Study on the Efficacy and Safety of Off-Label Dosing of Rivaroxaban in Chinese Nonvalvular Atrial Fibrillation Patients
Abstract
Objective: This prospective cohort study aimed to systematically evaluate the clinical outcomes associated with off-label rivaroxaban regimens (10 mg vs. 15 mg daily) in elderly Chinese patients with nonvalvular atrial fibrillation (NVAF).
Methods: A hospital-based observational cohort was established at Pudong Hospital, Shanghai, in 2021, enrolling 247 consecutive NVAF patients receiving rivaroxaban therapy after obtaining institutional ethics approval and informed consent. Multivariable logistic regression analyses were performed to identify independent predictors of clinical outcomes.
Results: The cohort comprised 155 patients in 10 mg daily group and 92 patients in 15 mg daily group, with a mean age of 79 years. During 12-month follow-up, the 15 mg daily group demonstrated superior antithrombotic efficacy with significantly lower incidence of thrombotic events (15 mg: 1.50% vs. 10 mg: 5.90%, p = 0.017). Both regimens showed comparable safety profiles regarding bleeding events (15 mg: 10.90% vs. 10 mg: 13.90%, p = 0.302). Multivariate analysis identified CHA2DS2-VASc score as the primary predictor of thrombosis, while lower BMI, elevated HAS-BLED score, and diabetes mellitus emerged as independent bleeding predictors (all p < 0.05).
Conclusion: Rivaroxaban 15 mg daily showed better thromboembolic protection than 10 mg without increasing bleeding risk, supporting its use in selected high-risk elderly NVAF patients.
1. Background
Atrial fibrillation (AF), characterized by chaotic atrial electrical activity replacing normal sinus rhythm, represents the most prevalent sustained cardiac arrhythmia [1]. Its global burden continues to escalate, currently affecting approximately 33.5 million individuals (prevalence 2.9%), with projections suggesting 720 million Asian cases by 2050 [2, 3]. According to the latest cross-sectional epidemiological study in China in 2022, the adult prevalence of AF is 1.6%, marking a 146% increase compared to surveys in 2004 [4]. Notably, nonvalvular AF (NVAF) refers to AF without mechanical heart valve implantation or significant mitral stenosis, accounting for approximately 65.2% of all AF cases [5].
AF has been demonstrated to elevate the risk of stroke and systemic embolic events, leading to heightened long-term disability and mortality rates [6]. Contemporary AF management prioritizes anticoagulation therapy as the cornerstone of stroke prevention, with nonvitamin K antagonist oral anticoagulants (NOACs) demonstrating superior safety-efficacy profiles compared to warfarin, with 19% relative risk reduction in stroke incidence [7, 8]. Rivaroxaban, as a representative of NOACs, exerts therapeutic effects with concentration-dependent pharmacodynamics, through inhibiting free FXa, FXa bound to prothrombin, and FXa associated with thrombus [9].
NOACs have predictable pharmacokinetics, high efficacy, short half-life, rapid elimination of effects after discontinuation, minimal food-drug interactions, low risk of intracranial hemorrhage, and do not require frequent monitoring [10]. However, due to age-related decline in kidney function or age-related renal impairment, the most significant pharmacokinetic characteristics of rivaroxaban are age and kidney function. Dosage recommendations have been approved for all adult patients to receive either 15 mg for CrCl 30–49 mL/min or 20 mg for CrCl ≥ 50 mL/min once daily depending on renal function by the Food and Drug Administration (FDA) of the United States, as well as the National Medical Products Administration (NMPA) of China.
Although anticoagulant activity of rivaroxaban is not routinely indicated, extra attention and monitoring are necessary in geriatric patients and those with renal dysfunction or experiencing hemorrhagic/thrombotic complications. Some large-scale studies conducted abroad have explored the impact of off-label doses of rivaroxaban on thromboembolism and bleeding risks in NVAF patients, however, significant ethnic variability in pharmacokinetic responses (15%–20% higher AUC in Asian vs. Caucasian populations) limits their generalizability to Chinese NVAF patients [11, 12] An evidence gap persists regarding optimal off-label dosing regimens in China’s real-world clinical practice, particularly concerning population-specific pharmacodynamic profiles and long-term safety outcomes.
