + PFD + SB431542 treatment, and LPS + PFD + SRI-011381 treatment. Cells were pretreated with SB431542 (10 μM) or SRI-011381 (10 μM) for 24 h before LPS and/or PFD treatment. Relative gene expression in different groups was quantified using real-time quantitative PCR (RT-qPCR).
2.5. Statistical Analysis
All data were summarized and expressed as mean ± standard deviation. One-way ANOVA (“analysis of variance”) was applied for multiple comparisons, with Tukey’s test as the posthoc test (Tukey’s multiple comparison test is one of the several tests that can be used to determine which means among a set differ from the rest). A value of p < 0.05 was used as the threshold of statistical significance.
3. Results
3.1. Establishment of a Rat Model of Intrauterine Infection to Assess Alveolar Development
A rat model of intrauterine infection was established using i.p. injection of LPS into pregnant rats. The placenta and uterine wall of pregnant rats showed hyperemia, edema, and massive infiltration of neutrophils, certificating that the model was well established. Reduced lung volume, congestion, and structural destruction on the lung surface were observed in the LPS group (Figure 1).
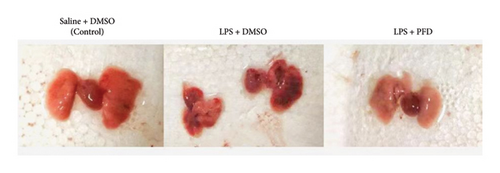
These results indicate that this model was successful to reflect intrauterine infections. Our data showed that in the control group, the alveolar wall and alveolar space of 3-day-old newborn rats were narrower than those in 1-day-old newborns. The number of alveoli was also increased, indicating the normal development of alveoli after birth. However, alveolar formation was significantly delayed in 3-day-old newborns from the LPS + DMSO-treated pregnant rats, as evidenced by the reduced number of alveoli and narrow alveolar space. These data suggest that intrauterine infection can interfere with alveolar development.
3.2. Protective Effects of PFD on Neonatal Lung Tissues During Intrauterine Infection
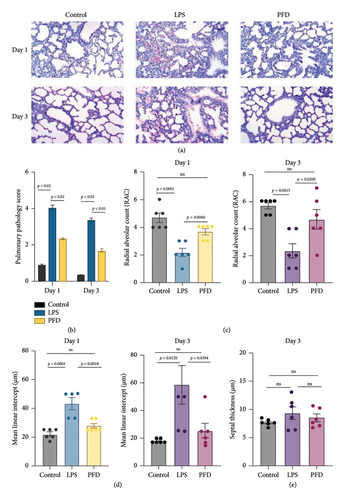
Compared to the lung tissues from the LPS group, the PFD administration group displayed an increased number of alveoli, a larger cavity of alveolar space, and a lower degree of injury in the lung tissues. There was a reduction of the pulmonary pathology score after PFD administration (Figure 2(a)). Overall, pulmonary pathology scores indicated that LPS treatment caused severe damages in the neonatal lung tissues when compared to the control group (p < 0.05). The RAC was decreased, the MLI was increased, and the lung injury score was high in intrauterine inflammation (LPS-treated pregnant rats) induced lung injuries in newborns (Figures 2(b), 2(c), and 2(d)). No significant alterations were found for the septal thickness (7.7 ± 0.6) μm−1 in Day 3 neonatal lungs from the control group compared with neonatal lungs from the LPS + DMSO-treated pregnant rats group (9.3 ± 2.7) μm−1 (p = 0.3022) (Figure 2(e)). The results indicate the protective effects of PFD against intrauterine infection-induced neonatal lung injuries.
