Wenjing Zhitong Decoction Alleviates Primary Dysmenorrhea Mediated by Pain Sensitization Via the ERα-BDNF Signaling Pathway
Abstract
Background: Wen-Jing-Zhi-Tong decoction (WJZTD) is a traditional Chinese medicine herbal decoction that has demonstrated clinical efficacy in treating primary dysmenorrhea (PDM) over decades. However, the underlying therapeutic mechanism of WJZTD requires further clarification.
Objective: This study aims to explore and elucidate the therapeutic mechanism of WJZTD in treating PDM through metabolomics, integrated network pharmacology, as well as in vitro and in vivo experiments.
Methods: A PDM rat model was established by estradiol benzoate and oxytocin for 3 consecutive estrous cycles, and then WJZTD treatment was administered for 3 consecutive estrous cycles. To evaluate the therapeutic effect of WJZTD on PDM, behavioral tests, histological analysis, and evaluation of serum prostaglandins (PGs) were performed. Moreover, immunofluorescence and Western blot analysis were carried out in dorsal root ganglion (DRG) tissues to explore the role of WJZTD in reducing pain sensitivity. Metabolomics and integrated pharmacology were utilized to identify potential bioactive targets, which were then validated through in vitro experiments.
Results: WJZTD significantly reduces pain sensitivity in PDM rats, treating the condition effectively by decreasing brain-derived neurotrophic factor (BDNF) protein expression, c-Fos-positive neurons in DRG, and serum prostaglandin PGF2α (PGF2α) levels. A nontargeted metabolic analysis identified 11 differential metabolites, suggesting ESR1 (estrogen receptor α, ERα) as a potential target. In vitro experiments confirmed WJZTD’s efficacy in treating PDM through regulating the ERα/BDNF signaling pathway.
Conclusion: WJZTD decreases the excitability of DRG neurons in rats with PDM through the ERα/BDNF pathway, which enhances pain sensitization and contributes to reducing dysmenorrhea.
1. Introduction
Primary dysmenorrhea (PDM) is a common gynecological disease without pelvic pathology, characterized by periodic, crampy, lower abdominal pain beginning just before or at the start of menstruation, affecting 50%–90% of women [1]. Studies showed that 15% of women with severe PDM resulting in absences from work or school [2] and other cross-sectional studies revealed similar evidence of dysmenorrhea affecting relationships, functioning, and productivity [1].
The pathogenesis of dysmenorrhea is closely related to the overproduction of prostaglandin F2α (PGF2α), which is synthesized by prostaglandin F2α (PGF2α) cascade displaying a cyclooxygenase-mediated pathway [3], causing uterine contractions.
Arachidonic acid originates from phospholipids by the lysosomes, whose activity stability is regulated by progesterone levels [4]. Several factors could regulate the expression of cyclooxygenase-2 (COX-2), one of which is estrogen levels [5]. Although women are likely more sensitive to chronic pain than men because of estrogen [6], the molecular role of estrogen and its receptors in metabolic tissues exhibits a tissue-specific aspect that necessitates further investigation.
Estrogen could bind to multiple receptors; notably cell signaling is mediated by estrogen receptors alpha and beta (ERα, ERβ), and both belong to the transcription factor nuclear receptor family [7] and are expressed broadly in numerous cells and tissues, encompassing the ovary, hypothalamus, muscle tissue, sensory neurons, and interneurons of the spinal and medullary dorsal horns [8]. Recently studies suggested that ERα-expressing neurons in the spinal cord dorsal horn appear to facilitate nociception and modulate the central sensitization [9].
Regardless of the expression of PGs, pain sensitivity in women with dysmenorrhea is also a finding that needs attention. Studies revealed that within not menstrual cycle, women with dysmenorrhea have enhanced pain perception of heat pain [10], pressure pain [11], and ischemic pain [12] than women without dysmenorrhea and are more sensitive to pain throughout the menstrual cycle [13]. This may be related to structural alterations in the brain [14], as well as adaptive neuroplasticity development and functional reorganization [15], caused by the periodic nociceptive input from the periphery. Kuner also found women with PDM that are not effective in conventional treatment might be mainly associated with the central mechanisms of pain [16].
The pain processing usually starts with nociceptors, including C-fibers and Aδ-fibers, which are primary afferents transmitting pain information to the central nervous system [17]. Nociceptor terminals are distributed across the skin, muscles, joints, and visceral organs, while their cell bodies reside in the DRG and the trigeminal ganglia. Inflammatory mediators such as prostaglandins, nerve growth factors, and cytokines including tumor necrosis factor-α (TNF-α or TNF), interleukin 1β (IL-1β), can activate or sensitize nociceptors by directly binding and stimulating various receptors [18]. Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, is stored in dense-core synaptic vesicles at the axon terminals and regulated by gonadotrophins, adrenal and gonadal steroids, neural activity, and epigenetic mechanisms [19]. When nerve injury or inflammation in the spinal cord, DRG, or other related areas, BDNF will overproduct and release from neurons. By binding to the high-affinity receptor tropomyosin receptor kinase B (TrkB), BDNF activates a series of signaling pathways that play an essential role in synaptic plasticity and central sensitization [20].. Evidence shows that PGs induce the expression of BDNF [19], and serum BDNF levels were significantly higher in PDM patients than in healthy controls [21].
Wen-Jing-Zhi-Tong decoction has been proven effective in treating dysmenorrhea with multicomponent, multitarget, and multi-effect characteristics, which alleviate the pain of PDM rat models via the BDNF/TrkB/ERK/CREB pathway [22]. It has been reported that superficial dorsal horn ERα activation mediates BDNF upregulation and nociceptive processing in visceral hypersensitivity [23]. However, ERα suppression impaired BDNF-induced downstream signaling molecules [24]. In the present study, we conduct behavioral tests to determine whether the PDM rat model is characterized by pain hypersensitivity and quantify the expression of BDNF and its downstream molecular pathways related to neuronal activity in DRG. Except for that, we screened several potential targets, including ESR1, of WJZTD in the treatment of PDM through UPLC-QE-LC/MS analysis. Afterward, the underlying molecular mechanisms of ERα-BDNF were explored in vitro.
2. Methods
2.1. Animals, Feeding, and Treatment
Animal studies were approved by the Experimental Animal Ethics Committee, Nanjing University of Chinese Medicine (ACU211205), and followed precisely the guidelines of Animal Research: Reporting of In Vivo Experiments (ARRIVE) (Supporting Material S1). [25]. And, 24 female SD rats (6–8 weeks of age, 160 ± 20 g, license no. SCXK (SU) 2020-0009) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (China). All rats were housed and maintained in an SPF standard laboratory environment (room temperature, 19 ∼ 25°C; 12 h light/dark cycle; 40%–70% relative humidity) with food and water ad libitum available. Behavioral tests were conducted in a sound-proofed and air-conditioned laboratory, with rats habituated to the environment for 1 hour at least.
