A Network Pharmacology-Based Strategy for Predicting Anti-Inflammatory Targets of Zao Ren An Shen Capsule in the Treatment of Asthma
Abstract
Backgrounds: The Zao Ren An Shen (ZRAS) Capsule, stemming from a traditional Chinese herbal formula, is recognized as a safe and effective sleep-regulating medication. Asthma often worsens during the night and early morning due to its distinct day–night rhythm, suggesting the potential for the ZRAS Capsule to intervene in asthma attacks and prognosis. Since inflammation is a primary mechanism in asthma, exploring the anti-inflammatory effects of the ZRAS Capsule is essential.
Methods: This study identifies the intersection of ZRAS Capsule-related targets and anti-inflammatory targets to uncover potential anti-inflammatory mechanisms. Core compounds and relevant genes were identified using protein–protein and compound–protein interaction networks. KEGG pathway and Gene Ontology analyses were performed on the anti-inflammatory targets to validate their role in mitigating asthma inflammation. Binding activities between the compounds and anti-inflammatory targets were assessed using western blot, qRT-PCR, molecular docking, and immunohistochemistry (IHC).
Results: Key compounds, including luteolin, (S)-Coclaurine, and zizyphusine, along with targets IL6, TNF, and MMP9, were identified. Their biological processes were linked to the IL-17 signaling pathway and TNF signaling pathway. Notably, the identified compounds inhibited the mRNA and protein expression of IL6, TNF, and MMP9.
Conclusion: Regulation of the IL-17/TNF signaling pathway is likely crucial for the protective effects of the ZRAS Capsule in asthma treatment.
1. Introduction
Asthma, a common chronic respiratory disease, affects approximately 350 million people globally [1]. Airway inflammation is recognized as the primary cause of asthma, leading to mucus production, airway remodeling, and bronchial hyperresponsiveness [2–4]. Asthma exhibits significant heterogeneity, encompassing various phenotypes or endotypes, such as type 2-low (predominantly neutrophilic asthma) and type 2-high (mainly eosinophilic asthma) [5]. In China, the eosinophilic phenotype accounts for up to 76.8% of severe asthma cases [6]. Currently, inhaled corticosteroids are the standard treatment worldwide, effectively controlling symptoms and preventing exacerbations. However, the long-term use of corticosteroids poses risks to human health [7, 8], highlighting the need for safer and more effective treatments.
The patent Chinese medication Zao Ren An Shen (ZRAS) Capsule is produced according to the standards of an insomnia-treating nonbenzodiazepine sedative [9]. Three major traditional Chinese medicine (TCM) ingredients are used to produce the capsule, including Suan Zao Ren (Ziziphi Spinosae Semen, 417 mg/capsule), Dan Shen (Salviae Miltiorrhizae Radix et Rhizoma, 167 mg/capsule), and Wu Wei Zi (Schisandrae Chinensis Fructus, 70 mg/capsule) [10]. TCM is also used as a complement or alternative in approximately 12% of patients suffering from insomnia [10].
Asthma presents a distinct circadian rhythm, with nighttime sleep quality significantly declining during attacks [11]. Improving sleep quality in asthma patients is associated with better symptom control [12]. ZRAS Capsules, formulated with Suanzaoren, Beiwuweizi, and Danshen as primary ingredients, are commonly utilized in TCM for the management of insomnia and anxiety. Recent research has demonstrated their efficacy in enhancing sleep quality and alleviating anxiety symptoms. The tranquilizing effects of these capsules are largely attributed to their ability to reduce neuronal excitability [10, 13]. Furthermore, the anti-inflammatory properties of Suanzaoren may play a crucial role in mitigating inflammation-related neuronal damage, thereby amplifying its calming effects. Given that inflammation is a key pathological mechanisms underlying asthma [14–17], the application of ZRAS Capsules in this context warrants further exploration. The study investigates whether the anti-inflammatory effects of ZRAS contribute to improved asthma prognosis using an integrated network pharmacological approach and validation in an ovalbumin-induced asthma mouse model.
2. Methods
2.1. Candidate Compounds and Targets of ZRAS
Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) and Integrative Pharmacology-Based Research Platform of Traditional Chinese Medicine (TCMIP) were used to search for chemical compounds of the three main ingredients of ZRAS. After that, candidate compounds with favorable absorption, distribution, metabolism, and excretion were chosen. In addition, predicted oral bioavailability ≥ 30% and druglikeness ≥ 0.18 were accepted as recommendation. Active ingredient-associated protein targets were also obtained from TCMSP. Next, using the UniProt database (https://www.uniprot.org/), we aligned the retrieved targets for standardization. Eventually, mismatches and duplicates were purged and valid gene symbols were obtained.
