Polypharmacy and Potential Drug–Drug Interactions in Patients With Atrial Fibrillation
Abstract
What is Known and Objective: The use of multiple medications, or polypharmacy, is associated with an elevated risk of potential drug–drug interactions (pDDIs), consequently heightening the probability of adverse drug reactions. Currently, there is a dearth of data regarding pDDIs in Chinese patients with atrial fibrillation (AF) within real-world scenarios. This study is designed to investigate the existing state of polypharmacy and pDDIs in Chinese AF patients and to analyze the factors influencing pDDIs.
Methods: This was a single-center retrospective investigation conducted at a tertiary hospital in China. Polypharmacy and pDDIs were examined based on the medications prescribed at the time of discharge. The pDDIs were assessed using the Lexi-Interact database and classified into Types A, B, C, D, and X.
Results and Discussion: The study encompassed 802 AF patients. The median age was 73 years (ranging from 64 to 80). The most (72.7%) were 65 years or older, and 53.9% were male. The incidence rates of polypharmacy and excessive polypharmacy were 74.8% and 29.8%, respectively. At discharge, 69.0% of patients had at least one clinically relevant pDDI. There were 1820 (84.2%), 261 (12.1%), and 81 (3.7%) interactions categorized as Types C, D, and X, respectively. The most prevalent Type C interaction was the combined use of antihypertensive medications. Among Type D interactions, the most common was the combination of anticoagulants and antiplatelet drugs. The most frequent Type X interaction involved drugs that augmented the hyperkalemic effect. Through multivariate analysis, advanced age (p = 0.008) and a greater number of medications (p < 0.001) were significant predictors of pDDIs.
What is New and Conclusions: Polypharmacy and pDDIs are widespread among AF patients. Advanced age and an increased number of drugs were determined to be predictive factors for pDDIs. The risk of DDIs can be reduced by decreasing the number of medications or opting for alternative drugs.
1. What is Known and Objective
Atrial fibrillation (AF) represents the most prevalent form of sustained cardiac arrhythmia in clinical practice, with a projected lifetime risk ranging from 22% to 26% [1, 2]. Globally, both the incidence and prevalence of AF are currently on the rise. AF is associated with an increased risk of myocardial infarction, heart failure, stroke, cognitive impairment or dementia, hospital admissions, and mortality [3–5]. The incidence of AF is positively correlated with age, and the accelerated aging process imposes a significant burden on both society and the healthcare system [6, 7]. The management of AF encompasses strategies for stroke and thrombosis prevention, symptom control through rate and rhythm management, and optimization of treatment for cardiovascular conditions and comorbidities [8]. Patients diagnosed with AF typically present with complex clinical profiles, involving multiple concurrent conditions and extensive medication regimens [9].
Multimorbidity, defined as the presence of two or more chronic diseases, is frequently observed in the elderly population and often necessitates polypharmacy [10]. Although a universal definition of polypharmacy is lacking, it is generally regarded as the concurrent use of at least five medications, with the use of 10 or more medications being considered excessive polypharmacy [11, 12]. Multimorbidity and polypharmacy are widespread globally and have garnered the attention of numerous researchers. For example, polypharmacy was reported in 41.2% of elderly adults in Switzerland [13], while in the United States, its prevalence increased from 24% to 39% among older adults between 1999 and 2012 [14]. In addition, the incidence of polypharmacy and excessive polypharmacy was found to be 49.7% and 24.3%, respectively, among nursing home residents [15], and 60.13% and 14.49% among community-dwelling elderly patients in China [16].
Polypharmacy augments the risk of potential drug–drug interactions (pDDIs), thereby heightening the likelihood of adverse drug reactions [17]. It is a common risk factor for emergency hospitalization in elderly patients due to adverse events and is also associated with an elevated mortality risk [18, 19]. In fact, epidemiological evidence suggests that 5%–20% of adverse drug events leading to hospitalization are attributable to drug–drug interactions (DDIs) [20]. DDIs occur when the effect of one drug on metabolism impacts the pharmacokinetics of another drug, resulting in reduced efficacy, an increased risk of adverse reactions, higher healthcare costs, and increased morbidity and mortality rates [21].
Currently, research on polypharmacy in patients with AF is limited [22–24]. Some foreign studies have examined pDDIs in AF patients undergoing anticoagulation therapy [25, 26]. However, there is a paucity of data on pDDIs in Chinese AF patients in real-world settings. Our study aims to investigate the current status of polypharmacy and pDDIs in Chinese AF patients and to analyze the factors influencing pDDIs. We anticipate that our data will contribute to the development of strategies for minimizing pDDIs. Furthermore, future research can expand on the findings of this study to further enhance the outcomes for AF patients.