2.2. PDM Rat Modeling Procedures
All animals were adapted to standard conditions for 7 days before experimentation. Employing vaginal smear, all female rats with regular 4-day estrus cycles were allocated to 4 groups according to the random number table method (n = 6 for each group): control, PDM model, ibuprofen (a nonsteroidal anti-inflammatory drug (NSAID) can alleviate dysmenorrhea by decreasing PGs’ secretion), and WJZTD groups. A vaginal smear was used to monitor the estrous cycle for the whole molding stage. Estradiol benzoate helps synchronize the estrous cycle in rats and increases the sensitivity of the uterus. Oxytocin (OT) can induce smooth muscle contractions in the uterine of rats. The combination of the two drugs induces dysmenorrhea, and rats with significant writhing reactions and significantly shortened writhing latency will be defined as a dysmenorrhea rat model. As shown in Figure 1(a), the rats in the PDM model, ibuprofen, and WJZTD groups were injected subcutaneously with each estradiol benzoate for 24 days. Then, 2.5 mg·kg−1 doses were injected once per estrous cycle (every 4 days), on 4th, 8th, 12th, 16th, 20th, 24th days, respectively. One h after injection, 10 U·kg−1 oxytocin was injected intraperitoneally to induce dysmenorrhea [26]. For the rest of the days, only 1.25 mg·kg−1 estradiol was injected once daily. The same amount of saline was injected at the corresponding time for the control group. When the writhing response of each rat, the dysmenorrhea rat model was established successfully. The criteria for evaluating the writhing body are based on the previous research [26].
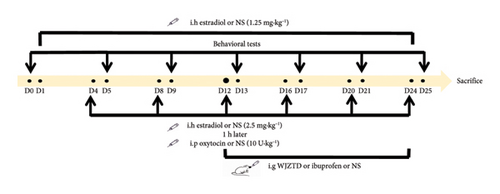
From the 13th day of the modeling procedure, the ibuprofen and WJZTD groups were, respectively, given corresponding drugs by intragastric administration, and the drug dose was calculated as the clinical dose of 70 kg adult using the conversion formula of rat and human surface area. Specifically, the ibuprofen group was administrated 0.07 g·kg−1 ibuprofen suspension once a day, and 12.15 g·kg−1 WJZTD was administrated once a day for the WJZTD group until the 24th day. Similarly, the control and PDM model groups were given a corresponding dose of saline. On the 25th day, the rats were anesthetized by intraperitoneal injection of 3% pentobarbital 45 mg kg−1 after a behavioral test. Blood was collected from the abdominal aorta and sacrificed by bleeding, and serum was separated; uterine tissue, T10-S4 DRGs, and colon tissue of rats were removed and transferred immediately in liquid nitrogen, and then stored at −80°C.
2.3. Behavioral Tests
Rats were accommodated individually into the clear behavior test box (74 ∗ 30 ∗ 31 cm) for at least 1 h before behavioral tests. The researchers were blinded to group assignment in the process of data collection and analysis.
2.3.1. Writhing Response Assessment
The writhing latency observed was the time from the intraperitoneal injection of oxytocin to the first writhing reaction. As shown in Table 1, according to the scoring standard by Schmauss et al. [27], writhing behavior is rated on a scale of 0–3 points over a 30 min observation period. The total writhing score is calculated as follows: writhing score = (Level 0 × 0) + (Level 1 × 1) + (Level 2 × 2) + (Level 3 × 3).
| Level | Behavioral performance | Score (point) |
|---|---|---|
| 0 | Normal posture (paw placed flat at the bottom of the box or normal probing behavior) | 0 |
| 1 | Body tilted to one side | 1 |
| 2 | Hind limb extension, hind paw dorsiflexion, body extension with frequent lateral pelvic rotation | 2 |
| 3 | Abdominal muscle contraction and hind limb backward extension | 3 |
2.3.2. Hot Plate Test
The paw withdrawal latency (PWL) was evaluated on a hot plate (Zheng Hua Biologic Apparatus Facilities, CHINA) to assess the nociception of rats. Rats were placed on a hot plate at 55°C to measure the latency of licking or withdrawing their hind paws. Rats were tested in a plexiglass bucket to prevent them from escaping. When the rat licks or withdraws the hind paw or does not respond within 50 s, it should be immediately removed from the plate.
2.3.3. Mechanical Allodynia
The Von Frey test was conducted to evaluate the mechanical threshold of rats. We used a series of Von Frey fiber filaments ranging from 0.6 g to 15 g (0.6, 1, 1.4, 2, 4, 6, 8, 10, and 15 g) to stimulate rat hindpaw on an elevated mesh-bottomed platform (RWD Scientific Instruments Service Company, CHINA) as previously described. [28]. Rapid shaking and/or biting, licking, or shaking of the paw, but not walking, could be defined as a positive response to the stimulus. Each fiber was applied 10 times; the positive claw response frequency was recorded and then imported into the up–down reader software for statistics and calculations; and finally, the force of the specific filament required to induce the 50% foot reduction response frequency was defined as paw withdrawal thresholds (PWTs).
2.4. Hematoxylin–Eosin (HE) Staining
The uterine tissue was isolated and subsequently fixed in 4% paraformaldehyde (PFA) for more than 24 h. Postdehydration, the tissues were embedded in paraffin and sectioned to a thickness of 5 μm. Staining with hematoxylin and eosin (Sigma, USA, Batch No. H9627 and 170) was carried out as previously detailed in references [29]. The observations and analyses were conducted using a light microscope (Nikon Eclipse E100, Tokyo, Japan).
2.5. Immunofluorescence
2.5.1. In Vivo
The DRGs of rats in each group were removed and postfixed in 4% PFA overnight and then cryoprotected in 20% sucrose in PBS at 4°C. After sinking to the bottom of the vessel, the DRG tissue is paraffin-embedded and cut into 5 -μm sections using a cryostat (RM2016, Leica, Germany). Sections were pretreated with 0.2% Triton X-100 (Beyotime, P0096, China) for 30 min and incubated with rabbit c-Fos antibody (1 : 100, 31254S, CST, USA) at 4°C overnight. The sections were immunostained with FITC-conjugated goat anti-rabbit IgG (1 : 200, RS0004, ImmunoWay, USA) at room temperature for 1 h and then stained by DAPI (C1005, Beyotime, China) for 15 min.
2.5.2. In Vitro
PC12 cells were fixed in 4% PFA for 20 min after being cultured and treated with drugs. Cells were incubated with 0.2% Triton X-100, primary, and secondary antibodies, and DAPI as described above.
All images were captured by confocal microscope (Nikon AX, Japan) at a 20× magnification and processed using ImageJ. The number of cells was counted by an investigator blinded to the experimental groups (The original images for microscopy were shown in Supporting Material S3).
2.6. Enzyme-linked Immunosorbent Assay (ELISA)
The level of PGF2α (AF9456-A, AiFang biological, China) and prostaglandin E2 (PGE2) (AF2062-A, AiFang biological, China) in serum separated from rat model was measured by incubating with ELISA reagents.
2.7. Colon Tissue Untargeted Metabolomics
The metabolomical raw data were acquired from the SHIMADZU-LC30 UPLC system and Thermo Scientific Q Exactive Plus Orbitrap LC-MS/MS spectrometer (Thermo Fisher Scientific, USA). The metabolite separation was performed with a Waters ACQUITY UPLC HSS T3 column (2.1 × 100 mm, 1.8 μm) (Waters, Milford, MA, USA). The mobile phase was 0.1% (V/V) formic acid–water (A) and acetonitrile (B), and the optimized elution gradient was implemented as follows: 0–2 min, 0% B; 2–6 min, 0%–48% B; 6–10 min, 48%–100% B; 10–12 min, 100% B; 12–12.1 min, 100%–0% B; 12.1–15 min, 0% B. MS-DIAL software was used to collect and analyze the mass spectrometry raw data detected in both negative and positive ion modes. The metabolites were identified by the HMDB, MassBank, GNPS databases and the self-built database. The metabolites were contrasted using orthogonal partial least squares discriminant analysis (OPLS-DA).