This prospective clinical investigation at Pudong Hospital in Shanghai, will systematically evaluate real-world rivaroxaban utilization in Chinese NVAF patients. Based on follow-ups, cases will be closely monitored for thromboembolism and bleeding. The study seeks to elucidate the risk-benefit profile of off-label anticoagulation strategies, ultimately generating evidence-based guidance for personalized dose optimization in this population.
2. Materials and Methods
2.1. Ethical Clearance
This real-world prospective research received approval from the Institutional Review Board of Pudong Hospital, affiliated with Fudan University (Approval No. QWJW-15/2021).
2.2. Study Design
This study selected patients with NVAF taking rivaroxaban in Shanghai, China as the subjects. A total of 247 patients were included to evaluate the efficacy and safety factors associated with nonguideline anticoagulation strategies.
2.2.1. Inclusion Criteria
Confirmed diagnosis of NVAF, meeting the diagnostic criteria for NVAF in the “2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation” [13]; current anticoagulation regimen: receiving rivaroxaban therapy for stroke prevention.
2.2.2. Exclusion Criteria
Severe renal impairment (defined as a creatinine clearance rate, CrCl, of less than 30 mL/min); cirrhosis or severe liver impairment (Child-Pugh B/C); history of malignancy; Severe bradycardia (such as sick sinus syndrome, high-degree atrioventricular block, etc.); coagulation abnormalities or platelet deficiencies leading to a risk of spontaneous bleeding; other conditions deemed ineligible by the investigators.
Rivaroxaban was obtained from Bayer AG, CSPC Pharmaceutical Group Limited, and Dongguan Yangzhikang Pharmaceutical Co., Ltd. Participants were stratified into two dosing cohorts: a high-dose group receiving 15 mg orally once daily and a low-dose group administered 10 mg daily, both regimens timed with morning meals. The effects were followed up and observed from the beginning of treatment.
2.3. Data Collection
This study extracted and analyzed clinical data from outpatient and inpatient cases in the Cardiology Department of Pudong Hospital, affiliated with Fudan University, from January 2021 to December 2021, through the Hospital Information System (HIS).
The extracted medical records encompassed patient demographics (gender, age, height, and body weight), clinical characteristics (diagnoses, comorbidities), rivaroxaban dosing regimens, and renal function parameters. CHA2DS2-VASc and HAS-BLED scores at admission were analyzed to assess thromboembolic risks and bleeding tendencies. Pretreatment evaluations included complete blood count analysis measuring hemoglobin (Hb), platelet count (PLT), along with biochemical profiling: alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (SCr), blood urea nitrogen (BUN), and uric acid (UA). Coagulation studies comprised activated partial thromboplastin time (APTT), prothrombin time (PT), fibrinogen (FIB), D-dimer quantification, thrombin time (TT), and international normalized ratio (INR), among other indicators. Data were collected by two investigators and input into collection forms, cross-checked for accuracy by another two investigators. Once collected, the data were kept confidential and only accessible to the primary researchers.
2.4. Follow-Up
Systematic follow-up protocols, combined outpatient clinic visits with telephone interviews, commenced at treatment initiation, spanning a 12-month observation period with endpoints’ assessment at 3-month intervals. Adverse drug events (ADEs) triggered immediate implementation of symptom-targeted interventions.
2.5. Outcome Definition
Incidence of thromboembolism was defined to determine the efficacy profiles for off-label dosage of rivaroxaban, including statistics on occurrences of stroke, myocardial infarction, other systemic circulation embolisms, and all-cause mortality during the follow-up period. While the incidence of bleeding events was defined as the safety outcome. Calculation of severe bleeding events characterized as meeting any of the following criteria for significant bleeding: ① fatal bleeding; ② retroperitoneal, intracranial, intraocular, intraspinal, intra-articular, or pericardial bleeding, or symptomatic muscle compartment syndrome with bleeding; ③ resulting in a reduction in Hb of at least 2.0 g/dL requiring blood transfusion; moderate bleeding events (such as genitourinary bleeding, soft tissue muscle bleeding, oral bleeding, intra-articular bleeding, and other nonhemodynamically significant bleeding); and mild bleeding events (i.e., other bleeding events that do not meet the criteria for severe or moderate bleeding, e.g., nontreatment-requiring nosebleeds) during the follow-up period.