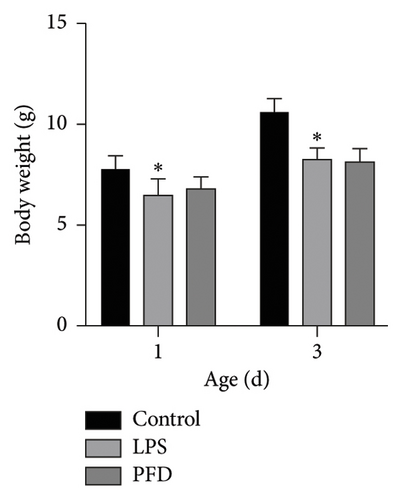
There were no significant changes in the activity or appetite of pregnant rats before or after treatment with saline, LPS, or PFD. However, one pregnant rat died upon LPS and PFD treatment, respectively. Natural delivery time for the three groups of pregnant rats was between 20 and 22 days, with 35/37 newborns (two were deceased shortly after birth) from the PFD group, 34 (34/36) newborns (two were deceased shortly after birth) from the LPS group, and 41 newborns from the control group. The body and lung tissue weights of the newborns at 1 and 3 days were lower in the LPS group when compared to the control group, while no significant difference was observed between PFD and LPS groups. Further, the ratio of lung/body weight in neonates at 1 and 3 days after birth was also reduced compared to the control group (Figures 3, 4).

3.3. Altered Expression of Extracellular Matrix Proteins in Neonatal Lung Tissues Was Reversed by PFD Administration
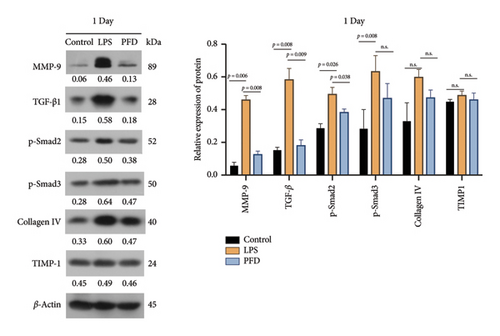
Aberrant expression of six extracellular matrix proteins was observed in the lung tissues of newborns in the control group (administrated with an equal volume of sterile saline), the LPS group (pregnant rats were i.p.-injected with 0.6 mg/kg of LPS), and the PFD administration group (pregnant rats in the PFD group were injected with PFD (200 mg/kg) 2 h before and 12 h after the LPS injection). The other two groups of rats were injected with an equal volume of the vehicle (dimethyl sulfoxide) as a control of PFD administration in parallel. Specifically, one day after birth, the levels of MMP-9 and TGF-β1 in lung tissues of newborns in the LPS group were elevated in comparison to the control group (p < 0.01), and the administration of PFD significantly reduced the levels of these proteins (Figure 5). The protein level of pSmad2 was also upregulated in the LPS group (p < 0.05), whereas it was reduced by PFD administration (p < 0.05). p-Smad3 expression was increased upon LPS treatment (p < 0.01). Collagen IV and TIMP-1 levels showed no statistical differences (Figure 5).
Three days after birth, the levels of MMP-9, TGF-β1, and pSmad3 in lung tissues of newborns were significantly upregulated in the LPS group in comparison to the control group (p < 0.01 or p < 0.05), and their levels were decreased by PFD administration (p < 0.05). Moreover, the levels of pSmad2, TIMP-1, and Collagen IV were mildly induced by LPS treatment and showed a trend of decrease after the administration of PFD (Figure 6).