2.8. Network Pharmacology Analysis
The Swiss Target Prediction (https://www.swisstargetprediction.ch) and BAT-man database (https://bionet.ncpsb.org.cn/batman-tcm/index.php) were used to predict possible targets of differential metabolites for WJZTD versus PDM and PDM versus Control groups. Meanwhile, the genes associated with PDM were searched by Mendelian Inheritance in Man (OMIM, https://omim.org/) and GeneCards (https://www.genecards.org/) online databases. The protein–protein interaction (PPI) analysis of potential targets for treating PDM in WJZTD was performed using the STRING database (https://cn.string-db.org). The network topology parameters of these predicted targets, including degree centrality (DC), betweenness centrality (BC), closeness centrality (CC), and eigenvector centrality (EC), were assessed using the CytoNCA algorithm on the Cytoscape3.9.1 software. Finally, gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were conducted using the Metascape database (https://metascape.org/), and bubble graphs were created with a bioinformatics mapping website (https://www.bioinformatics.com.cn/).
2.9. Molecular Docking Simulation
Molecular docking was employed to simulate the theoretical interactions between potential bioactive components and key genes, allowing for predictions of their binding patterns and affinities. Download the structural file of WJZTD’s potential bioactive ingredients from the PubChem database. OpenBabelGUI software was used to convert the file from SDF format to PDB format. Next, the PDB file for the three-dimensional structure of the key protein ESR1 (PDB ID: 1 × 7R) from the Protein Data Bank (RCSB) (https://www.rcsb.org/) was retrieved. Using AutoDockTools 1.5.6, polar hydrogens were added, and the grid box size was adjusted before saving the PDB file in pdbqt format. AutoDock Vina was then utilized for the molecular docking simulation, and the minimum binding energy was calculated. Finally, the results were visualized using PyMOL.
2.10. Assessment of Cell Viability
The CCK-8 kit (BS350B, Biosharp, China) was used to determine the survival rate of PC12 cells at different time points and different drug culture concentrations. PC12 cells (100 μL) were plated into 96-well plates (1 × 104 cells/well) and cultured with different concentrations of drugs. Then, cells were incubated with fresh medium with 10% CCK-8 solution in the dark for 1–4 h. The absorbance at 450 nm was measured by an Infinite M200 PRO spectrophotometer (Tecan, Switzerland).
2.11. Cell Culture and Drug Treatment
Rat pheochromocytoma PC-12 cells were obtained from Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China). Roswell Park Memorial Institute (RPMI)-1640 (Gibco, USA) medium supplemented with FBS (10%) (Gibco, USA), penicillin (100 U/mL), and streptomycin (100 μg/mL) was provided. Cells were incubated in a Thermo 311GP incubator at 37°C under 5% CO2.
Cells were treated with MPP (A-specific antagonists of ERα) (5 μM, M288006, Aladdin, USA), PPT (A-specific agonists of ERα) (10 nM, ab120161, Abcam, UK), BDNF (50 ng/mL, 092,261, PeproTech, USA) for 12 h, and 10% concentration of WJZTD-treated rat serum for 24 h (Supporting Material S2).
2.12. Preparation of Rat Serum Specimens
SD rats were randomly divided into a control group and a WJZTD administration group (n = 6 per group). The WJZTD group rats were given WJZTD by intragastric administration at a dose of 24.3 g·kg−1 twice a day for 3 consecutive days, while the control group was administered with saline. The rats were anesthetized with 3% pentobarbital (45 mg/kg, intraperitoneally injected), and blood samples were collected from the abdominal aorta 1 h after the last administration. After resting at room temperature for 1 h, the serum was isolated by centrifugation at 3000 RPM at 4°C for 15 min and then inactivated in a water bath at 57°C for 30 min. Ultimately, the serum underwent filtration using 0.22 μm microporous membranes and was subsequently stored at −80°C for follow-up experimentation [30].
2.13. BDNF-siRNA Transfection
Cells (3 × 105 cells/well) were divided into 5 groups (control, NC group, siRNA-1 group, siRNA-2 group, siRNA-3 group) seeded in six-well plates. Small interfering RNAs (siRNAs, GenePharma, Shanghai, China) and Lipofectamine 3000 reagents (Invitrogen, USA) were used to transfect cells targeting BDNF (NM_24225). Transfection efficiency was measured by Q-PCR and Western blotting analysis. Each siRNA oligo sequence used is shown in Table 2.
| Name | Direction | Oligonucleotides sequence 5′–3′ |
|---|---|---|
| Negative control | Sense | UUCUCCGAACGUGUCACGUTT |
| Antisense | ACGUGACACGUUCGGAGAATT | |
| siRNA-1 | Sense | CGCCCAUGAAAGAAGCAAATT |
| Antisense | UUUGCUUCUUUCAUGGGCGTT | |
| siRNA-2 | Sense | GAACUACCCAAUCGUAUGUTT |
| Antisense | ACAUACGAUUGGGUAGUUCTT | |
| siRNA-3 | Sense | GAAGAGUGAUGACCAUCCUTT |
| Antisense | AGGAUGGUCAUCACUCUUCTT | |
2.14. Western Blot Analysis
Protein concentration of DRG tissues and PC12 cells was measured by BCA Protein Assay Kit (Yeasen, China) and combined with 5× loading buffer (Yeasen, China), followed by incubation at 100°C for 5 min. Protein samples were loaded and resolved by dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), then electrotransferred to the polyvinylidene fluoride (PVDF) membrane (Millipore, USA), blocked with quick block solution for 30 min at room temperature. The membrane was incubated with diluted primary antibodies: anti-BDNF (1 : 5000, ab108319, Abcam), anti-ERα (1 : 1000, ab32063, Abcam), anti-c-Fos (1 : 1000, 31,254, Cell Signaling Technology), anti-nNOS (1 : 1500, ab76067, Abcam), β-tubulin (1 : 1000, 2128, Cell Signaling Technology), and β-Actin (1 : 1500, GB15001-100, ServiceBio) at 4°C overnight. Afterward, membranes were incubated with HRP-conjugated secondary antibody (1 : 10,000, SA00001-2, Proteintech) for 1 h at room temperature. The target protein bands were visualized by the electrochemiluminescence method and quantified using ImageJ software (The original images for blots are shown in Supporting Material S3).
2.15. Flow Cytometry Assay
After being treated with different drugs, the cell membrane potential of PC12 wells was evaluated by BBcellProbe M09 (BestBio, China) analysis kit. Cells were collected and resuspended with an M09 fluorescent staining working solution. After incubation in the dark for 1 h, the fluorescence intensity was detected by flow cytometry (Beckman Gallios, USA), and the results were analyzed using FlowJo 10.8.1 software.
2.16. Statistical Analysis
GraphPad Prism 8.0.1 software was used for data analysis and visualization. One-way or two-way analysis of variance (ANOVA) was conducted to compare differences between groups, with statistical significance defined as a p-value of less than 0.05. All results are presented as the mean ± standard deviation (SD), and details of the statistical methods used can be found in the figure legends for each study.