2. Methods
2.1. Study Design
This cross-sectional retrospective investigation was carried out within the timeframe from January 1st to December 31st, 2018, at Beijing Tongren Hospital, a tertiary care teaching facility in China, equipped with 1700 beds. The study received approval from the Ethics Committee of Beijing Tongren Hospital (Approval no.: TRECKY2019-124). Given its retrospective nature, the necessity for obtaining written informed consent from patients was waived.
The study included patients with a discharge diagnosis of AF, excluding those who died during hospitalization and those with no medication prescribed at discharge. For patients with multiple admissions, only the first admission was considered. Finally, a total of 802 participants were included in the analysis (Figure 1).
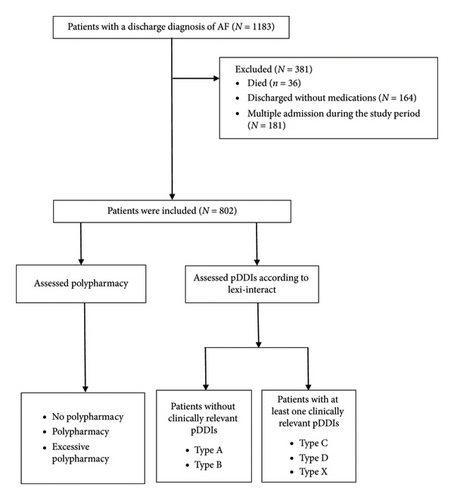
2.2. Data Source
Clinical information was retrieved from the electronic hospital information system for subsequent analysis. This encompassed demographic particulars such as age, gender, total length of hospital stay, department of admission, and diagnosis, in addition to laboratory test results and discharge medications. The age-adjusted Charlson comorbidity index (aCCI) was utilized to measure the level of comorbidity among the patients [27].
2.3. Defining Polypharmacy
The degree of polypharmacy was appraised based on the quantity of medications prescribed at the time of discharge and was classified as follows: absence of polypharmacy (< 5 drugs), polypharmacy (5–9 drugs), and excessive polypharmacy (≥ 10 drugs) [12].
2.4. Defining pDDIs
The Lexi-Interact database was utilized to dissect pDDIs, which were grouped into the following categories: Type A (no known interaction), Type B (no action needed), Type C (monitor therapy), Type D (consider therapy modification), and Type X (avoid combination) [28]. Types C, D, and X were deemed clinically relevant pDDIs.
2.5. Statistical Analyses
Continuous variables following a normal distribution were presented as means ± standard deviations, while those with skewed distributions were displayed as medians ± interquartile ranges (IQRs). Categorical variables were represented as percentages (frequencies). Categorical variables across different groups were compared using the χ2 test or Fisher’s exact test. Significant variables (p < 0.05) identified on univariate logistic regression analysis were subjected to further analysis via multivariate logistic regression. Statistical analyses were performed using SPSS Version 22.0, with p < 0.05 considered statistically significant.
3. Results
3.1. Characteristics of Patients With AF
A total of 802 patients diagnosed with AF were incorporated into the study. The median age was 73 years (ranging from 64 to 80), and 583 (72.7%) of the participants were 65 years or older. In addition, 53.9% of the overall study cohort were males. aCCI was 5 (ranging from 3 to 7). The most prevalent comorbidities observed were hypertension (69.0%), hyperlipidemia (56.5%), and coronary heart disease (42.0%) (Table 1).
| Characteristic | Total (n = 802) | Clinically relevant pDDIs (n = 553) | Nonclinically relevant pDDIs (n = 249) | p value | OR (95% CI) |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 73 (64.80) | 74 (64.81) | 71 (61.80) | 0.026 | |
| Gender | |||||
| Male, n (%) | 432 (53.9) | 292 (52.8) | 140 (56.2) | 0.368 | 0.871 (0.645.1.177) |
| Length of stay at hospital, days, median (IQR) | 10 (7.15) | 11 (8.16) | 9 (6.13) | < 0.001 | |
| aCCI, median (IQR) | 5 (3.7) | 5 (4.7) | 4 (2.5) | < 0.001 | |
| Hypertension, n (%) | 553 (69.0) | 415 (75.0) | 138 (55.4) | < 0.001 | 2.419 (1.764, 3.316) |
| Hyperlipidemia, n (%) | 453 (56.5) | 348 (62.9) | 105 (42.2) | < 0.001 | 2.328 (1.716, 3.158) |
| Diabetes, n (%) | 278 (34.7) | 235 (42.5) | 43 (17.3) | < 0.001 | 3.540 (2.447, 5.122) |
| Stroke/TIA, n (%) | 232 (28.9) | 182 (32.9) | 50 (20.1) | < 0.001 | 1.952 (1.366, 2.791) |
| Coronary heart disease, n (%) | 337 (42.0) | 275 (49.7) | 62 (24.9) | < 0.001 | 2.984 (2.140, 4.159) |
| NYHA/Killip Class II–IV, n (%) | 284 (35.4) | 245 (44.3) | 39 (15.7) | < 0.001 | 4.283 (2.927, 6.268) |
| Infectious diseases, n (%) | 170 (21.2) | 143 (25.9) | 27 (10.8) | < 0.001 | 2.868 (1.842, 4.464) |
| Renal insufficiency, n (%) | 126 (15.7) | 112 (20.3) | 14 (5.6) | < 0.001 | 4.263 (2.392, 7.597) |
| Liver insufficiency, n (%) | 63 (7.9) | 43 (7.8) | 20 (8.0) | 0.901 | 0.965 (0.555.1.678) |
| Number of medications at discharge, median (IQR) | 7 (4.10) | 9 (6.12) | 4 (3.5) | < 0.001 |
- Note: aCCI = age-adjusted Charlson comorbidity index; TIA = transient cerebral ischemia.