2.6. Statistical Analysis
The statistical analysis was performed utilizing R (v 4.30). Continuous variables were expressed as median (interquartile range, IQR) and analyzed using the Mann–Whitney U test. Categorical variables were expressed as n (%) and analyzed using the chi-square test or Fisher’s exact test. Balance between the groups was assessed using standardized mean differences (SMD). An absolute SMD of ≤ 0.2 was considered to indicate acceptable balance [14]. An inverse propensity of treatment weighting (IPTW) was used to balance the baseline covariates between the two cohorts. The efficacy and safety of off-label rivaroxaban dosing 10 or 15 mg daily were evaluated using a generalized linear model. Logistic regression models were constructed using forward selection stepwise analysis. A significance level of p < 0.05 indicated quantitatively meaningful differences.
3. Results
3.1. Baseline Data and Follow-Up Information
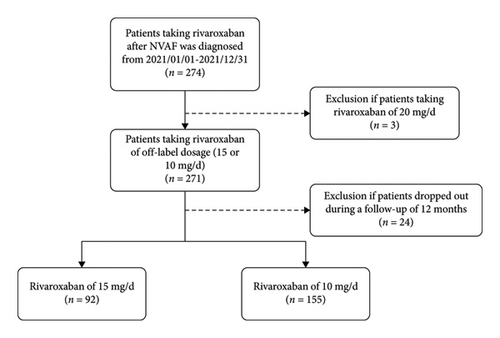
A total of 274 patients in all taking rivaroxaban with recruited, and 3 cases (1.09%) with 20 mg daily were excluded. Follow-up through combined telephone interviews and clinical visits revealed an 8.86% attrition rate (24/271), comprising 7 discontinuations (2.58%) in the 15 mg cohort versus 17 (6.28%) in the 10 mg group. Final analysis included 247 completer patients, stratified as 15 mg daily (n = 92, 37.25%) and 10 mg daily (n = 155, 62.75%) treatment arms (Figure 1). Statistically significant differences were observed between the two cohorts in terms of age, CHA2DS2-VASc score, TT, CrCl, and HDL (SMD before IPTW > 0.2). Upon adjustment by IPTW, patients’ characteristics were well balanced (SMD after IPTW ≤ 0.2), as shown in Table 1.
| Characteristic | Rivaroxaban of 15 mg/d (n = 92) | Rivaroxaban of 10 mg/d (n = 155) | SMD before IPTW | SMD after IPTW |
|---|---|---|---|---|
| Age (years) | 77.00 (71.00–84.00) | 80.00 (72.00–85.00) | −0.23 | −0.05 |
| Male, n (%) | 39 (42.39%) | 63 (40.65%) | 0.04 | −0.09 |
| BMI (kg/m2) | 24.22 (22.40–25.53) | 23.51 (21.93–25.58) | 0.04 | −0.02 |
| Hypertension, n (%) | 73 (79.35%) | 115 (74.19%) | 0.12 | 0.08 |
| Diabetes, n (%) | 32 (34.78%) | 44 (28.39%) | 0.14 | 0.17 |
| Hyperlipidemia, n (%) | 15 (16.30%) | 18 (11.61%) | 0.14 | 0.07 |
| CHA2DS2-VASc | 4.00 (2.75–5.00) | 4.00 (3.00–5.00) | −0.27 | 0.04 |
| HAS-BLED | 2.00 (1.75–2.00) | 2.00 (2.00–3.00) | −0.11 | 0.02 |
| HGB (g/L) | 129.00 (114.75–142.00) | 126.00 (114.50–139.00) | 0.18 | −0.11 |
| PLT (109/L) | 181.50 (145.75–210.65) | 170.00 (130.50–206.50) | 0.11 | −0.03 |
| APTT (s) | 30.60 (27.75–34.45) | 31.20 (27.70–35.20) | −0.13 | 0.00 |
| D-dimer (mg/L) | 0.54 (0.33–1.07) | 0.52 (0.28–1.23) | −0.08 | −0.05 |
| FIB (g/L) | 3.21 (2.79–3.76) | 3.22 (2.71–4.06) | −0.06 | 0.08 |
| PT (s) | 12.27 (11.60–13.24) | 12.42 (11.80–13.30) | −0.13 | 0.07 |
| INR | 1.05 (0.99–1.12) | 1.05 (1.01–1.13) | −0.09 | 0.12 |
| TT (s) | 17.67 (16.95–18.62) | 17.90 (17.10–19.10) | −0.24 | −0.09 |
| ALT (U/L) | 20.00 (13.00–26.00) | 18.00 (12.20–25.00) | 0.12 | −0.06 |
| AST (U/L) | 23.00 (19.00–27.15) | 23.00 (18.50–30.40) | −0.08 | −0.06 |
| BUN (mmol/L) | 6.65 (5.20–8.10) | 6.60 (5.30–8.50) | −0.18 | −0.07 |