3. Results
3.1. WJZTD Exerts Significant Effects on Alleviating the Pain of the PDM Rat Model
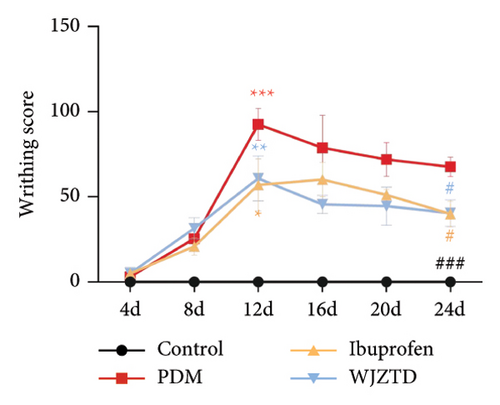
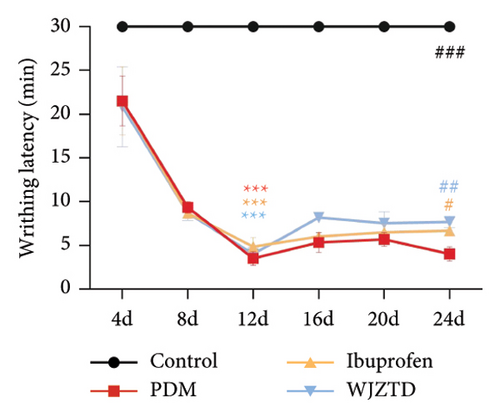
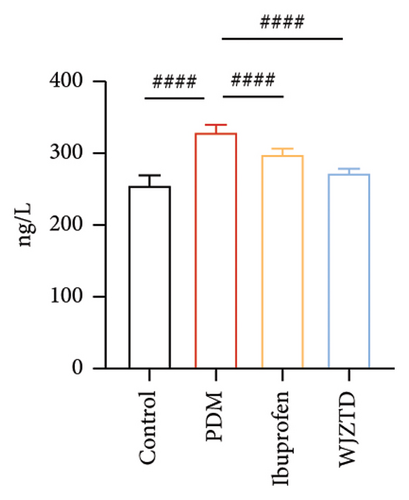
To explore the potential effect of WJZTD in the treatment of PDM, we evaluated the level of PGF2α, PGF2α/PGE2 in serum, writhing score, and latency, which are defined as the characteristic hallmark of the OT-induced model of PDM [31]. When the third oxytocin injection induced dysmenorrhea on the 12th day, the PDM model, ibuprofen, and WJZTD groups’ writhing score and latency differed significantly from the control group (writhing score: p < 0.001, p < 0.05, p < 0.01; writhing latency: p < 0.001). However, following 3 estrus cycles (12 days) of ibuprofen and WJZTD treatments, both groups experienced a callback with a significant difference compared with the PDM model group (writhing score: p < 0.05; writhing latency: p < 0.05, p < 0.01) (Figures 1(b), 1(c)). Concomitantly, groups treated with ibuprofen and WJZTD also had significant lower serum PGF2α and PGF2α/PGE2 levels than the model group (p < 0.0001) (Figure 1(d)). These observations implied that PDM model is successful and the therapeutic impact of WJZTD on PDM.
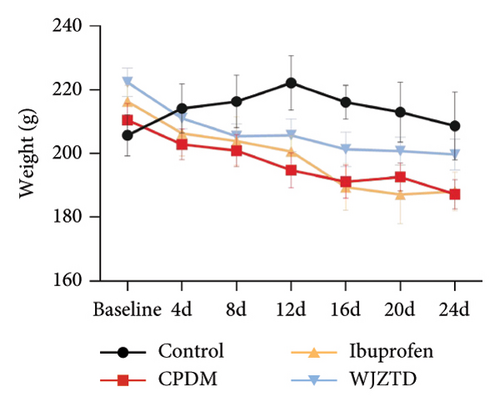
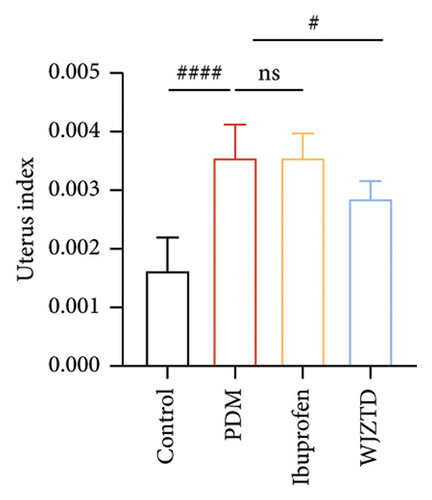
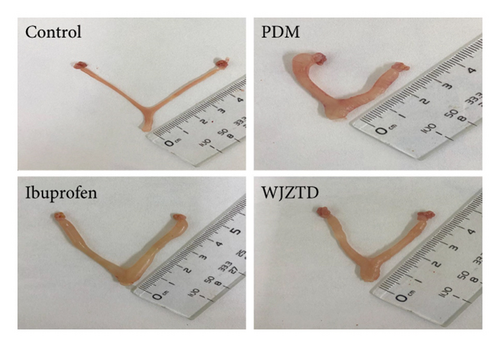
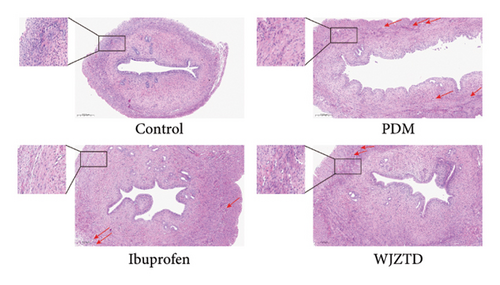
According to earlier research, rats with dysmenorrhea developed edema and hyperemia in their uterine tissue. As shown in Figures 1(e), 1(f), 1(g), on the premise that there was no statistical difference in body weight among the groups, we reached the same conclusion, and it was noted that WJZTD could significantly recover uterine tissue compared to PDM group (p < 0.05). Furthermore, HE staining revealed severe pathological damages in the PDM group’s uterine tissue. As previously reported [22], uterine tissue separated from the PDM group displayed clear histomorphological alterations, including discontinuous smooth muscle cells, disorganized muscle fibers, and uterine spiral artery congestion, as compared to the control group (Figure 1(h)).
3.2. WJZTD Reduced PDM Rat Model Pain Hyperalgesia
According to studies, PDM patients have lower pain thresholds for tenderness, heat, and cold [32] and are more sensitive to pain [12]. The central nervous system receives repeated pain signals during monthly menstruation, which causes an enhanced sensitivity to painful stimuli. This is the reason for this heightened sensitivity [4]. We conducted a behavioral test of mechanical allodynia and heat sensitivity one day following each oxytocin injection during the modeling process to investigate if the development of a dysmenorrhea model is associated with increasing pain sensitivity.
As shown in Figures 2(a), 2(b), we assessed the PWT and paw retraction latency to find out how WJZTD affected pain sensitivity. The findings demonstrated that on day 13 of behavioral testing, the mechanical and thermal pain thresholds of the other three groups remained significantly lower than those of the control group (PWL: PDM group: p < 0.01, ibuprofen group: p < 0.01, WJZTD group: p < 0.001; PWT: PDM group, ibuprofen group, WJZTD group: p < 0.001). On the 25th day of behavioral testing, the rats in the WJZTD group had reversed pain hypersensitivity when compared to the PDM group (PWL: p < 0.01; PWT: p < 0.05).