- Abbreviations: IQR = interquartile range; pDDIs = potential drug–drug interactions.
3.2. Polypharmacy and pDDIs
A total of 6128 medications were prescribed at discharge. The median number of medications prescribed was 7 (ranging from 4 to 10), with a range from 1 to 25. Specifically, 25.2%, 45.0%, and 29.8% of the patients were prescribed 1–4, 5–9 (classified as polypharmacy), and ≥ 10 medications (excessive polypharmacy), respectively. The drugs most frequently associated with Type X interactions were potassium chloride, spironolactone, and amiodarone. Meanwhile, for Type D interactions, the top three drugs were aspirin, dabigatran, and insulin (Table 2).
| Category X | N | Category D | N |
|---|---|---|---|
| Potassium chloride | 64 | Aspirin | 49 |
| Spironolactone | 56 | Dabigatran | 49 |
| Amiodarone | 11 | Insulin | 46 |
| Flupentixol–melitracen | 9 | Clopidogrel | 43 |
| Tiotropium | 5 | Acarbose | 42 |
| Moxifloxacin | 3 | Warfarin | 40 |
| Esomeprazole | 2 | Rivaroxaban | 33 |
| Clopidogrel | 2 | Calcium carbonate | 28 |
| Levofloxacin | 2 | Amiodarone | 25 |
| Nicergoline | 1 | Sodium bicarbonate | 23 |
| Tamsulosin | 1 | Iron preparations | 20 |
| Atorvastatin | 1 | Allopurinol | 13 |
| Ciclosporin | 1 | Ginkgo biloba | 9 |
| Rifampicin | 1 | Simvastatin | 9 |
| Omeprazole | 1 | Cefuroxime | 9 |
| Nabumetone | 1 | Heparin | 7 |
| Diclofenac | 1 | Methylprednisolone | 7 |
| Amlodipine | 6 | ||
| Digoxin | 6 | ||
| Fluconazole | 6 | ||
| Prednisone | 5 | ||
| Sitagliptin | 5 | ||
| PPI | 5 | ||
| Ibuprofen | 4 | ||
| Levothyroxine | 4 | ||
| Nabumetone | 3 | ||
| Celecoxib | 3 | ||
| Repaglinide | 3 | ||
| Torsemide | 3 | ||
| Moxifloxacin | 2 | ||
| Famotidine | 2 | ||
| Glimepiride | 2 | ||
| Zolpidem | 1 | ||
| Oxycodone acetaminophen | 1 | ||
| Diclofenac sodium | 1 | ||
| Escitalopram oxalate | 1 | ||
| Dexamethasone | 1 | ||
| Fosinopril | 1 | ||
| Lanthanum carbonate | 1 | ||
| Levofloxacin | 1 | ||
| Vildagliptin | 1 | ||
| Glipizide | 1 | ||
| Pioglitazone | 1 |
- Abbreviations: pDDIs = potential drug–drug interactions; PPI = proton pump inhibitor.
A total of 2162 clinically relevant pDDIs were detected in 553 (69.0%) patients, with a range of 1–26 interactions per patient. There were 1820 (84.2%), 261 (12.1%), and 81 (3.7%) Type C, D, and X interactions, respectively. Most pDDIs (80.6%) were considered to have moderate severity. The most frequent reliability rating was fair, representing 68.8% of pDDIs (Table 3).
| Type C (n = 1820) | Type D (n = 261) | Type X (n = 81) | Total (n = 2162) | |
|---|---|---|---|---|
| Severity | ||||
| Major, n (%) | 163 (9.0) | 106 (40.6) | 70 (86.4) | 339 (15.7) |
| Moderate, n (%) | 1592 (87.5) | 139 (53.3) | 11 (13.6) | 1742 (80.6) |
| Minor, n (%) | 65 (3.6) | 16 (6.1) | 0 (0.0) | 81 (3.7) |
| Reliability rating | ||||
| Excellent, n (%) | 50 (2.7) | 19 (7.3) | 0 (0.0) | 69 (3.2) |
| Good, n (%) | 462 (25.4) | 67 (25.7) | 58 (71.6) | 587 (27.2) |
| Fair, n (%) | 1290 (70.9) | 175 (67.0) | 23 (28.4) | 1488 (68.8) |
| Poor, n (%) | 18 (1.0) | 0 (0.0) | 0 (0.0) | 18 (0.8) |
- Abbreviation: pDDIs = potential drug–drug interactions.