| SCr (μmol/L) | 76.80 (64.00–86.25) | 80.00 (66.96–98.00) | −0.17 | −0.06 |
| CrCl (mL/min) | 75.70 (64.36–86.14) | 73.80 (57.63–82.44) | 0.27 | 0.03 |
| UA (mmol/L) | 0.36 (0.29–0.44) | 0.39 (0.30–0.47) | −0.11 | 0.01 |
| TC (mmol/L) | 3.74 (3.32–4.37) | 3.67 (3.15–4.26) | 0.18 | −0.05 |
| TG (mmol/L) | 1.09 (0.82–1.38) | 1.10 (0.84–1.38) | 0.02 | −0.07 |
| HDL (mmol/L) | 1.11 (0.94–1.30) | 1.04 (0.87–1.22) | 0.28 | 0.08 |
| LDL (mmol/L) | 2.26 (1.75–2.74) | 2.25 (1.77–2.69) | 0.04 | −0.10 |
- Note: HGB, hemoglobin; PLT, platelets; FIB, fibrinogen; TT, whole blood clotting time; ALT, alanine aminotransferase, AST, aspartate aminotransferase; BUN, blood urea; SCr, serum creatinine; CrCl, creatinine clearance; TG, triglycerides; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol.
- Abbreviations: APTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time; TC, total cholesterol; UA, uric acid.
3.2. Comparison of Thromboembolic and Bleeding Events in the Two Cohorts of Patients
3.2.1. Comparison of Thrombotic Events Between the Two Cohorts
Throughout the follow-up period, a total of 11 out of 247 patients experienced efficacy endpoint events. In the 10 mg daily rivaroxaban group, there were 9 efficacy endpoint events, comprising 8 cases of stroke or other systemic circulation embolisms, 0 myocardial infarction, and 1 event of all-cause mortality. In the 15 mg daily rivaroxaban group, there were 2 efficacy endpoint events, consisting of 2 cases of stroke or other systemic circulation embolisms, 0 myocardial infarction, and 0 events of all-cause mortality. Upon analysis after IPTW, the occurrence rates of thromboembolic events between the two cohorts were assessed using a generalized linear model. The 15 mg daily rivaroxaban was more effective than 10 mg daily to prevent the occurrence of thrombotic events in elderly Chinese NVAF patients (OR, 0.25; 95% CI, 0.08 to 0.78), with the incidence of thrombotic events was 1.50%, which was lower than that in 10 mg daily rivaroxaban (5.90%) with statistical significance (p = 0.017). Details can be found in Table 2.
| Category | Rivaroxaban of 15 mg/d (n = 92) (%) | Rivaroxaban of 10 mg/d (n = 155) (%) | OR (95% CI) | p value |
|---|---|---|---|---|
| Incidence of thrombotic events | 1.50 | 5.90 | 0.25 (0.08–0.78) | 0.017 |
| Incidence of bleeding events | 10.90 | 13.90 | 0.75 (0.44–1.29) | 0.302 |
3.2.2. Comparison of Bleeding Events Between the Two Cohorts
Over the course of the follow-up period, a total of 32 safety endpoint events occurred among 247 patients. The follow-up revealed 4 cases of severe bleeding, 8 cases of moderate bleeding, and 10 cases of mild bleeding in the 10 mg daily group. In the 15 mg daily group, there were 1 case of severe bleeding, 5 cases of moderate bleeding and 4 cases of mild bleeding. The comparison of the overall occurrence of bleeding between the two cohorts after IPTW showed no statistically significant difference (10.90% in the 15 mg group and 13.90% in the 10 mg group, p = 0.302), as shown in Table 2. The off-label dosage of 15 mg daily rivaroxaban had showed a comparable safety to 10 mg daily rivaroxaban in elderly Chinese NVAF patients (OR = 0.75; 95% CI, 0.44 to 1.29).