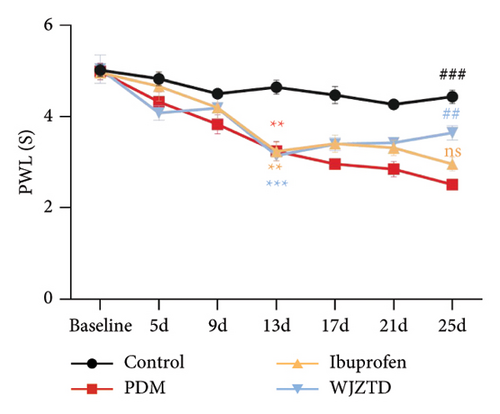
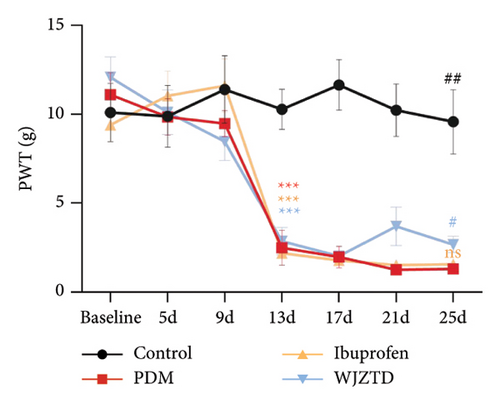
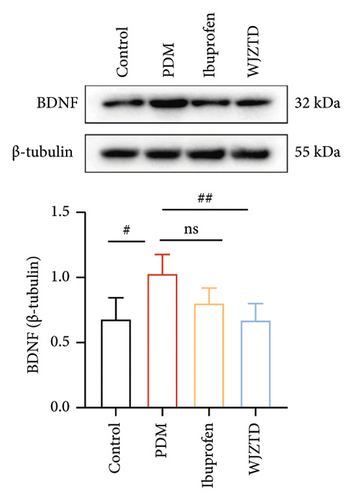
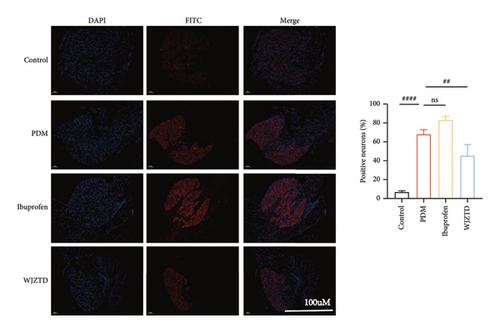
We assessed the protein levels of BDNF in DRG to see if it mediates pain sensitivity. According to our findings, the PDM group’s BDNF protein expression of DRG was substantially higher than that of the WJZTD group (p < 0.01) and the control group (p < 0.05) (Figure 2(c)). Similarly, c-Fos-positive cells are thought to be biomarkers of neuronal activation under nociceptive stimuli [33], with a significant increase in immunofluorescence staining of DRGs in the PDM group compared to control group (profen (Figure 2(d)) (p < 0.0001) and WJZTD (p < 0.001), whereas there was no significant difference in ibuprofen group. In a rat model of PDM, these behavioral data and the downregulation of c-Fos and BDNF were found to be important markers of WJZTD decrease of hyperalgesia.
3.3. WJZTD Improved the Rat Colonic Metabolomic Profiles During PDM
To further identify the mechanism and targets of WJZTD in treating PDM, we carried out a nontargeted colonic metabolomics analysis. OPLS-DA is a crucial technique for assessing the overall variations in metabolic profiles between groups as previously investigated, with R2X, R2Y, and Q2 being important predictive parameters for this analysis. We believe that when Q2 > 0.5, the closer R2Y and Q2 are to 1, the more reliable and stable the model is [34]. As illustrated in Figure 3(a), the metabolites of the four groups can be categorized into distinct regions (Q2 0.633, R2Y 0.991). A total of 1824 differential metabolites between four groups were screened in both ESI-positive and ESI-negative ion modes. Predicted bioactive chemical compounds that WZJTD may treat PDM were identified by comparing the identity of metabolites between PDM versus control groups and WJZTD versus PDM groups with a p-value less than 0.05. Fold change (FC) > 1.2 is seen as an increase among them, while FC < 0.667 is regarded as a drop. The 11 distinct bioactivities’ metabolites, such as 2S-3′-(2-hydroxy-3-methylbut-3-enyl) abyssinone II, jasmonic acid, beilschmiedic acid N, and others, were identified using this screening process (Figure 3(b)).
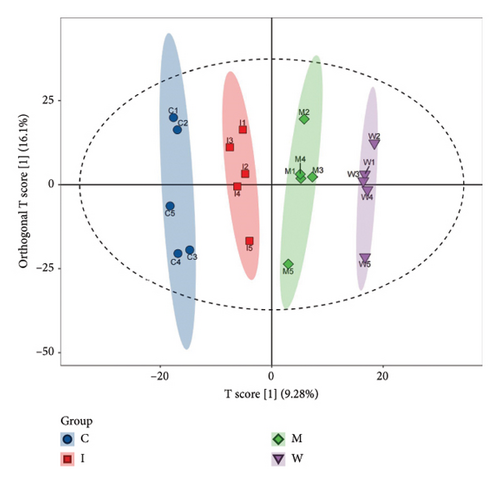
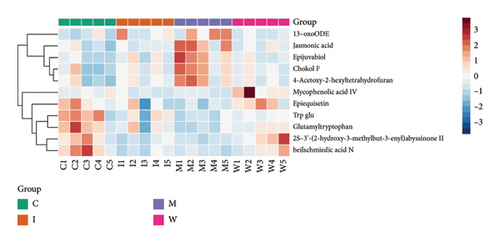
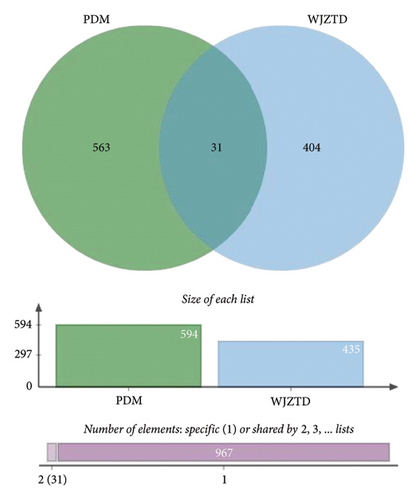
After removing targets with probability = 0, 435 targets of 11 differential metabolites were obtained from the Swiss Target Prediction and BAT-man database. At the same time, after deduplication, 594 related genes of PDM were confirmed from the OMIM and GeneCards databases. In conclusion, we identified 31 potential shared targets between WJZTD components and PDM (Figure 3(c), Table 3).