Among the Type X interactions, the most prevalent were those involving hyperkalemia (2.6%), QTc interval prolongation (0.5%), and anticholinergic properties that enhanced the ulcerogenic effect of oral solid potassium (0.4%). Of these interactions, 69 (85.2%) were pharmacodynamic, while 12 (14.8%) were pharmacokinetic. In addition, 4.9% and 1.2% of the patients were observed to have cytochrome P (CYP) 450 and organic anion transporting polypeptide (OATP) interactions, respectively. For Type D interactions, the most frequent was the combination of anticoagulants with antiplatelet drugs that increased the risk of bleeding (3.6%), followed by the combined use of antidiabetic drugs that increased the risk of hypoglycemia (2.1%), and then by drugs that increased the risk of bleeding with warfarin (1.6%). Here, 138 (52.9%) were pharmacodynamic interactions, and 123 (47.1%) were pharmacokinetic. A prevalence of 19.5% and 2.3% of patients with CYP and P-glycoprotein (P-gp) interactions was noted. Among Type C interactions, the most common was the concomitant use of antihypertensive drugs causing hypotension (9.3%), followed by those that reduced the therapeutic efficacy of antidiabetic agents (6.6%), and then by those that enhanced hypoglycemia (4.7%) (Tables 4, 5, and 6).
| Interacting drugs | N (%) | Nature of interaction | Potential consequence | Management strategies |
|---|---|---|---|---|
| Spironolactone + potassium chloride | 56 (2.6) | Additive hyperkalemic effect | Hyperkalemic | Monitor potassium concentration |
| Amiodarone + flupentixol/moxifloxacin/levofloxacin | 11 (0.5) | QT-prolonging agent combination | QTc prolongation and arrhythmias | Avoid concomitant use |
| Potassium chloride + flupentixol–melitracen/tiotropium | 8 (0.4) | Decreased gastric motility due to anticholinergics and increased contact time | Ulcerogenic | Replacement with liquid or effervescent potassium preparations |
| Clopidogrel + omeprazole/esomeprazole | 2 (0.09) | CYP2C19 substrate + CYP2C19 inhibitor | Reduced clopidogrel’s effect | Replacement with rabeprazole or pantoprazole |
| Nicergoline + tamsulosin | 1 (0.05) | Additive pharmacologic effects | Hypotension | Avoid concomitant use |
| Atorvastatin + cyclosporine | 1 (0.05) | CYP3A and OATP1B1 substrate + CYP3A and OATP1B1 inhibitor | Atorvastatin-related toxicity | Change to pravastatin or fluvastatin or an alternative type of LDL-lowering medication. |
| Esomeprazole + rifampicin | 1 (0.05) | CYP2C19 substrate + CYP2C19 inducer | Reduced esomeprazole’s effect | Avoid concomitant use |
| Nabumetone + diclofenac | 1 (0.05) | Concurrent use of NSAIDs | NSAIDs-related toxicity | Avoid concomitant use |
- Note: CYP = cytochrome P450.
- Abbreviations: NSAIDs = nonsteroidal anti-inflammatory drugs; OATP = organic anion transporting polypeptide; pDDIs = potential drug–drug interactions.