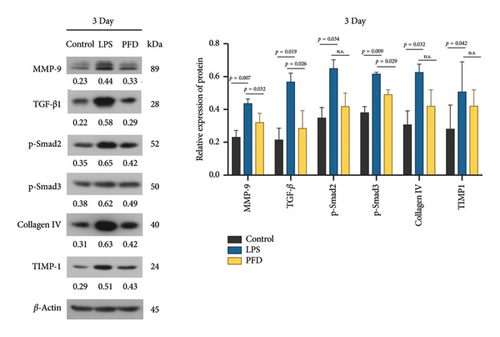
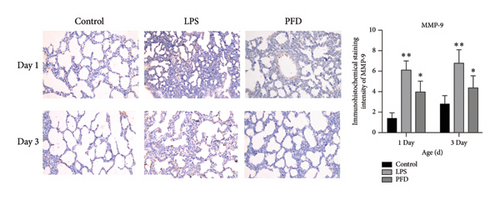
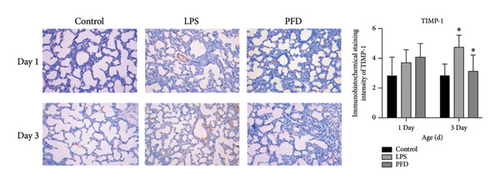
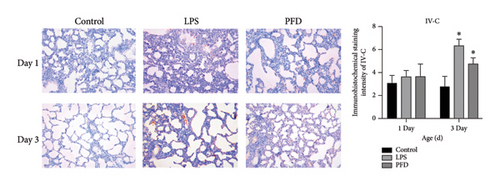
Next, we examined the MMP-9, TIMP-1, and Collagen IV (IV-C) expressions in the lung tissues using IHC. We found that the expression of MMP-9 in the LPS group was enhanced in the lung tissues of 1- and 3-day-old newborns than the controls (p < 0.05), whereas PFD intervention downregulated the expression (p < 0.05). The expressions of TIMP-1 and IV-C were enhanced in the lung tissues of 3-day-old newborn rats in the LPS + DMSO group in comparison to the control group (p < 0.05) (Figures 7, 8, 9).
3.4. In vitro Lung Epithelial Cell Model for PFD Efficacy Evaluation and Mechanism Study
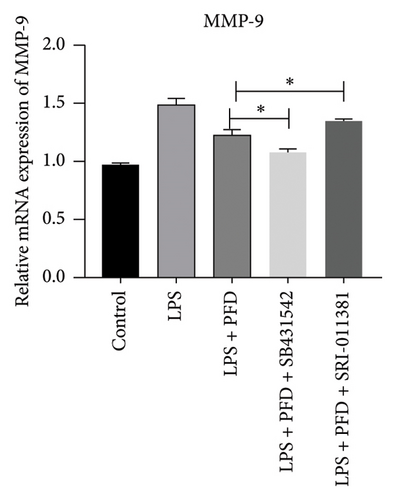
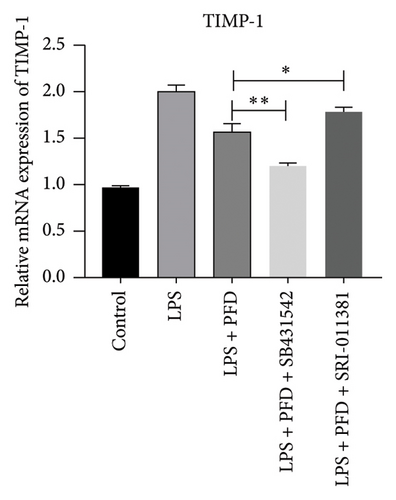
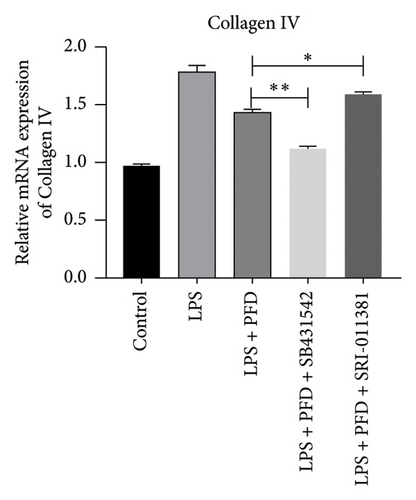
Three experimental conditions were set up using MLE-12 cells: control group (treated with PBS), LPS group (treated with 4 μg/mL), and LPS + PFD group (4 and 200 μg/mL). RT-qPCR was performed to analyze gene expression changes. LPS treatment caused significant increases in MMP-9, TIMP-1, and Collagen IV mRNA levels, and the presence of PFD abrogated their upregulation (Figure 10). Similar results were observed for mRNA levels of TGF-β1, Smad2, and Smad3 (Figure 11).