| Targets | Probability | Differential metabolites | Reference m/z | Formula | Rt (min) | Adduct type | p-value (WJZT vs. CPDM) | Fold change | UP/DOWN) |
|---|---|---|---|---|---|---|---|---|---|
| CYP1B1 | 0.106165761 | 2S-3′-(2-hydroxy-3-methylbut-3-enyl)abyssinone II | 407.18649 | C25H28O5 | 10.728 | [M−H]− | 0.01506749 | 2.168236238 | UP |
| ESR1 | 0.156149437 | 10.728 | [M−H]− | 0.01506749 | 2.168236238 | UP | |||
| PIK3CA | 0.106165761 | 10.728 | [M−H]− | 0.01506749 | 2.168236238 | UP | |||
| CYP19A1 | 0.16447208 | 10.728 | [M−H]− | 0.01506749 | 2.168236238 | UP | |||
| ESR2 | 0.156149437 | 10.728 | [M−H]− | 0.01506749 | 2.168236238 | UP | |||
| PGR | 0.106165761 | 10.728 | [M−H]− | 0.01506749 | 2.168236238 | UP | |||
| CXCL8 | 0.042894212 | Jasmonic acid | 211.13287 | C12H18O3 | 6.815 | [M+H]+ | 0.019851042 | 0.499213707 | DOWN |
| PTGS2 | 0.106542926 | Beilschmiedic acid N | 433.20209 | C27H30O5 | 10.945 | [M−H]− | 0.020137709 | 1.752201571 | UP |
| MMP9 | 0.106542926 | 10.945 | [M−H]− | 0.020137709 | 1.752201571 | UP | |||
| MTOR | 0.106542926 | 10.945 | [M−H]− | 0.020137709 | 1.752201571 | UP | |||
| SLC10A2 | 0.112041901 | Epijuvabiol | 267.19669 | C16H28O3 | 8.072 | [M−H]− | 0.022198274 | 0.488144539 | DOWN |
| CYP17A1 | 0.112041901 | 8.072 | [M−H]− | 0.022198274 | 0.488144539 | DOWN | |||
| MEN1 | 0.104671941 | Trp glu | 334.1398 | C16H19N3O5 | 4.708 | [M+H]+ | 0.029613841 | 1.284488672 | UP |
| PPARG | 0.758865242 | 4.708 | [M+H]+ | 0.029613841 | 1.284488672 | UP | |||
| OXTR | 0.104671941 | 4.708 | [M+H]+ | 0.029613841 | 1.284488672 | UP | |||
| HLA-A | 0.128898633 | 4.708 | [M+H]+ | 0.029613841 | 1.284488672 | UP | |||
| SSTR1 | 0.104671941 | 4.708 | [M+H]+ | 0.029613841 | 1.284488672 | UP | |||
| OPRM1 | 0.104671941 | 4.708 | [M+H]+ | 0.029613841 | 1.284488672 | UP | |||
| SCN9A | 0.042894212 | 4-acetoxy-2-hexyltetrahydrofuran | 213.14906 | C12H22O3 | 7.179 | [M−H]− | 0.034998296 | 0.548312024 | DOWN |
| TERT | 0.097874534 | 13-oxoODE | 295.2269 | C18H30O3 | 8.022 | [M+H]+ | 0.036436757 | 0.35762941 | DOWN |
| TNF | 0.115736675 | Mycophenolic acid IV | 387.1813 | C22H28O6 | 5.437 | [M−H]− | 0.046344393 | 4.451007647 | UP |
| BCL2 | 0.115736675 | 5.437 | [M−H]− | 0.046344393 | 4.451007647 | UP | |||
| JAK3 | 0.115736675 | 5.437 | [M−H]− | 0.046344393 | 4.451007647 | UP | |||
| MMP2 | 0.115736675 | 5.437 | [M−H]− | 0.046344393 | 4.451007647 | UP | |||
| MMP14 | 0.115736675 | 5.437 | [M−H]− | 0.046344393 | 4.451007647 | UP | |||
| INSR | 0.115736675 | 5.437 | [M−H]− | 0.046344393 | 4.451007647 | UP | |||
| AGTR1 | 0.115736675 | 5.437 | [M−H]− | 0.046344393 | 4.451007647 | UP | |||
| CASP3 | 0.115736675 | 5.437 | [M−H]− | 0.046344393 | 4.451007647 | UP | |||
| MMP1 | 0.115736675 | 5.437 | [M−H]− | 0.046344393 | 4.451007647 | UP | |||
| CCR1 | 0.115736675 | 5.437 | [M−H]− | 0.046344393 | 4.451007647 | UP | |||
| PPARA | 0.115736675 | 5.437 | [M−H]− | 0.046344393 | 4.451007647 | UP | |||
3.4. ESR1 Plays a Core Role in WJZTD Treatment of PDM
To identify the most significant target, we imported 31 targets into the STRING database and found that ESR1 exhibited the highest values for DC, BC, CC, and EC according to the CytoNCA algorithm. We believe this target is likely to play a crucial role in the molecular mechanism of WZITD therapy for pain management in PDM (Figures 4(a), 4(b)).
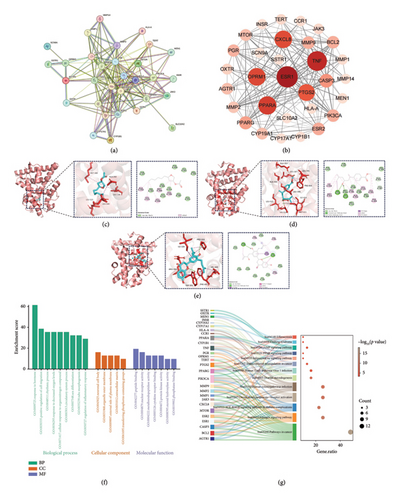
Meanwhile, the molecular docking method also produced similar results. Table 4 shows that the binding energies of the three metabolites with ESR1 are all below −4.25 kcal/mol, indicating a strong affinity. Epijuvabiol and 2S-3′-(2-hydroxy-3-methylbut-3-enyl) abyssinone II have binding energies under −7 kcal/mol, reflecting an even stronger affinity for ESR1 [35]. As shown in Figures 4(c), 4(d), 4(e), ESR1 binds to compounds through hydrogen bonding and hydrophobic interactions.
| Compound | CID | Target gene | PDB ID | Protein pocket coordinates | Grid box size | Protein affinity (kcal/mol) |
|---|---|---|---|---|---|---|
| 2S-3′-(2-hydroxy-3-methylbut-3-enyl)abyssinone II | 74,336,650 | ESR1 | 1 × 7R | X = 22.367 | X = 48 | −8.3 |
| Epijuvabiol | 14,396,628 | Y = 27.669 | Y = 46 | −7.5 | ||
| 4-acetoxy-2-hexyltetrahydrofuran | 61,457 | Z = 13.248 | Z = 58 | −6 |
To further confirm our findings, we conducted GO and KEGG analyses on these 31 common targets (Figures 4(f), 4(g)). As previously discussed, these targets are significantly involved in the biological process related to hormone response, with the estrogen signaling pathway being particularly important. Additionally, it is noteworthy that the biological annotations corresponding to the minimum p-value in cellular component analysis indicate neuron cell bodies. This suggests that WJZTD may help reduce pain sensitivity in PDM treatment.
3.5. BDNF Deletion Impaired the Activation of ERα and Its Downstream Pain Sensitization-Related Signaling Molecules
Based on the previous studies, ERα (ESR1) expressed in the spinal cord can regulate the processing of pain sensations [36]. Activation of ER can counteract NGF deprivation-induced cell death in DRG neurons [37], while post-siRNA treatment of ERα in the spinal cord can significantly reduce the expression of ERα and BDNF mRNA and protein [23]. To further explore the roles of ERα and BDNF in a rat model of PDM with pain sensitization, we conducted in vitro experiments using a highly differentiated PC12 cell line with neuroendocrine function [38].
After culturing cells with BDNF (50 ng/mL) for 12 h, the PC-12 cells were then incubated with PPT (10 nM) to activate ERα and MPP (5 μM) to antagonize it. As shown in Figures 5(a), 5(b), 5(c), 5(d), 5(e), compared with the control group, treatment with BDNF significantly increased the expression of ERα (p < 0.05), BDNF (p < 0.05), c-Fos (p < 0.05), and neuronal nitric oxide synthase (nNOS) (p < 0.01) proteins. Additionally, PPT treatment further enhanced these changes (ERα: p < 0.05, BDNF: p < 0.05, c-Fos: p < 0.05, nNOS: p < 0.01), while MPP treatment did not reverse them.
