Linezolid-Induced Lactic Acidosis: Avoiding Concomitant Use With Metformin and Monitoring Linezolid Trough Concentration
Abstract
Objective: Serum lactic acidosis has been reported as a serious adverse effect associated with linezolid. This study aims to explore the risk factors of linezolid-induced lactic acidosis.
Methods: Patients admitted to a 3600-bed university hospital, who received linezolid treatment and had at least one steady-state concentration of linezolid, were retrospectively reviewed to analyze the incidence of linezolid-induced lactic acidosis. Meanwhile, univariate and multivariate logistic regression analyses were conducted to determine the risk factors of lactic acidosis.
Results: A total of 95 adult patients were included in the study. 18.95% (18 out of 95) of patients developed lactic acidosis during linezolid treatment. Importantly, patients who concurrently used linezolid and metformin had a high risk of developing lactic acidosis (90.9%, 10 out of 11). After excluding these patients from the original database, 9.52% (8 out of the 84) of the patients developed lactic acidosis. In the population not receiving concurrent metformin treatment, univariate analysis showed that patients who developed lactic acidosis had higher linezolid Cmin and serum creatinine levels or lower creatinine clearance, and multivariate analysis showed that Cmin (OR: 1.114; 95% CI: 1.012–1.226; p = 0.027) was an independent risk factor for lactic acidosis.
Conclusion: The concurrent use of linezolid and metformin raises the risk of lactic acidosis. Therapeutic drug monitoring of linezolid based on Cmin is recommended for decreasing the risk of lactic acidosis during linezolid treatment.
1. Introduction
Linezolid is a member of the oxazolidinone class of antibiotics [1]. It is primarily used to treat various Gram-positive bacterial infections, including vancomycin-resistant enterococci (VRE) and methicillin-resistant Staphylococcus aureus (MRSA) [2]. It is also effective against Nocardia species, multidrug-resistant tuberculosis, and other mycobacterial infections [3]. Linezolid has a bioavailability close to 100%, and it exhibits strong tissue penetration with relatively stable overall tissue distribution. Moreover, approximately 65% of the drug undergoes elimination through the nonrenal pathway, while approximately 30% is eliminated via the renal pathway [4, 5].
Hematological toxicity is a major concern of linezolid treatment in clinic practices, including thrombocytopenia, anemia, and leukopenia (2.4%–64.7%) [6–8]. Recently, a large multicentre cohort study evaluated linezolid toxicity [9]. In this study, the most common linezolid toxicities experienced were thrombocytopenia, anemia, and intolerance such as nausea and vomiting, and other serious side effects such as lactic acidosis and peripheral neuropathy were also identified. Moreover, the benefit of linezolid TDM in minimizing toxicity has been proposed in relation to hematological toxicities [9]. However, the linezolid concentration thresholds associated with severe metabolic and neurological toxicities are less evident. In recent years, there has been an increasing concern about linezolid-induced lactic acidosis [10]. Lactic acidosis, characterized by low pH and the accumulation of lactate in tissues and blood [11], can also be a potentially lethal side effect of linezolid [12]. Reports on linezolid-induced lactic acidosis are mainly case studies [1, 13–15]. In addition, there is a cohort study which reported an incidence of 6.8% for linezolid-related lactic acidosis [13]. Although the incidence is relatively low, occurring of lactic acidosis is usually life-threatening. The toxic mechanism of linezolid-induced lactic acidosis is currently believed to be associated with inhibition of mitochondrial protein synthesis and genetic polymorphism. To the best of our knowledge, there is limited research on the analysis of risk factors associated with linezolid-induced lactic acidosis. Im JH et al. found that a longer duration of linezolid use (> 6 weeks) was a risk factor for lactic acidosis [16]. In this study, we aim to investigate the incidence of lactic acidosis induced by linezolid in hospitalized patients, especially on the drugs concurrently used with linezolid which may further injure mitochondria.