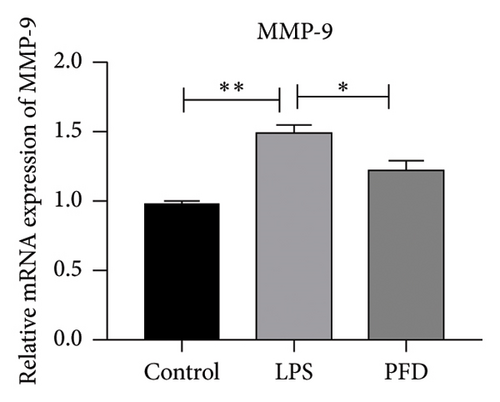
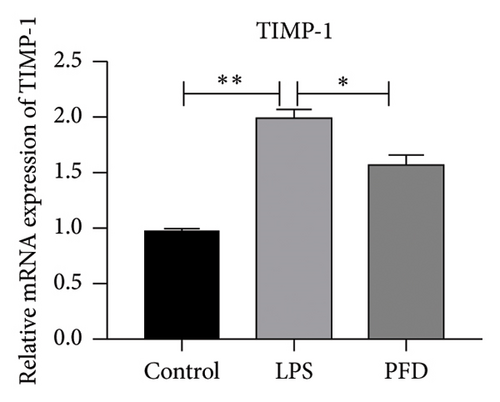
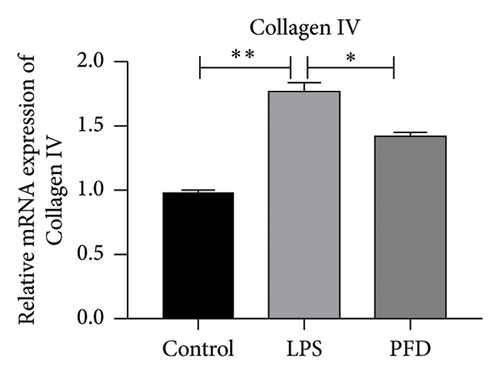
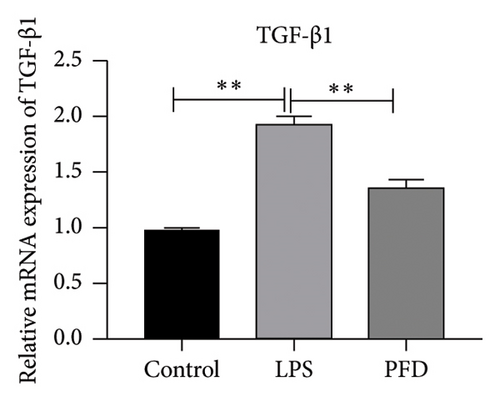
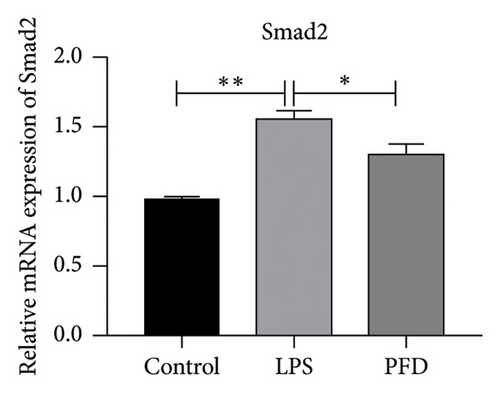
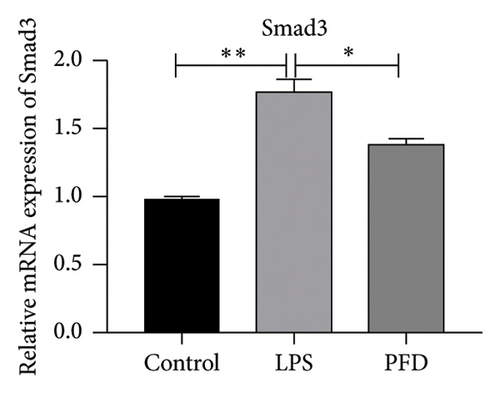
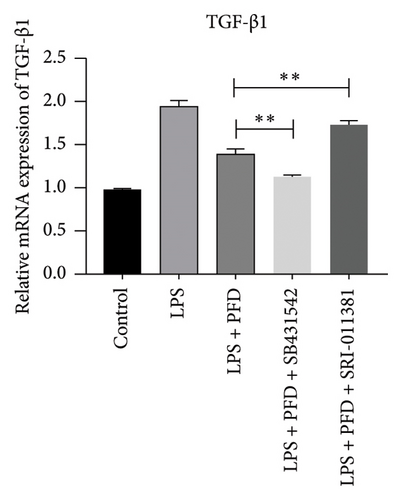
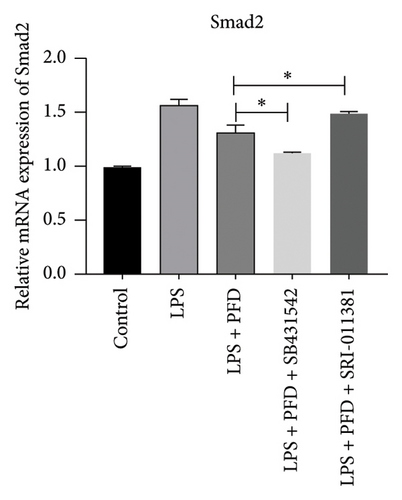
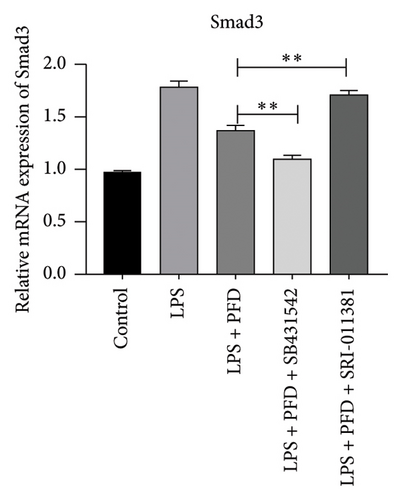
In order to examine the role of TGF-β1 signaling in PFD treatment, the TGF-β1/Smad3 inhibitor (SB431542) and agonist (SRI-011381) were used together with LPS and PFD in MLE-12 cells. TGF-β1 inhibitor treatment further reduced the mRNA levels of TGF-β1, Smad2, Smad3, MMP-9, Collagen IV, and TIMP-1 upon LPS and PFD combination treatment. However, their expressions were increased after the treatment of the TGF-β1 agonist (Figures 12, 13, 14). Together, these data indicate that PFD suppresses LPS-induced expression of extracellular matrix proteins such as MMP-9, Collagen IV, and TIMP-1 through the TGF-β1-dependent signaling pathway.
4. Discussion
The infection of pathogenic bacteria, viruses, atypical pathogens, fungus, and protozoa in pregnant females not only causes chorioamnionitis and premature birth but also leads to fetal inflammatory response syndrome (FIRS) which triggers damages in lung tissues, nervous system, immune organs, and liver. Based on the pregnant rat model of intrauterine infection, several studies have found that intrauterine infection could affect fetal nutrient absorption, intrauterine growth, and lung development of neonatal rats. These data were consistent with previous findings [22, 23].