3.3. Influence of Clinical Factors on Thrombotic and Hemorrhagic Events in Two Cohorts
Logistic regression analysis was conducted for each clinical factor regarding thrombotic and hemorrhagic events. It was found that for thrombotic events, only CHA2DS2-VASc Score was identified as a significant predictor (OR = 2.37, 95% CI: 1.20–4.66, p = 0.013), Gender approached statistical significance (OR = 4.04, 95% CI: 0.83–19.55, p = 0.083). All other variables showed no significant association with thrombotic events (p > 0.05), as shown in Table 3. Regarding bleeding events, three significant predictors were identified. Lower BMI was associated with increased bleeding risk (OR = 0.83, 95% CI: 0.71–0.97, p = 0.021). Higher HAS-BLED score significantly predicted bleeding events (OR = 2.16, 95% CI: 1.25–3.74, p = 0.006), with each one-point increase more than doubling the bleeding risk. Diabetes was a strong risk factor (OR = 3.01, 95% CI: 1.23–7.41, p = 0.016), with diabetic patients having threefold higher bleeding odds. Other variables were not significantly associated with bleeding events, as shown in Table 4.
| Variable | Coefficient | Std_err | OR (95% CI) | p value |
|---|---|---|---|---|
| Intercept | 1.22 | 7.46 | 3.40 (0.00–7.65 ∗ 106) | 0.870 |
| Age | −0.02 | 0.06 | 0.98 (0.87–1.10) | 0.701 |
| Gender | 1.40 | 0.81 | 4.04 (0.83–19.55) | 0.083 |
| BMI | −0.10 | 0.10 | 0.91 (0.75–1.11) | 0.346 |
| Hypertension | −0.89 | 1.02 | 0.41 (0.06–3.01) | 0.382 |
| Diabetes | −1.55 | 1.00 | 0.21 (0.03–1.50) | 0.120 |
| Hyperlipidemia | 1.05 | 0.86 | 2.86 (0.53–15.51) | 0.222 |
| CHA2DS2-VASc score | 0.86 | 0.35 | 2.37 (1.20–4.66) | 0.013 |
| Dosage | −1.00 | 0.89 | 0.37 (0.06–2.13) | 0.265 |
| HGB | −0.02 | 0.02 | 0.98 (0.95–1.02) | 0.364 |
| PLT | 0.00 | 0.01 | 1.00 (0.99–1.01) | 0.957 |
| APTT | −0.01 | 0.06 | 0.99 (0.87–1.12) | 0.863 |
| D-Dimer | 0.12 | 0.17 | 1.13 (0.80–1.58) | 0.493 |
| PT | −0.12 | 0.70 | 0.88 (0.23–3.45) | 0.859 |
| INR | 0.06 | 7.94 | 1.06 (0.00–6.12 ∗ 106) | 0.994 |
| ALT | 0.05 | 0.05 | 1.05 (0.96–1.14) | 0.319 |
| AST | −0.07 | 0.05 | 0.94 (0.84–1.04) | 0.219 |
| CrCl | 0.00 | 0.02 | 1.00 (0.97–1.04) | 0.887 |
| TC | 0.06 | 0.49 | 1.07 (0.40–2.80) | 0.899 |
| Variable | Coefficient | Std_err | OR (95% CI) | p value |
|---|---|---|---|---|
| Intercept | 3.34 | 4.66 | 28.10 (0.00–2.62 ∗ 106) | 0.474 |
| Age | −0.05 | 0.04 | 0.95 (0.88–1.02) | 0.158 |
| Gender | 0.03 | 0.46 | 1.03 (0.42–2.55) | 0.953 |
| BMI | −0.19 | 0.08 | 0.83 (0.71–0.97) | 0.021 |
| Hypertension | 0.44 | 0.71 | 1.55 (0.38–6.26) | 0.537 |
| Diabetes | 1.10 | 0.46 | 3.01 (1.23–7.41) | 0.016 |
| Hyperlipidemia | −0.30 | 0.69 | 0.74 (0.19–2.85) | 0.666 |
| HAS-BLED score | 0.77 | 0.28 | 2.16 (1.25–3.74) | 0.006 |
| Dosage | −0.35 | 0.46 | 0.70 (0.29–1.72) | 0.440 |
| HGB | 0.00 | 0.01 | 1.00 (0.98–1.02) | 0.999 |
| PLT | 0.00 | 0.00 | 1.00 (1.00–1.01) | 0.304 |
| APTT | 0.05 | 0.04 | 1.05 (0.97–1.14) | 0.257 |
| D-Dimer | −0.12 | 0.15 | 0.89 (0.66–1.20) | 0.447 |
| PT | −1.63 | 0.97 | 0.20 (0.03–1.32) | 0.094 |
| INR | 16.00 | 9.66 | 8.84 ∗ 106 (0.05–1.47 ∗ 1015) | 0.098 |
| ALT | −0.01 | 0.03 | 0.99 (0.93–1.05) | 0.789 |
| AST | 0.03 | 0.03 | 1.03 (0.97–1.10) | 0.321 |
| CrCl | 0.00 | 0.01 | 1.00 (0.98–1.03) | 0.804 |
| TC | 0.29 | 0.30 | 1.34 (0.75–2.39) | 0.323 |
4. Discussion
Global demographic shifts toward aging populations and lifestyle modifications have driven a marked rise in AF prevalence, demonstrating a steep age-dependent escalation from sporadic occurrence in young adults (< 40 years; < 1%) to substantial prevalence in octogenarians (≥ 80 years; 10%–17%) [13]. The major adverse effects of AF are thrombosis formation and embolism. The annual thrombotic event rate in NVAF patients is 5%, accounting for 15%–20% of all cerebral embolic events. AF-associated strokes exhibit quintupled mortality-disability risks, characterized by 5-year recurrence persistence and elevated early mortality. Consequently, thromboembolism prophylaxis remains paramount in AF management.