2. Methods
2.1. Patients and Ethics
This retrospective observational study aims to investigate the incidence and risk factors of linezolid-related lactic acidosis in adult hospitalized patients who received linezolid treatment at the First Affiliated Hospital of Wenzhou Medical University from July 2019 to December 2021. The inclusion criteria were as follows: (1) ≥ 18 years, (2) received intravenous and/or oral linezolid, and (3) at least one steady-state concentration of linezolid was collected. The exclusion criteria were as follows: (1) received renal replacement therapy or extracorporeal membrane oxygenation; (2) died within 24 h after being treated with linezolid; and (3) developed hypoxia, sepsis, septic shock, acute blood loss, or diabetic ketoacidosis.
The patient data including demographic characteristics (gender, age, height, and weight), laboratory parameters (baseline hemoglobin, total bilirubin, albumin (ALB), linezolid concentration, lactate levels pre- and postlinezolid administration, alanine aminotransferase, and aspartate aminotransferase), comorbidities, and concomitant medications were collected from the electric hospital database.
This study was designed in accordance with legal requirements and the Helsinki Declaration and was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University, China ([2022] 043).
2.2. Measurement of Linezolid Plasma Concentrations
Linezolid plasma samples were collected at a steady state (Css, attained after at least five continuous doses). Cmax was obtained 30 min after the end of intravenous infusion or 2 h after oral administration, and Cmin was obtained 30 min prior to the next dose. Plasma concentration quantification was carried out using a validated high-performance liquid chromatography–tandem mass spectrometry (LC–MS/MS) assay. The intra- and interday assay coefficients of variation were less than 10%, and the lower limit of quantification was 0.1 mg/L.
2.3. Definition of Lactic Acidosis
“Definite” lactic acidosis was characterized by a serum pH below 7.25 and a serum lactate level exceeding 4 mmol/L. “Probable” lactic acidosis was defined when metabolic acidosis was confirmed, but serum lactate levels were not identified, or when elevated serum lactate levels were confirmed, but serum pH values were not identified [16]. Lactic acidosis induced by linezolid was determined in accordance with the probable grade of the Naranjo criteria for adverse drug reactions [17].
2.4. Statistical Analysis
Data processing and analysis were performed using SPSS 26.0 software. The assessment of continuous variables for normality or non-normality distribution was conducted using the Kolmogorov–Smirnov test. Non-normally distributed variables were expressed as the median and interquartile range (IQR) and compared by the Mann–Whitney U-test. Normally distributed variables were presented as mean ± standard deviation (SD) and compared by Student′s t-test. The categorical variables were analyzed by a chi-square test. Univariate analyses were performed separately for each of the risk factors in patients who developed linezolid-related lactic acidosis and those who did not (absence = 0 or presence = 1), and the ones with a p < 0.1, who underwent a pairwise correlation comparison to avoid confounding and collinearity of variables. For covariates with confounding or collinearity, the one with the smallest p value was included in further multivariate logistic regression analysis to determine the independent risk factors for linezolid-related lactic acidosis [18]. Receiver operating characteristic (ROC) curves were generated in order to evaluate the predictive capability of the indicators encompassed within the multivariate logistic regression model. All statistical tests were two-tailed, and a p value < 0.05 was considered statistically significant.
3. Results
The demographic and clinical characteristics of the patients are presented in Table 1. A total of 95 adult patients (58 males and 37 females) with a mean age ± SD of 63.78 ± 12.01 years were included. The plasma concentration database consisted of 94 Cmin measurements with a median (IQR) value of 6.62 mg/L (3.48–9.85 mg/L), and 22 Cmax measurements with a mean ± SD value of 14.82 ± 8.59 mg/L.