| Interacting drugs | N (%) | Nature of interaction | Potential consequence | Management strategies |
|---|---|---|---|---|
| Antiplatelets + anticoagulants | 78 (3.6) | Antithrombotic agent combination | Bleeding | Monitor signs of bleeding |
| Insulin + acarbose/sitagliptin | 46 (2.1) | Hypoglycemic agent combination | Hypoglycemic | Monitor glucose; drugs’ dosage reduction |
| Warfarin + amiodarone/allopurinol/fluconazole/ibuprofen/diclofenac/ginkgo biloba | 35 (1.6) | CYP 2C9 and CYP3A4 substrate + CYP 2C9 and/or CYP3A4 inhibitor; inhibit the hepatic metabolism of warfarin; affect platelet aggregation | Bleeding | Monitor INR; warfarin dosage reduction |
| Iron preparations + calcium carbonate/sodium bicarbonate | 16 (0.7) | Antacid-mediated increase in gastrointestinal pH | Decreased absorption of iron salts | Separate oral administration; monitor the therapeutic effects of iron preparations |
| Dabigatran + calcium carbonate/sodium bicarbonate | 15 (0.7) | Decrease the absorption of dabigatran | Reduced dabigatran’s effect | Administer dabigatran 2 h prior to the antacid; monitor clinical response to dabigatran |
| Calcium carbonate + prednisone/methylprednisolone | 13 (0.6) | Decrease the bioavailability of corticosteroids | Reduced corticosteroids’ effect | Separate at least for 2 h; monitor the therapeutic effects of corticosteroid |
| Cefuroxime + PPIs/famotidine/calcium carbonate/sodium bicarbonate | 9 (0.4) | Decreased absorption of cefuroxime (due to increased gastric pH) | Reduced cefuroxime’s effect | Ensure oral cefuroxime axetil is taken with food to minimize the interaction |
| Simvastatin + amlodipine/amiodarone | 9 (0.4) | CYP3A4 substrate + CYP3A4 inhibitor | Simvastatin-related toxicity | Limit simvastatin dose to 20 mg daily; monitor toxic effects (e.g., myopathy and liver function test) |
| Aspirin + ginkgo biloba | 6 (0.3) | Additive antiplatelet effect | Bleeding | Monitor signs of bleeding |
| Digoxin + amiodarone | 6 (0.3) | P-gp substrate + P-gp inhibitor | Digoxin-related toxicity | Digoxin dosage reduction; monitor serum concentrations and toxic effects (e.g., gastrointestinal symptoms and cardiac arrhythmias) |
| Aspirin + ibuprofen/nabumetone/celecoxib | 3 (0.1) | Concurrent use of a COX inhibitor | Bleeding | Monitor signs of bleeding |
| Clopidogrel + fluconazole | 3 (0.1) | CYP2C19 substrate + CYP2C19 inhibitor | Reduced clopidogrel’s effect | Monitor platelet reactivity index |
| Clopidogrel + repaglinide | 3 (0.1) | CYP2C8 inhibitor + CYP2C8 substrate | Hypoglycemia | Limit repaglinide dose to 4 mg daily; monitor glucose |
| Loop diuretics + ibuprofen/celecoxib | 3 (0.1) | Loop diuretics increase the concentrations of prostaglandins; NSAIDs would block this activity | Nephrotoxic | Monitor renal function |
| Iron preparations + quinolones (e.g., levofloxacin/moxifloxacin) | 3 (0.1) | Decreased absorption (due to the formation of an insoluble complex) | Reduced quinolones’ effect | Separate for at least 4 h |
| Levothyroxine + calcium carbonate | 3 (0.1) | Adsorption of levothyroxine to calcium in the GI tract | Reduced levothyroxine’s effect | Separate for at least 4 h |
| Sulfonylureas/DPP-IV inhibitors | 2 (0.1) | Hypoglycemic agent combination | Hypoglycemia | Monitor glucose; drugs’ dosage reduction |
| Amiodarone + fluconazole | 1 (0.05) | CYP3A4 substrate + CYP3A4 inhibitor | QTc prolongation and arrhythmias | Monitor ECG |
| Dabigatran + nabumetone | 1 (0.05) | Anticoagulant and antiplatelet agent combination | Bleeding | Monitor signs of bleeding |
| Fosinopril + calcium carbonate | 1 (0.05) | Direct inhibition of fosinopril absorption | Reduced fosinopril’s effect | Separate for at least 2 h |
| Levothyroxine + iron preparations | 1 (0.05) | Decreased levothyroxine absorption (due to formation of a ferric-thyroxine complex) | Reduced levothyroxine’s effect | Separate for at least 4 h |
| Lanthanum + sodium bicarbonate | 1 (0.05) | Antacid components may bind with lanthanum | Reduced lanthanum’s effect | Separate for at least 2 h |
| Nabumetone + escitalopram | 1 (0.05) | Reduction of gastroprotective prostaglandins and antiplatelet effects of both NSAIDs and SSRIs; NSAIDs may reduce SSRIs exposure | Bleeding; reduced escitalopram’s effect | Monitor signs of bleeding; therapeutic effect of escitalopram |
| Sulfonylureas + thiazolidinediones | 1 (0.05) | Hypoglycemic agent combination | Hypoglycemia | Monitor glucose |
| Zolpidem + oxycodone acetaminophen | 1 (0.05) | Additive or synergistic CNS depressant effect | CNS depression | Monitor CNS depression (e.g., respiratory depression, hypotension, and sedation); drugs’ dosage reduction |
- Note: CYP = cytochrome P450.