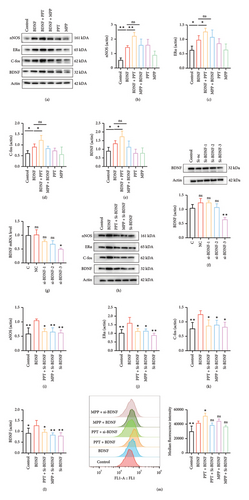
Then, PC-12 cells were subjected to transfection with three different siRNAs (siRNA1, siRNA2, siRNA3) to knock down BDNF expression, and the transfection efficiency was analyzed by qPCR and Western blotting (Figures 5(f), 5(g)). The results showed that downregulation of BDNF significantly reduced the expression of proteins c-Fos (p < 0.05) and nNOS (p < 0.01) related to neuronal excitability [39], while treatment with PPT and MPP did not upregulate this change (Figures 5(h), 5(i), 5(j), 5(k), 5(l)).
Neuronal membrane depolarization results in neuronal excitation. To further investigate the involvement of the ERα and BDNF in pain sensitization, we measured the membrane potential of cells under various culture treatments using flow cytometry, as shown in Figure 5(m). Consistent with our previous findings, cells cultured with BDNF and PPT exhibited significantly enhanced excitability. In contrast, the knockdown of BDNF reversed the depolarization of the cells.
These findings indicate that ERα plays a role in pain sensitization, with BDNF being the key protein involved in this process.
3.6. WJZTD Decreased Hyperexcitability in PC12 Cells by Interacting Directly Through ERα/BDNF
Finally, we aimed to investigate whether WJZTD reduces neuronal excitability and pain sensitivity through the ERα/BDNF pathway, thereby alleviating dysmenorrhea. PC12 cells were cultured with 10% WJZTD-drug serum under various conditions, as illustrated in Figure 6(a). The number of c-Fos-positive cells was used as a measure of cell excitability.
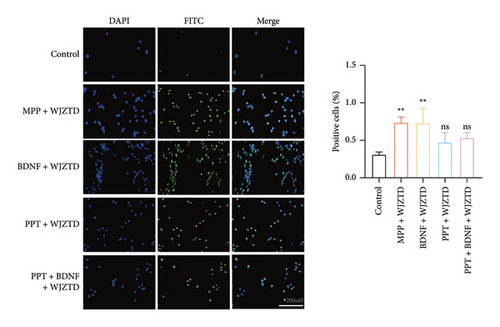
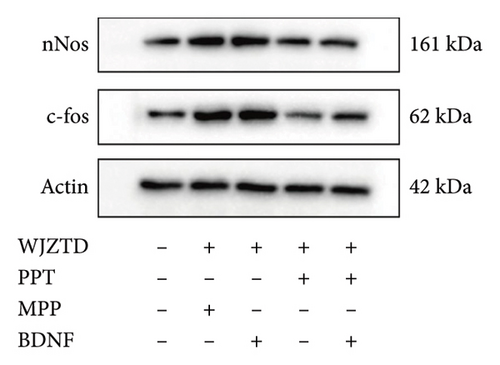
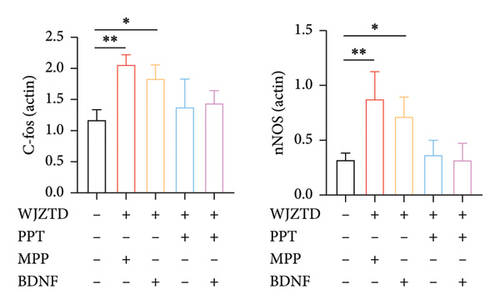
Our results showed that, compared to the control group, the WJZTD-drug serum significantly reduced the expression of c-Fos-positive cells when ERα was stimulated by PPT. However, there was no significant effect when the ERα was antagonized (p < 0.01). Additionally, the significant increase in the number of positive cells induced by BDNF culture was not influenced by the WJZTD-drug serum (p < 0.01). However, this situation changed after the activation of the ERα.
As shown in Figures 6(b), 6(c), under the same treatment conditions, when the ERα was antagonized, WJZTD could not reduce the levels of c-Fos and nNOS proteins in PC12 cells. However, when the ERα is activated, the expression of these proteins significantly decreases, showing no notable difference compared to the control group. This is consistent with the previous experimental results and indicates that WJZTD can reduce neural excitability through ERα/BDNF, and its target is ERα.
4. Discussion
Dysmenorrhea can be classified into two categories, PDM is not brought on by pelvic organic disorders, while secondary dysmenorrhea can be produced by pathological factors of the uterus and pelvic cavity, such as endometriosis and pelvic inflammatory disease [40]. Despite being a common gynecological condition, PDM is frequently misdiagnosed, untreated, or treated incorrectly [41, 42].
Pain sensitivity and inflammatory response brought on by PG release are parts of the recognized pathophysiology of PDM [4]. NSAIDs are recommended as the first-line of clinical treatment for PDM because they inhibit enzymes that release PGs and reduce pain [43]. Clinically, oral contraceptives (OCs) are also efficient at preventing ovulation and thinning the endometrium. However, it is worth noting that 15% of women suffer from dysmenorrhea either do not respond to PG inhibitors or may experience intolerance to them [44]. Additionally, the risk of severe gastrointestinal events linked to NSAIDs should be taken seriously [45], as well as the connection between OCs and the risk of venous thromboembolism [46].
Research indicates that various experimental harmful regulatory stimuli on the skin, muscles, or internal organs of human volunteers can result in hyperalgesia, while electrophysiological or imaging techniques show secondary changes in brain activity and abnormal increases in neuronal excitability [47, 48]. Women who regularly suffer from menstrual pain also tend to have a lower pain threshold [10, 12, 49]. Recently, it has been proven that pain sensitivity is linked to the adoption of analgesic interventions, and lowering the pain threshold can alleviate refractory pain [50, 51]. BDNF, a member of the growth factor neurotrophic factor family, is synthesized by DRG neurons. It plays a crucial role in the survival of nerve cells and is involved in pain perception and sensitization. A series of trauma- and drug-induced persistent pain models have investigated that an increase in BDNF expression is associated with central sensitization and the development of pain [52, 53].
In traditional Chinese medicine, blood stasis is usually considered a crucial pathological factor in PDM, and promoting blood circulation to resolve stasis is regarded as a treatment principle [54]. Additionally, the classic theory in traditional Chinese medicine states that “all pains and sores are attributed to the heart,” suggesting that soothing the nerves and tranquilizing the mind can help reduce pain based on this belief. WJZTD is an effective clinical decoction used to treat dysmenorrhea. It is composed of several ingredients, including Cinnamomum cassia Presl [Lauraceae; CINNAMOMI RAMULUS], Cervus elaphus Linnaeus [Cervidae; CERVICORNU], Cuscuta chinensis Lam [Convolvulaceae; CUSCUTAE SEMEN], Dioscorea opposita Tunb. [Dioscoreaceae; DIOSCOREAE RHIZOMA], Pueraria lobata (Willd.) Ohwi [Fabaceae; PUERARIAE LOBATAE RADIX], Paeonia suffruticosa Andr. [Ranunculaceae; MOUTAN CORTEX], Foeniculum vulgare Mill. [Apiaceae; FOENICULI FRUCTUS], Salvia miltiorrhiza Bge. [Lamiaceae; SAL VIAE MILTIORRHIZAE RADIX ET RHIZOMA], Artemisia argyi Levl. Et Vant. [Asteraceae; ARTEMISIAE ARGYi FOLIUM], and et al., which have the effects of warming yang and promoting blood circulation, calming the mind, and soothing the spirit. It has shown good efficacy in relieving dysmenorrhea in clinical applications [55]. Previous studies have confirmed that WJZTD can alleviate pain in dysmenorrhea model rats. This study explores the analgesic mechanisms of WJZTD in dysmenorrhea, specifically focusing on its role in reducing pain sensitivity by regulating the ERα/BDNF pathway.