In amniotic cavity, microbial infection can trigger the onset of local inflammations and elevated production of proinflammatory factors including IL-1, IL-6, and IL-8 [24, 25]. In our current study, we showed that LPS treatment decreased the number of alveoli and secondary alveolar septain of neonatal rats. The thickening of alveolar septa and massive inflammatory cell infiltration were also observed, which are consistent with a previous report [26, 27]. Importantly, PFD intervention significantly ameliorated the damages induced by LPS treatment in newborn rats. Protein levels of MMP9, TIMP-1, and Collagen IV were increased by LPS treatment in neonatal lung samples, whereas PFD intervention downregulated their expression. MMP-9 and TIMP-1 (the inhibitor of MMP) play important roles in the development and extracellular matrix remodeling of various organs [28]. The release of various inflammatory mediators causes damage to vascular endothelium, which is often accompanied by the decrease of extracellular matrix enzyme type IV collagenase and impaired lung function [29, 30]. Our current study revealed that LPS-induced intrauterine infection in pregnant rats elevated the TIMP-1 level in 3-day-old neonatal lung samples. These data suggested that upregulation of MMP-9 and TIMP-1 was implicated in pathological lung injuries during intrauterine infection, which may be responsible for the injury in lung tissues. Importantly, PFD administration lowered their expression and alleviated the associated lung injuries.
The expression of MMP-9 is regulated by different extracellular factors, transcriptional factors, and inflammatory factors. TGF-β1/Smad signaling is a multifunctional signaling cascade [31] which modulates the expression of MMP-9 and TIMP-1 [32]. A previous study showed that the MMP9 level was reduced in trophoblasts upon TGF-β1 treatment, whereas TGF-β1-antagonizing antibody increased its expression [33]. Another study [34] has demonstrated that upregulated TGF-β1 expression in lung tissues is responsible for excessive extracellular matrix deposition, alveolar damages, and interstitial thickening in a mouse model of lung fibrosis. However, increased collagen deposition can negatively regulate TIMP-1 expression and activation [35]. Our data revealed an upregulation of TGF-β1, Smad2, and Smad3 upon intrauterine infection, which is consistent with the pathogenic role of MMP-9 and TIMP-1 in pulmonary fibrosis. Future work is needed to validate whether TGF-β1 and its downstream effectors Smad2/Smad3 are implicated in the regulation.
PFD is developed as an antipulmonary fibrosis medication that downregulates various growth factors and procollagens [36]. In addition, it also serves as an inhibitor for profibrotic and proinflammatory cytokine to suppress the activity of fibroblasts and collagen deposition [35, 37–39]. PFD has been reported to reduce vascular permeability and the accumulation of inflammatory cells [19, 37]. Our data further revealed that PFD was able to alleviate LPS-induced neonatal lung damages and improve the conditions of pulmonary alveoli without obvious toxicity. Ruwanpura, Thomas, and Bardin [40] established an airway-sensitized mouse model, and they showed that PFD administration can reduce the production of inflammatory cytokines in the airways. In a mouse model of pulmonary fibrosis, PFD alleviated pulmonary fibrosis by suppressing the expression of multiple inflammatory cytokines such as interleukin 1 beta (IL-1β), interleukin 6 (IL-6), and interleukin 12 p40 (IL-12p40) [41]. A retrospective review of the clinical safety of PFD suggested that PFD could be well-tolerated in most patients [42]. Our study showed that PFD administration could suppress the expressions of MMP-9, collagen IV, and TIMP-1 and alleviate LPS-induced pulmonary damages. These effects seem to be mediated by the TGF-β1-dependent signaling pathway. We further tested this in vitro lung epithelial cell mode: PFD attenuates the LPS-induced inflammatory response and reduces the expressions of MMP-9 through the TGF-β1/Smad2/3 signaling pathway. TGF-β1 is one of the major profibrotic cytokine implicated in different pathogenic conditions [43]. Smads are the signal transducers mediating the intracellular signaling cascade of TGF-β1 [44]. The TGF-β1/Smad signaling pathway has been recognized as one of the main pathways responsible for the chronic inflammatory response and fibrosis in the respiratory tract. In our cell model, we found that the TGF-β1 signaling inhibitor enhanced the effect of PFD on extracellular matrix-related genes, while