- Abbreviations: CNS = central nervous system; COX = cyclooxygenase; DPP = dipeptidyl peptidase; GI = gastrointestinal; pDDIs = potential drug–drug interactions; P-gp = P-glycoprotein; PPIs = proton pump inhibitors; SSRIs = selective serotonin reuptake inhibitors.
| Interacting drugs | N (%) | Nature of interaction | Potential consequence | Management strategies |
|---|---|---|---|---|
| Blood pressure lowering agent combination (e.g., CCB/ACEI/ARB/ARNI/β-blocker/α-blocker/diuretic/terazosin/amiodarone) | 200 (9.3) | Antihypertensive agent combination | Hypotension | Monitor blood pressure |
| Antidiabetic agent + hyperglycemic agent (e.g., metformin/acarbose/insulin/sitagliptin/glipizide/vildagliptin and methylprednisolone/prednisone/torsemide/furosemide/indapamide/olanzapine/tacrolimus) | 143 (6.6) | Hyperglycemia-associated agent + antidiabetic agent | Reduced antidiabetic effect | Monitor blood glucose |
| Antidiabetic agent combination (e.g., metformin/glimepiride/glipizide/gliclazide/acarbose/repaglinide) | 102 (4.7) | Hypoglycemic agent combination | Hypoglycemia | Monitor blood glucose |
| Bradycardia-causing agent combination (e.g., metoprolol/bisoprolol/digoxin/propafenone/sotalol/diltiazem/amiodarone) | 96 (4.4) | Bradycardia-causing agent combination | Bradycardic | Monitor ECG |
| Spironolactone + ACEI/ARB | 83 (3.8) | Additive hyperkalemic effect | Hyperkalemia | Monitor potassium concentration |
| β-blocker + antidiabetic agent (e.g., metoprolol/bisoprolol/sotalol and glimepiride/glipizide/gliclazide/insulin) | 79 (3.7) | Beta-blockers mask symptoms of hypoglycemia (due to suppressing adrenergic response to hypoglycemia) | Hypoglycemia | Monitor blood glucose |
| Antiplatelet agent combination (e.g., aspirin/clopidogrel/sarpogrelate) | 69 (3.2) | Antiplatelet agent combination | Bleeding | Monitor signs of bleeding |
| Potassium salts + ACEI/ARB | 68 (3.1) | Additive hyperkalemic effect | Hyperkalemia | Monitor potassium concentration |
| Clopidogrel + pantoprazole | 63 (2.9) | CYP2C19 substrate + CYP2C19 inhibitor | Reduced clopidogrel’s effect | Monitor platelet reactivity index |
| Clopidogrel + CCB (e.g., amlodipine/lacidipine/nifedipine/diltiazem) | 50 (2.3) | Inhibition of CYP3A4 (responsible for the activation of clopidogrel) | Reduced clopidogrel’s effect | Monitor platelet reactivity index |
| Dabigatran + P-glycoprotein inhibitors (e.g., amiodarone/ciclosporin) | 49 (2.3) | P-gp substrate + P-gp inhibitor | Bleeding | Monitor signs of bleeding |
| Digoxin + loop diuretics (e.g., torsemide/furosemide) | 45 (2.1) | Digoxin and potassium compete for binding to the cardiac sodium–potassium ATP pump | Digoxin-related toxicity | Monitor serum concentrations and toxic effects (e.g., gastrointestinal symptoms and cardiac arrhythmias) |
| Atorvastatin + amiodarone | 42 (1.9) | CYP3A4 substrate + CYP3A4 inhibitor | Atorvastatin-related toxicity | Monitor toxic effects (e.g., myopathy and liver function test) |
| Digoxin + spironolactone | 38 (1.8) | Decreased digoxin clearance | Digoxin-related toxicity | Monitor serum concentrations and toxic effects (e.g., gastrointestinal symptoms and cardiac arrhythmias) |
| Warfarin + statins (e.g., pravastatin/fluvastatin/rosuvastatin/simvastatin) | 38 (1.8) | CYP2C9 substrate + CYP2C9 inhibitor | Bleeding | Monitor signs of bleeding |
| Aspirin + ACEI | 36 (1.7) | Salicylates attenuate the beneficial systemic vasodilatory effects of ACE inhibitors | Nephrotoxic | Monitor renal function |
| Aspirin + spironolactone | 36 (1.7) | Aspirin-mediated inhibition of the tubular secretion of canrenone (active metabolite of spironolactone) | Reduced spironolactone’s effect | Monitor spironolactone efficacy |
| Warfarin + torsemide | 34 (1.6) | Both torsemide and warfarin are substrates for CYP2C9 (competition for the metabolic pathway) | Bleeding | Monitor signs of bleeding |
| Rivaroxaban + amiodarone | 33 (1.5) | P-gp substrate + P-gp inhibitor | Bleeding | Monitor signs of bleeding |
| Rosuvastatin + clopidogrel | 30 (1.4) | BCRP substrate of + BCRP inhibitors | Rosuvastatin-related toxicity | Monitor toxic effects (e.g., myopathy and liver function test) |
- Note: CYP = cytochrome P450.