| Characteristic | Value |
|---|---|
| No. of patients | 95 |
| Age (years), median (IQR) | 66.00 (24.00–87.00) |
| Gender (male/female) | 58/37 |
| Height (cm), mean ± SD | 164.04 ± 8.14 |
| Weight (kg), mean ± SD | 62.82 ± 12.39 |
| Laboratory data at baseline | |
| T-bil (μmol/L), median (IQR) | 9.00 (5.00–55.00) |
| AST (U/L), median (IQR) | 24.00 (9.00–273.00) |
| ALT (U/L), median (IQR) | 20.00 (3.00–243.00) |
| ALB (g/L), mean ± SD | 32.76 ± 6.14 |
| SCr (μmol/L), median (IQR) | 78.00 (38.00–887.00) |
| CrCL (mL/min), mean ± SD | 69.11 ± 40.66 |
| HGB (g/L), mean ± SD | 100.24 ± 22.72 |
| PLT (× 109/L), median (IQR) | 251.00 (66.00–744.00) |
| Hypertension (positive/negative) | 54/41 |
| Diabetes (positive/negative) | 66/29 |
| Diseased kidney (positive/negative) | 45/50 |
| Liver dysfunction (positive/negative) | 38/57 |
| Cardiac insufficiency (positive/negative) | 33/62 |
| Metformin (positive/negative) | 11/84 |
| Duration of linezolid treatment, median (IQR) | 8.00 (2.00–47.00) |
| Days of occurrence of lactic acidosis after initiating linezolid treatment, median (IQR) | 12.00 (4.00–32.00) |
| Lactate value before administration (mmol/L), mean ± SD | 2.08 ± 0.66 |
| Lactate value after administration (mmol/L), mean ± SD | 2.77 ± 0.99 |
| Linezolid plasma concentration | |
| Cmax (mg/L), mean ± SD | 14.82 ± 8.59 |
| Cmin (mg/L), median (IQR) | 6.76 (0.20–32.93) |
| Type of infection | |
| Pneumonia, n (%) | 20 (21.05) |
| Bacteremia, n (%) | 1 (1.05) |
| Skin and soft tissue infection, n (%) | 58 (61.05) |
| Urinary tract infection, n (%) | 4 (4.21) |
| Bloodstream infection, n (%) | 6 (6.32) |
| Abdominal infection, n (%) | 3 (3.16) |
| Intracranial infection, n (%) | 3 (3.16) |
| Other infections, n (%) | 17 (17.89) |
- Note: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CrCL, creatinine clearance, calculated by CKD-EPI formula; Cmax, maximum concentration; Cmin, minimum concentration; SCr, serum creatinine; T-bil, total bilirubin.
18.95% (18 out of 95) of the patients developed linezolid-related lactic acidosis and were confirmed by the Naranjo criteria. In addition, 14 of the 18 cases of lactic acidosis were classified as definite, while the remaining 4 cases were classified as probable. The median time of occurrence of lactic acidosis after linezolid treatment was 12.00 days (Table 1). It is worth noting that patients who concurrently used linezolid and metformin had a high risk of developing lactic acidosis (90.9%, 10 out of 11). In addition, females and high baseline platelet (PLT) count were also considered as significant risk factors for linezolid-related lactic acidosis according to the univariate analysis (Table 2). To avoid confounding and collinearity of variables, we conducted Spearman correlation analysis for the covariates with p < 0.1 in Table 2. PLT count was associated with metformin (p = 0.039), and metformin with the smaller p value was selected for further analysis. In the final multivariate regression model, female, ALB, and metformin were included as candidate variables. The multivariate stepwise logistic regression analysis showed that females (OR: 7.144; 95% CI: 1.330–38.375; p = 0.022) and concurrent use of metformin (OR: 150.531; 95% CI: 13.050–1736.323; p < 0.001) were independent risk factors for linezolid-related lactic acidosis (Table 3).