Given the limited evidence on the factors influencing the safety and effectiveness of off-label doses of rivaroxaban in NVAF patients in China, we conducted this prospective study, systematically evaluating safety-efficacy determinants across varied regimens. Distinctive methodological strengths include: (1) Population specificity. Our study focused on a Chinese population, enabling better reflection of the characteristics of China’s unique AF phenotype. (2) Data comprehensiveness. Real clinical data provides a more comprehensive analysis of various factors’ relationships with off-label doses of rivaroxaban compared to subgroup screening data.
This cohort study demonstrated superior thromboprophylaxis efficacy of 15 mg daily rivaroxaban versus 10 mg regimen in Chinese NVAF patients (n = 247), with comparable safety profiles. Further analysis of the above results indicates the following: (1) The majority of participants in our research were elderly NVAF, 65.18% aged ≥ 75 years. In China, the lifetime risk of AF among the elderly population above 75 is twice as high as that among the population above 50 [4]. The dose selection of rivaroxaban for the elderly Chinese NVAF patients has always been a challenging issue as they have a lower body mass, a clinical background of multimorbidity and polypharmacy, reduced renal function that made the elderly having a complex risk of thrombosis and bleeding [15]. About 73.2% of elderly patients with AF had more than 2 comorbidities, while in our study, more than half had hypertension (n = 188, 76.11%) and nearly one-third had diabetes (n = 76, 30.77%) with appropriate therapy. Hypertension and diabetes, the risk factors for cardiovascular disease, exhibited strong AF correlations, indicating that these comorbidities can lead to poorer vascular conditions, concomitantly elevating thromboembolic risk and major bleeding potential (2) Rivaroxaban exhibits CrCl-dependent pharmacokinetics, where diminished renal clearance (CrCl < 50 mL/min) results in elevated plasma concentrations under equivalent dosing regimens [16]. Current guidelines recommend dose reduction when CrCl declines below 50 mL/min, positioning renal function as a modifiable protective determinant, particularly in bleeding-prone populations. Our cohort demonstrated preserved renal function across treatment arms, with median CrCl levels exceeding 70 mL/min in both dosage groups. (3) Meanwhile, the CHA2DS2-VASc and HAS-BLED score showed that the most patients had high risk of thrombosis and low risk of bleeding. Considering these points, the widely use of low-dose rivaroxaban, 10 mg daily may increase the risk of inadequate anticoagulant treatment for Chinese elderly patients with high risk of thrombosis and low risk of bleeding.
Additionally, a multifactor logistic regression analysis was conducted on clinical factors influencing endpoint events, revealing that CHA2DS2-VASc score and HAS-BLED score showed statistically significant impact on efficacy or safety endpoint events (p < 0.05). As they were widely used as risk assessment tools for AF-related ischemic stroke and bleeding, our study validated their utility in assessing risks among Chinese elderly patients. The lower BMI and a history of Diabetes were identified as risk factors associated with bleeding events (p < 0.05). BMI was an important variable in drug distribution and plasma concentration levels. The association between lower BMI and increased bleeding risk is plausible, potentially reflecting relatively higher drug exposure. Clinically, patients with low BMI frequently exhibited a clustering of hemorrhage-predisposing comorbidities, such as advanced age, frailty, malignancy, and chronic kidney disease. Furthermore, renal function may be overestimated due to their reduced muscle mass, potentially leading to inappropriate dosing of rivaroxaban [17]. These findings underscored the necessity for meticulous anticoagulant dosing strategies in low BMI populations.