- Abbreviations: ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; ARNI = angiotensin-receptor/neprilysin inhibitor; BCRP = breast cancer resistance protein; CCB = calcium channel blocker; CNS = central nervous system; NSAIDs = nonsteroidal anti-inflammatory drugs; pDDIs = potential drug–drug interactions; P-gp = P-glycoprotein.
3.3. Influencing Factors of pDDIs
On univariate analysis, advanced age (p = 0.026), longer hospitalization days, higher aCCI, hypertension, hyperlipidemia, diabetes, stroke/transient ischemic attack, coronary heart disease, NYHA/Killip Class II-IV, infectious diseases, renal insufficiency, and an increased number of medications (p < 0.001 for all) were all associated with an increased risk of pDDIs. On multivariate analysis, advanced age (p = 0.008) and an increased number of medications (p < 0.001) were identified as strong predictors of pDDIs (Table 1).
4. Discussions
Polypharmacy is common in clinical practice, particularly among patients with cardiovascular disease. In the United States, polypharmacy was observed in 95% of elderly patients with cardiovascular disease, with 69% experiencing hyperpolypharmacy and 77.5% encountering at least 1 severe pDDI [29].
AF represents the most prevalent clinical arrhythmia disorder; however, research on drug interactions among Chinese AF patients is scarce. This study aimed to examine polypharmacy and drug interactions in individuals diagnosed with AF. The key findings of this study are as follows. First, the prevalence of polypharmacy and excessive polypharmacy was 74.8% and 29.8%, respectively, and 69.0% of patients with AF had at least 1 clinically relevant pDDI at discharge. Second, 1820 (84.2%), 261 (12.1%), and 81 (3.7%) patients had Type C, D, and X interactions, respectively. Among Type C interactions, the most common was a combination of antihypertensive drugs that increased the risk of hypotension. Among Type D interactions, the most frequent was the combination of anticoagulants with antiplatelet drugs, increasing the risk of bleeding. Among Type X interactions, the most common were drugs that increased hyperkalemia. Third, advanced age and an increased number of medications were identified as significant predictors of pDDIs.
The incidence of polypharmacy was relatively high. A prospective study of AF outpatients initiating vitamin K antagonist therapy reported that 32.9% of patients had polypharmacy [30]. Another investigation on 338,810 elderly patients with AF (≥ 75 years) using the MarketScan Medicare Supplemental database from 2007 to 2015 revealed a prevalence of 52% for polypharmacy [23]. A post hoc analysis conducted in 2015 of results from ARISTOTLE showed that polypharmacy was observed in 76.5% of patients with AF [31]. In a China AF Registry study, 49.4% were found to have polypharmacy [32]. The differences in findings between these studies could be attributed to variations in study populations, comorbidities, and prescription patterns.
Research on the drug interactions of warfarin has demonstrated that 58% of individuals were taking drugs that could potentially interact with warfarin, leading to a significant increase in the risk of severe bleeding [33]. In a study involving patients with AF who were newly prescribed NOACs, 26.5% experienced drug interactions related to NOACs. These interactions could potentially reduce the effectiveness of antithrombotic therapy or increase bleeding risk [34]. A cross-sectional study assessing the prevalence of DDI with NOACs among elderly individuals revealed a 16.9% prevalence of pharmacokinetic DDIs at admission, which increased to 20.7% at discharge [35]. In this study, the proportion of pDDIs was 69.0%; this was higher compared to previous studies, likely because previous studies exclusively focused on patients taking anticoagulant medications. This study investigated the overall pDDIs in patients with AF; this scope is seldom explored, yet it holds great significance in minimizing DDI.
Type C interactions accounted for 84.2% of pDDIs in this study, the most common of which was the combined use of antihypertensive drugs, which increased the risk of hypotension. In clinical practice, increasing the dose of monotherapy does not typically cause a significant additional lowering of blood pressure but may actually lead to an increased drug toxicity. Monotherapy may not be sufficient for most patients, and combination therapy may be required to achieve the recommended blood pressure targets [36]. These findings may serve as a reminder for healthcare professionals to implement close monitoring for drugs with Type C pDDIs.
The most common Type D pDDI was the combination of anticoagulants and antiplatelet drugs, which increased the risk of bleeding. This was followed by the combined use of antidiabetic drugs, which increased the risk of hypoglycemia, and then by drugs that increased the risk of bleeding with warfarin. Antiplatelet therapy is necessary for patients with coronary heart disease to reduce the risk of myocardial ischemic events, whereas anticoagulation therapy is crucial for patients with AF who are at high risk for thromboembolism. It is recommended to formulate specific antithrombotic therapies based on different clinical situations [37]. In this study, the prevalence of AF with coronary heart disease was 42.0%, and thus, the concomitant use of antiplatelet and anticoagulant drugs is considered appropriate. However, plasma warfarin concentrations are increased by amiodarone, a CYP2C9 inhibitor, and fluconazole, an inhibitor of both CYP2C9 and CYP3A. When these drugs are used together, caution should be exercised to reduce the dosage of warfarin and closely monitor INR levels [38].