| Lactic acidosis | |||
|---|---|---|---|
| None (n = 77) | Yes (n = 18) | p value | |
| Age (years), median (IQR) | 67.00 (24.00–87.00) | 63.50 (49.00–77.00) | 0.179c |
| Gender (female, n (%) | 26 (33.77) | 11 (61.11) | 0.032b |
| Height (cm), mean ± SD | 164.26 ± 7.94 | 163.11 ± 9.15 | 0.593a |
| Weight (kg), mean ± SD | 62.85 ± 11.95 | 62.69 ± 14.49 | 0.962a |
| Duration of linezolid treatment, median (IQR) | 8.00 (2.00–47.00) | 9.50 (4.00–28.00) | 0.466c |
| Lactate value before administration, mean ± SD | 2.08 ± 0.67 | 2.08 ± 0.65 | 0.969a |
| Lactate value after administration, mean ± SD | 2.45 ± 0.73 | 4.03 ± 0.81 | ≤ 0.001a |
| Cmax (mg/L), mean ± SD | 15.17 ± 8.87 | 14.00 ± 8.63 | 0.789a |
| Cmin (mg/L), median (IQR) | 6.76 (0.20–29.90) | 6.70 (0.70–32.93) | 0.643c |
| HGB (g/L), mean ± SD | 99.62 ± 22.22 | 102.89 ± 25.29 | 0.586a |
| PLT (× 109/L), median (IQR) | 242.00 (66.00–612.00) | 317.00 (150.00–744.00) | 0.008c |
| T-bil (μmol/L), median (IQR) | 9.00 (5.00–55.00) | 7.00 (6.00–17.00) | 0.731c |
| AST (U/L), median (IQR) | 25.00 (12.00–273.00) | 22.50 (9.00–41.00) | 0.441c |
| ALT (U/L), median (IQR) | 20.00 (3.00–243.00) | 20.00 (4.00–45.00) | 0.672c |
| ALB (g/L), mean ± SD | 32.34 ± 6.52 | 34.53 ± 3.83 | 0.067a |
| SCr (μmol/L), median (IQR) | 78.00 (38.00–887.00) | 78.50 (39.00–794.00) | 0.857c |
| CrCL (mL/min), mean ± SD | 67.96 ± 39.56 | 74.06 ± 45.94 | 0.569a |
| Hypertension (yes), n (%) | 43 (55.84) | 11 (61.11) | 0.685b |
| Diabetes (yes), n (%) | 52 (67.53) | 14 (77.78) | 0.395b |
| Diseased kidney (yes), n (%) | 37 (48.05) | 8 (44.44) | 0.783b |
| Liver dysfunction (yes), n (%) | 30 (38.96) | 8 (44.44) | 0.669b |
| Cardiac insufficiency (yes), n (%) | 26 (33.77) | 7 (38.89) | 0.681b |
| Metformin (yes), n (%) | 1 (1.30) | 10 (55.56) | ≤ 0.001b |
- Note: ALT, alanine aminotransferase; ALB, albumin; AST, aspartate aminotransferase; Cmax, maximum concentration; Cmin, minimum concentration; CrCL, creatinine clearance; SCr, serum creatinine; T-bil, total bilirubin.
- aStudent’s t-test.
- bChi-square test.
- cMann–Whitney test.
| Risk factor | Multivariate logistic regression analysis | ||
|---|---|---|---|
| OR | 95% CI | p value | |
| Female | 7.144 | 1.330–38.375 | 0.022 |
| ALB | 1.069 | 0.952–1.201 | 0.259 |
| Metformin | 150.531 | 13.050–1736.323 | ≤ 0.001 |
| Characteristic | Value |
|---|---|
| No. of patients | 84 |
| Age (years), median (IQR) | 66.50 (24.00–87.00) |
| Gender (male/female) | 52/32 |
| Height (cm), mean ± SD | 163.71 ± 7.98 |
| Weight (kg), mean ± SD | 61.94 ± 12.03 |
| Laboratory data at baseline | |
| T-bil (μmol/L), median (IQR) | 8.50 (5.00–55.00) |
| AST (U/L), median (IQR) | 24.50 (12.00–273.00) |
| ALT (U/L), median (IQR) | 19.00 (3.00–243.00) |
| ALB (g/L), mean ± SD | 32.50 ± 6.46 |
| SCr (μmol/L), median (IQR) | 82.50 (38.00–887.00) |
| CrCL (mL/min), mean ± SD | 64.66 ± 40.24 |
| HGB (g/L), mean ± SD | 98.26 ± 22.80 |
| PLT (× 109/L), mean ± SD | 254.90 ± 108.24 |
| Hypertension (positive/negative) | 46/38 |
| Diabetes (positive/negative) | 55/29 |
| Diseased kidney (positive/negative) | 42/42 |
| Liver dysfunction (positive/negative) | 33/51 |
| Cardiac insufficiency (positive/negative) | 30/54 |
| Duration of linezolid treatment, median (IQR) | 8.00 (2.00–47.00) |
| Lactate value before administration, median (IQR) | 2.00 (0.80–3.60) |
| Lactate value after administration, median (IQR) | 2.60 (0.70–5.80) |
| Linezolid plasma concentration | |
| Cmax (mg/L), mean ± SD | 16.38 ± 8.94 |
| Cmin (mg/L), median (IQR) | 6.87 (0.20–32.93) |
- Note: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cmax, maximum concentration; Cmin, minimum concentration; CrCL, creatinine clearance; SCr, serum creatinine; T-bil, total bilirubin.