Many studies have explored methods for establishing mouse models of recurrent PDM, yet there is no widely accepted approach [31, 56]. In this study, we induced dysmenorrhea once every 4 days, corresponding to the duration of one estrous cycle in rats. Through behavioral observation, we established a chronic dysmenorrhea model by identifying dysmenorrhea caused by three consecutive estrous cycles. Similar to clinical treatments, we set the duration of our medication intervention to three estrous cycles. Behavioral tests and the elevated expression of BDNF and c-Fos in DRG tissues indicated that, like chronic pain associated with inflammatory bowel disease [57] and rheumatoid arthritis [58], pain sensitization plays a role in the pathogenesis of PDM. Furthermore, WJZTD can significantly reverse these changes.
Integrated pharmacology and untargeted metabolomics are disciplines and methods for screening bioactive compounds and exploring their interactions with gene targets [59, 60]. WJZTD is a traditional Chinese medicine compound made up of multiple medicinal herbs, characterized by its multiple components, targets, and pathways. To explore its potential targets and mechanisms for treating PDM, we screened 11 metabolites with possible biological activity, including both primary and secondary metabolites, using untargeted metabolism of rat colon tissue. Based on network pharmacology analysis, 31 targets of WJZTD therapy for PDM have been identified, corresponding to 8 differential metabolites. These include the flavonoid metabolite 2S-3′-(2-hydroxy-3-methylbut-3-enyl) abyssinone II, which exhibits antioxidant effects [61]; jasmonic acid, a derivative of fatty acids and a type of lipid hormone; terpenoid compounds such as beilschmiedic acid N, epijuvabiol, and mycophenolic acid IV; ester compounds like 4-acetoxy-2-hexyltetrahydrofuran, and Trp Glu, a dipeptide composed of tryptophan (Trp) and glutamic acid (Glu). Additionally, the primary metabolite 13-Ox-OODE, isolated from Artemisia argyi, has been proven to exhibit multiple biological activities in human colon epithelial cells [62].
According to the CytoNCA algorithm and molecular docking analysis, ESR1 has been identified as a potential core therapeutic target, which has been further confirmed by GO and KEGG analyses. In addition, the ESR1 gene is a common target of 2S-3′-(2-hydroxy-3-methylbut-3-enyl) abyssinone II, epijuvabiol, and 4-acetoxy-2-hexyltetrahydrofuran. Coincidentally, the compound 2S-3′-(2-hydroxy-3-methylbut-3-enyl) abyssinone II, which has the lowest docking binding energy with ESR1 molecules, has the smallest p value among the 8 differential metabolites in the WJZT group versus PDM group.
Estrogen, the main sex hormone in women, likely plays a significant role in pain management. It is associated with several chronic pain disorders, including dysmenorrhea, migraines, and fibromyalgia, which are more prevalent in women than in men. This gender difference in pain perception typically begins during adolescence and gradually decreases after menopause [6]. Research indicates that estrogen has a complex role in brain function by activating estrogen receptors (ERs) found in glial and neuronal cells [63, 64]. BDNF is present in neurons of the adult rat DRG and serves as a regulatory factor at the first synapse in the spinal dorsal horn pain transmission pathway [65]. Studies confirm that estrogen influences the expression of BDNF mRNA in hippocampal pyramidal cells in a dose-dependent manner, and BDNF colocalizes with ERα [66]. Additionally, the degenerative changes in estrogen receptors of neural stem cells can lead to a decrease in the expression of neurotrophic factor family proteins (NGF, BDNF, and Trk) [67].
The differentiated PC12 cell line exhibits neurogenic characteristics, such as neuronal morphology and neuroendocrine properties, making it the preferred model for neurobiological research [68]. This study confirmed through in vitro validation that ERα may increase cell excitability via BDNF. Additionally, WJZTD can reduce the secretion of BDNF, c-Fos, and nNOS in PC12 cells by interacting with the ERα. This action mitigates pain sensitization and helps in treating PDM.
Collectively, this study confirms that WJZTD downregulates BDNF protein expression by targeting ERα, reduces neuronal excitability, alleviates pain sensitivity, and improves PDM. Correspondingly, while NSAIDs, such as ibuprofen, can relieve dysmenorrhea, they do not reduce pain sensitivity.
Limitations of this study include the following: Firstly, the method of inducing dysmenorrhea in dysmenorrhea models is widely accepted in many experiments. However, the modeling time used in this study is still preliminary and requires further experimental validation to evaluate its stability. Second, the primary and secondary metabolites of WJZTD need to be more clearly distinguished, and it is essential to explore the medicinal chemical components they belong to to clarify the bioactive substances. Third, the mechanism of action of the ERα/BDNF pathway requires further in vivo validation, or the biological role of this pathway could be clarified by extracting primary DRG cells. Finally, since WJZTD has characteristics of multiple components, targets, and pathways, the mechanisms underlying its effectiveness in treating dysmenorrhea still need further investigation.
5. Conclusion
This study indicates that WJZTD improves pain sensitization therapy for dysmenorrhea, potentially through the ERα/BDNF pathway. WJZTD significantly enhances abnormal levels of PG and pain-sensitive behaviors associated with PDM in rats. It also increases the expression of BDNF and c-Fos in the DRG, both of which are involved in pain sensitization. Moreover, untargeted colonic metabolomics and integrated pharmacological analyses suggest that WJZTD may target ERα. Through in vitro validation, we investigated the interaction between ERα and BDNF, as well as their roles in pain sensitization. This study provides a new theoretical framework for using WJZTD in the treatment of PDM.
Nomenclature
-
- WJZTD
-
- Wen-Jing-Zhi-Tong decoction
-
- PDM
-
- Primary dysmenorrhea
-
- PGs
-
- Prostaglandins
-
- DRG
-
- Dorsal root ganglion
-
- BDNF
-
- Brain-derived neurotrophic factor
-
- ERα
-
- Estrogen receptor α
-
- AA
-
- Arachidonic acid
-
- COX-2
-
- Clooxygenase-2
-
- PWL
-
- Paw withdrawal latency
-
- PWTs
-
- Paw withdrawal thresholds
-
- HE
-
- Hematoxylin–eosin
-
- OPLS-DA
-
- Orthogonal partial least squares discriminant analysis
-
- PPI
-
- Protein–protein interaction
-
- DC
-
- Degree centrality
-
- BC
-
- Betweenness centrality
-
- CC
-
- Closeness centrality
-
- EC
-
- Eigenvector centrality
-
- GO
-
- Gene ontology
-
- KEGG
-
- Kyoto Encyclopedia of Genes and Genomes
-
- CCK8
-
- Cell counting kit-8 assay
-
- ESR1
-
- Estrogen receptor 1
-
- NSAIDs
-
- Nonsteroidal anti-inflammatory drugs
-
- OCs
-
- Oral contraceptives
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Yajie Qin: writing – review & editing, writing—original draft, and data curation.
Xiaotian Yang: writing – original draft and data curation.
Xingran Tang: investigation.
Huijin Zhao: methodology.
Huifang Zhou: supervision.
Yajie Qin and Xiaotian Yang contributed equally to this work.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81973898) and the Postgraduate Scientific Research Innovation Plan of Jiangsu Province in 2024 (Grant Nos: KYCX24_2217 and KYCX24_2222).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
All the data used and analyzed during the current study are available from the corresponding author upon reasonable request.