Among Type X interactions, the most prevalent involved drugs that increased hyperkalemia, followed by those with QTc interval prolongation, and then by those with anticholinergic properties that increased the ulcerogenic effect of oral solid potassium. Since potassium salts may enhance the hyperkalemic effect of spironolactone, the combination of these two drugs should be avoided unless there is evidence of hypokalemia. Moreover, the concurrent use of flupentixol–melitracen, a commonly prescribed antidepressant, and quinolone antibiotics with amiodarone may increase the risk of QT prolongation, potentially leading to life-threatening arrhythmias. It is advisable to avoid using these medications together if possible. Lastly, patients taking medications with significant anticholinergic properties are advised to avoid solid oral potassium chloride formulations. Instead, liquid or effervescent forms of potassium can be used as an alternative.
DDIs encompass both pharmacodynamic and pharmacokinetic interactions. Pharmacodynamic interactions occur when two or more drugs exhibit either additive or synergistic pharmacological effects or demonstrate antagonistic activities. It appears that pharmacodynamic interactions are more prevalent than pharmacokinetic interactions [39]. We obtained similar results in this study. In this research, common pharmacodynamic interactions include the concurrent use of antithrombotic drugs or antihypertensive drugs. Pharmacokinetic drug interactions arise when one drug affects the absorption, distribution, metabolism, or excretion of another [40]. Many examples are presented in this study, such as the reduced absorption of oral quinolones due to the formation of an insoluble complex with iron and the decreased absorption of cefuroxime caused by the elevated gastric pH induced by proton pump inhibitors (PPIs). The most frequent pharmacokinetic DDIs involve the CYP monooxygenation system or the P-gp or multidrug resistance protein transport system [38]. In this study, the drug treatment for AF patients mainly involves CYP3A4, CYP2C19, CYP2C9, CYP2C8, P-gp, OATP, and breast cancer resistance protein (BCRP). DDIs may occur when CYP inducers or inhibitors are coadministered with drugs metabolized by CYP enzymes. Clinicians and pharmacists can predict treatment responses and modify treatment plans before drug administration, thus helping patients avoid adverse events.
In order to effectively reduce the incidence of DDIs, it is essential to investigate the various risk factors influencing their occurrence. Our results indicated that older age and an increased number of medications were significant predictors of DDI. The prevalence of diseases tends to increase with age, resulting in a higher prescription of medications, subsequently elevating the risk of DDIs [41]. To mitigate this risk, it is crucial to minimize the number of prescribed medications while maintaining effective treatment. Furthermore, replacing medications that are prone to interactions with those that have fewer or no known interactions can assist in preventing adverse reactions.
The strength of this study lies in the fact that its analysis of consecutive patients hospitalized with AF reflects the real-world treatment of AF patients. This study explored the incidence and risk factors of pDDIs in AF patients, as well as analyzed the drugs related to different categories of pDDIs, thus serving as a reminder for physicians and pharmacists alike. This study provides valuable insights into optimizing drug treatment and reducing adverse drug events for patients with AF. However, the limitations of this study should also be acknowledged. First, this was a retrospective study in a single institution. Second, this study did not assess the clinical significance of the detected pDDIs in accordance with disease treatment guidelines. Third, a previous study evaluated five drug interaction screening programs (Lexi-Interact, Micromedex, iFacts, Epocrates, and Medscape) and found that Lexi-Interact performs better than others [42]. However, none of the drug interaction screening programs was considered ideal. Therefore, the involvement of an expert specialist is necessary to make a final clinical judgment.
5. What is New and Conclusions
A high incidence of polypharmacy and pDDIs was discovered among the real-world population of Chinese patients with AF. Most drug interactions were classified as Type C, thus highlighting the importance of close monitoring to prevent potential consequences. For Type D and X interactions, adjusting the treatment in accordance with guidelines can be particularly challenging in clinical practice. Advanced age and an increased number of drugs were identified as predictive factors for pDDIs. Thus, it is advisable to reduce unnecessary medication use and consider alternatives with fewer interactions in order to minimize drug-related adverse events.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Ying Bai contributed to the design, data collection and analysis, and manuscript writing and revision. Jianqi Wang was responsible for collecting data and analysis. Guangyao Li was responsible for data analysis. Zhen Zhou was responsible for statistical analysis. All authors provided comments and assisted with revisions to the manuscripts.
Funding
No funding was received for this manuscript.
Acknowledgments
We sincerely thank and extend our gratitude to all the authors who participated in this study.
Open Research
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.