| Lactic acidosis | |||
|---|---|---|---|
| None (n = 76) | Yes (n = 8) | p value | |
| Age (years), median (IQR) | 67.00 (24.00–87.00) | 64.00 (49.00–77.00) | 0.360c |
| Gender (female), n (%) | 26 (34.21) | 6 (75.00) | 0.061b |
| Height (cm), mean ± SD | 164.14 ± 7.92 | 159.63 ± 7.86 | 0.128a |
| Weight (kg), mean ± SD | 62.73 ± 11.99 | 54.44 ± 10.22 | 0.063a |
| Duration of linezolid treatment, median (IQR) | 8.00 (2.00–47.00) | 7.50 (4.00–28.00) | 0.795c |
| Lactate value before administration, median (IQR) | 2.00 (0.80–3.60) | 1.95 (1.20–3.50) | 0.703c |
| Lactate value after administration, median (IQR) | 2.45 (0.70–4.00) | 4.15 (3.20–5.80) | ≤ 0.001c |
| Cmax (mg/L), mean ± SD | 15.17 ± 8.87 | 24.85 ± 3.32 | 0.158a |
| Cmin (mg/L), median (IQR) | 6.62 (0.20–29.90) | 11.82 (4.47–32.93) | 0.027c |
| HGB (g/L), mean ± SD | 99.38 ± 22.26 | 87.63 ± 26.67 | 0.167a |
| PLT (× 109/L), mean ± SD | 251.33 ± 112.09 | 288.88 ± 53.34 | 0.354a |
| T-bil (μmol/L), median (IQR) | 9.00 (5.00–55.00) | 7.00 (7.00–16.00) | 0.516c |
| AST (U/L), median (IQR) | 24.50 (12.00–273.00) | 23.50 (16.00–31.00) | 0.766c |
| ALT (U/L), median (IQR) | 20.00 (3.00–243.00) | 15.50 (4.00–39.00) | 0.200c |
| ALB (g/L), mean ± SD | 32.28 ± 6.54 | 34.59 ± 5.57 | 0.341a |
| SCr (μmol/L), median (IQR) | 78.00 (38.00–887.00) | 219.00 (58.00–794.00) | 0.029c |
| CrCL (mL/min), mean ± SD | 67.66 ± 39.74 | 36.18 ± 35.43 | 0.034a |
| Hypertension (yes), n (%) | 42 (55.26) | 4 (50.00) | 1.000b |
| Diabetes (yes), n (%) | 51 (67.11) | 4 (50.00) | 0.564b |
| Diseased kidney (yes), n (%) | 37 (48.68) | 5 (62.50) | 0.710b |
| Liver dysfunction (yes), n (%) | 30 (39.47) | 3 (37.50) | 1.000b |
| Cardiac insufficiency (yes), n (%) | 26 (34.21) | 4 (50.00) | 0.618b |
- Note: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cmax, maximum concentration; Cmin, minimum concentration; CrCL, creatinine clearance, SCr, serum creatinine; T-bil, total bilirubin.
- aStudent’s t-test.
- bChi-square test.
- cMann–Whitney test.
| Risk factor | Multivariate logistic regression analysis | ||
|---|---|---|---|
| OR | 95% CI | p value | |
| Cmin (mg/L) | 1.114 | 1.012–1.226 | 0.027 |
The ROC curve (Figure 1) was constructed using Cmin as the factor, and the area under the ROC curve (AUC) for Cmin was 0.755 (95% CI: 0.580–0.930; p = 0.027), the maximum Youden index for the combined predictive factors was 0.479, and the optimal cutoff value on the ROC curve was 7.455 mg/L (Table 7).