its agonist attenuated the effect of PFD. These data strongly indicate that the TGF-β1 signaling pathway mediates the protective effect of PFD in pulmonary fibrosis. At the cellular level, fibroblasts are the important player in tissue fibrosis, which can differentiate into myofibroblasts to boost extracellular matrix deposition in the alveolar septum [45]. PFD is able to reduce inflammation and alleviate lung injury through multiple pathways and signal pathways. In previous studies, it was reported that IKβ, IκBα, and NF-κB proteins are down-regulated by PFD after bleomycin-induced pulmonary fibrosis in rats [46]. MMP-9 is a well-known member in MMPs and plays a vital role in fibrotic disease progressions through different signaling pathways. Myofibroblasts modify ECM turnover by modulating the balance between MMPs and their natural inhibitors (tissue inhibitor of MMP; TIMPs). Levels of TIMP-1/MMPs affect lung diseases. We found that TGF-β1-p-Smad2/3 signaling was activated, and the ratio of MMP-9/TIMP-1 was increased in newborns with intrauterine infection which was consistent with previous studies. There have been studies reporting that lower levels of TIMP-1/MMPs (high levels of MMPs/TIMP-1) may cause lung injury [47]. In conclusion, inhibition of inflammatory cytokine production and various upstream and downstream pathways may explain the clinical benefits after PFD treatment, which is our next research direction. We sought paths and directions of future treatment for intrauterine infection-induced lung injury through mechanism research with PFD, a representative drug of antifibrosis drug.
Increasing evidence have shown that intrauterine infections affect lung development in newborns, especially on the preterm infants. Intrauterine infection-induced fetal lung injuries result in a high risk of morbidity and mortality in newborns. Herein, developing new strategies to prevent intrauterine infection-induced neonatal lung injuries is of great importance [48–51]. In this study, we demonstrated that intrauterine infections affected neonatal lung health, and PFD could alleviate the intrauterine infection-induced lung injury in neonates. However, to date, data on use in pregnant patients are insufficient, especially on the drug-associated risks for birth defects and miscarriage. Previous animal studies reported that there was no teratogenicity at doses up to 3 times the maximum recommended human dose. More clinical trials of PFD in pregnant patients, newborns, infants, and young children are needed for its further clinical applications. Exploring the early treatment with less side effects on the mother has a positive effect on improving the degree of lung injury in the newborn with intrauterine infection, reducing the occurrence of complications, and improving the long-term prognosis. Although one study concluded that there was no evidence of genotoxic effects when PDF was administered directly to pregnant rats and in rat neonates exposed transplacentally [52], whether PFD treatment has adverse effects on pregnant mice, intrauterine environment, and fetal mice needs further independent experiments, and the safe range of dosage and administration time should be studied in the future.
5. Conclusions
To conclude, the current study demonstrated a beneficial effect of PFD in alleviating pulmonary injury in newborns caused by intrauterine infection in pregnant rats. The mechanism was that PFD inhibited the LPS-activated TGF-β1/Smad signaling pathway, reversed LPS-upregulated TGF-β1, p-Smad2, and p-Smad3 expressions, rebalanced the ratio of MMP-9/TIMP-1 expressions, and prevented the formation of Collagen IV. These findings provide theoretical basis and insights for PFD-mediated intervention of lung injury in neonates with intrauterine infection.
Ethics Statement
All the animal experimental procedures gained approval of the Animal Care and Use Committee of Qingdao University (Qingdao, China). The experimental design and execution conformed to the Guidelines for the Care and Use of Laboratory Animals issued by the Chinese Council on Animal Research.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization, W.D. and R.S.; methodology, investigation, software, data curation, writing – original draft preparation, and visualization, W.D.; validation, R.S.; formal analysis, W.D.and R.L.; resources, R.L.and C.W.; writing – review and editing, W.D. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Weifang Health Commission Scientific Research Project (WFWSJK-2021-327).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