Notably, diabetes was identified as a strong independent predictor of bleeding (OR = 3.01, 95% CI: 1.23–7.41, p = 0.016). This suggested that patients with diabetes may have a heightened bleeding tendency. Numerous studies have highlighted that diabetes independently contributes to NVAF patients, leading to increased incidence and mortality. Further specific analysis revealed that with diabetes, concurrent AF was associated with increased risks for diabetes-related macrovascular complications, diabetic nephropathy, and diabetic foot [18], possibly due to underlying vasculopathy, subtle renal impairment not fully captured by CrCl, or other mechanisms. Particular caution was warranted when selecting anticoagulant doses in diabetic NVAF patients.
This study possessed several strengths, including its prospective design and real-world setting, which enhanced external validity. The use of IPTW helped to mitigate confounding by balancing baseline covariates between the groups. However, certain limitations must be acknowledged: (1) The single-center design and relatively modest sample size (n = 247) may limit the generalizability of our findings. (2) The loss to follow-up rate of 8.86% (n = 24) could potentially introduce bias. (3) As an observational study, residual confounding from unmeasured variables could not be entirely excluded, despite the use of IPTW. (4) We could not ascertain the specific reasons guiding physicians’ initial dose choices, which might correlate with unmeasured patient characteristics. (5) Finally, our comparison was limited to the 10 mg and 15 mg doses and did not include a 20 mg group or directly compare outcomes against standard guideline-recommended dosing strategies.
Notwithstanding these limitations, our findings suggested that in elderly Chinese NVAF patients, rivaroxaban 15 mg daily might offer a better efficacy profile than 10 mg daily, without a significant increase in bleeding risk. This supported considering the 15 mg dose for appropriate patients, based on a thorough individualized assessment that incorporates BMI, HAS-BLED score, and diabetes status. Future research involving larger, multicenter cohorts was warranted to validate these findings, and randomized controlled trials might ultimately be necessary to definitively establish the optimal risk-benefit balance of different dosing strategies in this population.
5. Conclusion
This prospective study conducted in elderly Chinese patients with NVAF demonstrated that rivaroxaban 15 mg daily was significantly more effective in preventing thrombotic events compared to the 10 mg daily dose over a 12-month follow-up. Importantly, this enhanced efficacy was achieved without a statistically significant increase in the overall incidence of bleeding events between the two cohorts, indicating comparable safety profiles in this population. The study also identified key predictors for clinical outcomes: higher CHA2DS2-VASc score were independently associated with an increased risk of thrombotic events, whereas lower BMI, higher HAS-BLED score, and the presence of diabetes mellitus were significant predictors of bleeding events. The selection of the optimal dose of rivaroxaban required careful individualized patient assessment, paying particular attention to established risk factors for thrombosis (CHA2DS2-VASc) and bleeding (HAS-BLED, BMI, and diabetes). Further validation in larger, potentially multicenter studies was warranted to corroborate these findings and guide precise anticoagulation strategies for this specific patient group.
Consent
The participants provided their written informed consent to participate in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Jingru Gong conceived and designed the study; Zhe Jiang and Wenmin Dong collected the data and contributed to the writing of the manuscript; Xiaoxiao Lu and Xiaolin Liu analyzed the data; all authors have read and approved the final manuscript.
Zhe Jiang and Wenmin Dong have contributed equally to this work and share first authorship.
Funding
This work was supported by the 2021 Pudong New Area Health and Family Planning Commission Project (Grant No. PW2021A-27).
Acknowledgments
This manuscript involved the use of artificial intelligence (AI)–assisted software during its preparation. Specifically, DeepSeek-R1 (version 1.0, DeepSeek AI) was employed to assist in editing and language polishing of the manuscript text. No AI tools were used for data collection, statistical analysis, or content generation related to scientific findings or interpretation.
All AI-assisted outputs were carefully reviewed, verified, and revised by the authors to ensure factual accuracy, scientific integrity, and adherence to the journal’ s standards. The final manuscript reflects the authors’ own intellectual work and responsibility.
Open Research
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.