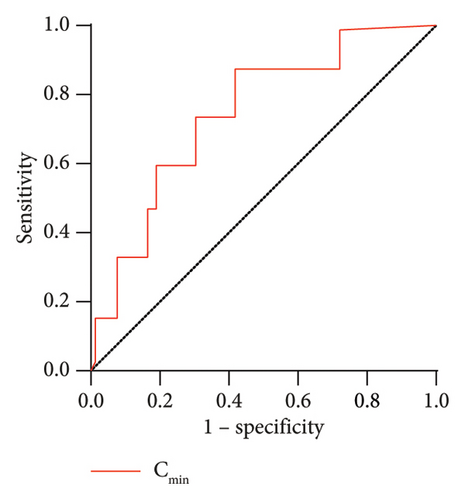
| Variance | Cutoff | Sensitivity (%) | Specificity (%) | Youden index | Area under the ROC curve (95% CI) | p value |
|---|---|---|---|---|---|---|
| Cmin (mg/L) | 7.455 | 0.857 | 0.622 | 0.479 | 0.755 (0.580–0.930) | 0.027 |
4. Discussion
Linezolid-induced lactic acidosis is a severe complication, which may result in fatalities and multiorgan failure. This study aims to investigate the incidence of lactic acidosis induced by linezolid and analyze the risk factors. Out of the 95 adult patients included in the study, 18 individuals developed linezolid-related lactic acidosis (18.9%, 18 out of 95). Importantly, we found that patients who concurrently used linezolid and metformin had an incredibly high risk (90.9%) of developng lactic acidosis. In addition, Cmin was another significant independent risk factor for lactic acidosis.
Signs and symptoms of lactic acidosis include vomiting, nausea, weight loss, tachypnea, and hyperventilation. Prolonged use of linezolid has been reported to be related to lactic acidosis [1]. In addition, female gender, renal impairment, pregnancy, obesity, and lipid dystrophy are recognized as risk factors associated with lactic acidosis [16]. Similarly, in our study, we found that females were more inclined to develop lactic acidosis compared to males. In this study, we excluded patients with sepsis, acute blood loss, or diabetic ketoacidosis, because they are at a very high risk of developing lactic acidosis due to the disease itself. In order to better analyze the risk factors for linezolid-induced lactic acidosis, those patients were excluded [16]. Moreover, infusions of adrenaline at doses commonly used in the treatment of septic shock could cause lactic acidosis [19], but none of the patients included in this study were concurrently using adrenaline and linezolid. As for the mechanism of linezolid-related lactic acidosis, linezolid can bind to mammalian ribosomes, resulting in reduced activity of respiratory chain complexes that contain mitochondrial DNA–encoded subunits and a decrease in the amount of protein of these complexes, and subsequently induce increased lactic acid levels [20]. In addition, De Vriese et al. have reported enzymatic activity in the mitochondria of affected tissues in a patient treated with linezolid.
The most notable finding in our study is that patients who concurrently used linezolid and metformin had an incredibly high risk of developing lactic acidosis (90.9%, 10 out of 11). Metformin is a long-known oral hypoglycemic agent, increasingly used in recent years as a first-line therapy in diabetes mellitus [21]. It has been reported that metformin can increase blood lactate levels [22]. Metformin facilitates increased lactate levels mainly through two processes: (i) increases the transformation of glucose to lactate by the intestinal mucosa and (ii) suppresses mitochondrial oxidative metabolism, leading to a decrease in hepatic gluconeogenesis from lactate, pyruvate, and alanine. Consequently, these lead to anaerobic glycolysis and the accumulation of lactate [22–24]. In addition, metformin is primarily excreted through the kidney, and the risk of lactic acidosis may be increased in patients with renal insufficiency due to metformin accumulation [25]. The incidence of metformin-associated lactic acidosis (MALA) is not clear. Reported estimates of MALA range from three to nine cases per 100,000 patient years [26–30]. However, concurrently using linezolid and metformin, which further inhibits mitochondrial function, leads to the accumulation of lactate, and significantly increases the risk of lactic acidosis. MALA can occur with both acute and chronic metformin exposure. Despite that the exposure threshold between metformin concentration and incidence of lactic acidosis remains unknown, the high concentrations of metformin, such as those seen in overdose or from significant drug accumulation due to renal failure, the risk of MALA significantly increases [31].
Due to the extremely high incidence of lactic acidosis when concurrently using linezolid and metformin, we excluded these patients from the original database in order to further explore other factors contributing to linezolid-related lactic acidosis. We acknowledge that excluding patients on metformin from the original cohort for analysis raises concerns about potential selection bias. The exclusion of patients on metformin was aimed to isolate the effect of linezolid. Including metformin patients would likely have overshadowed the specific contribution of linezolid to lactic acidosis, given that metformin already carries a high risk for this condition. While excluding metformin users could introduce selection bias, this step was crucial to avoid conflating the two drugs’s effects. 9.52% (8 out of the 84) of the patients developed lactic acidosis. The incidence rate of lactic acidosis during linezolid treatment is relatively low and falls within the category of rare adverse reactions. While lactic acidosis is infrequent in linezolid treatment, it is crucial for healthcare professionals to regularly monitor patients′ lactate levels. Furthermore, the outcomes of the univariate analysis revealed that patients who developed lactic acidosis had significantly higher SCr levels and linezolid Cmin, or lower CrCL values, implying a potential association between lactic acidosis and renal insufficiency and higher linezolid exposure. Current research studies have reported that linezolid concentrations are significantly higher in patients with renal insufficiency due to the decreased clearance of linezolid [32, 33]. The therapeutic target for linezolid recommends maintaining a trough concentration of 2–8 mg/L [34]. Almost all studies define the upper therapeutic threshold based solely on the development of thrombocytopenia [34]. In our study, the multivariate logistic regression analysis showed that the linezolid Cmin was the only independent risk factor identified for lactic acidosis. When Cmin ≥ 31.90 mg/L, the estimated probability of lactic acidosis occurrence exceeds 50%. However, the incidence of linezolid-induced lactic acidosis is generally low. Therefore, we performed a ROC curve analysis, which identified an optimal cutoff value of 7.455 mg/L, closely aligning with the current upper therapeutic threshold for linezolid. In addition, the concentration data of linezolid were collected at steady-state, meaning that the trough concentration of linezolid was maintained at a stable level. Therefore, even though the trough concentration data were not collected on the same day when lactic acidosis occurred, the concentration did not change significantly on the day lactic acidosis occurred. In general, based on the findings of this study, we concluded that elevated linezolid concentrations were an independent risk factor for lactic acidosis, based on the subgroup analysis that excluded metformin. Although this limits the generalizability of the findings to metformin users, it was necessary to clarify the role of linezolid without the confounding effect of metformin. However, we acknowledge that the results may not apply to patients who are coadministered metformin, and future studies with large populations could explore this combined risk.
There are some limitations in the method of this study. First, the limited number of cases weakened the statistical comparisons between groups and its retrospective nature may restrict the application of some conclusions. In order to accurately determine the mechanism and influencing factors of linezolid-induced lactic acidosis, a larger sample size of prospective randomized controlled studies is needed. Second, we did not take into account other potential drug–drug interactions during linezolid treatment. For instance, medications that disrupt oxidative phosphorylation (e.g., antiretroviral drugs and propofol) may potentially elevate lactic acid levels and lead to lactic acidosis [16]. In addition, concurrent use of drugs that interfere with mitochondrial function (such as aminoglycosides, omeprazole, amiodarone, or amlodipine) alongside linezolid may increase the risk of mitochondrial toxicity [35–37].
In general, concurrently using metformin and linezolid extremely elevates the risk of lactic acidosis. Furthermore, Cmin is an independent risk factor for linezolid-associated lactic acidosis. Therefore, it is necessary to avoid concurrently using linezolid and metformin and regularly monitor the concentration of linezolid and lactic acid levels during linezolid treatment.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Committees of the First Affiliated Hospital of Wenzhou Medical University, China ([2022] 043].
Consent
Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Disclosure
A preprint has previously been published [38].
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Y.-J.C., J.F., L.-W.Z., H.-N.Z., and X.-B.Y. conceptualized and planned the work that led to the manuscript. Y.-J.C., J.F., L.-W.Z., and C.C. collected and analyzed the data. Y.-J.C., H.-N.Z., X.-B.Y., G-.Y.L., and X.-H.Z. drafted the manuscript. The final submitted version of the manuscript was reviewed and approved by all the authors. Y.-J.C. and J.F. contributed equally to this work.
Funding
This work was supported by the “Science and Technology Plan Project of Wenzhou Municipality, China” (Y20211026).
Acknowledgments
We gratefully acknowledge the financial support provided by the “Science and Technology Plan Project of Wenzhou Municipality, China” (Y20211026). Furthermore, we would like to acknowledge the valuable insights provided by the preprint available at SSRN [38].
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