2.2. Candidate Targets Associated With Inflammation and Asthma
The Therapeutic Target Database (https://db.idrblab.net/ttd/, TTD), Pharmacogenomics Knowledgebase (https://www.pharmgkb.org/, PharmGKB), DrugBank Online (https://go.drugbank.com/, DrugBank), Online Mendelian Inheritance in Man (https://omim.org/, OMIM), and Human Gene Database (https://www.genecards.org/, GeneCards) were used. Disease-related gene sets were established by intersecting all disease-associated search results, and their intersection was then visualized in R v4.2.3.
2.3. Compound–Target Network Construction
Using the Cytoscape software (Version 3.7.0), we linked candidate compounds to candidate targets to construct the candidate compound–candidate target network. Nodes were used to represent the compounds and proteins and edges were used to show the interaction between these molecular species.
2.4. Pathway Analyses
In order to examine the biological functions of common targets of drug and disease, we performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses. Molecular function (MF), cellular component (CC), and biological process (BP) were included in the GO enrichment analysis as the primary categories. KEGG enrichment analysis revealed complex biological pathways. We performed both analyses in R software v4.2.3 and the corresponding data packages with q value < 0.05 serving as the screening criterion.
2.5. Animal Experiments
We purchased 30 male BALB/c mice (weight 18 ± 2 g) from Shanghai BK/KY Biotechnology Co., Ltd (Shanghai, China). They were maintained in an animal facility with temperature of 16°C–26°C, a light/dark cycle of 12/12 h and humidity of 40%–70%. Standard chow was fed, and clean water was provided for the mice to drink freely. Those mice were randomly divided into five groups (n = 5 each): normal group, model group (OVA), OVA + ZRAS group (ZRAS), and OVA + dexamethasone group (DEX). A total of 0.3 g/kg of ZRAS was administered intragastrically to the mice in the ZRAS group (convert the human drug dosage to mouse dosage using the body surface area method). A total of 2 mg/kg of DEX was administered to the mice in the DEX group [18]. The mice in OVA, DEX, and ZRAS groups were intraperitoneally injected with 200 μL sensitizer on days 0, 7, 14, and 21. From Day 28 to Day 32, each mouse was given a nasal drip of activator (50 μL/day), and corresponding medication was given to each treatment group by gavage. Mice were euthanized 24 h after the completion of the last stimulation on Day 33. All animal experiments were performed according to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, which were approved by the Research Ethics Committee for Experimental Animal Center of Huai’an No. 1 People’s Hospital (no.: DW-P-2023-001-22).
2.6. Histological Analysis
Harvested lung samples (upper lobe of the right lung) was fixed in 4% paraformaldehyde, embedded in paraffin wax, and sectioned. Tissue sections were then stained using hematoxylin and eosin (HE). Pathological changes under optical microscope were analyzed.
2.7. qRT-PCR
Expression of TNF, IL6, MMP, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) on the mRNA level in the lung tissues were measured. According to the manufacturer’s instructions (EZBioscience, US), TRIzol was used to extract total RNA. Then, using the RevertAid First Strand cDNA Synthesis Kit (TAKARA, China), the isolated RNA was reverse-transcribed into cDNA. The LightCycler 96 Real-Time PCR System (Roche) was used to conduct quantitative real-time-PCR (qRT-PCR). The qRT-PCR analysis for each sample was conducted in triplicates with the 2-ΔΔCT method. Normalization was conducted against the GAPDH expression.
2.8. Immunohistochemical Assay
Antigen retrieval was performed, and 5% goat serum was used to block the lung sections. Then, the sections were incubated with primary antibody (anti-IL6, anti-MMP9, or anti-TNF) at 4°C overnight. After that, they were incubated with HRP-conjugated goat anti-rabbit IgG secondary antibody for 30 min at 37°C. The 3, 3′-diaminobenzidine (DAB) peroxidase substrate kit (Servicebio) was used to visualize the positive stains. Finally, an Olympus microscope (Mantra, PerkinElmer) was used to photograph the slides.
2.9. Statistical Analysis
Statistical analyses were performed using Prism 8 software (GraphPad, La Jolla, California, United States). All values are expressed as the mean ± SEM and will undergo normality testing. One-way ANOVA and Tukey’s post hoc test were used to determine the differences between groups. p < 0.05 was considered statistically significant.
3. Results
3.1. Active Ingredients and Target Screening
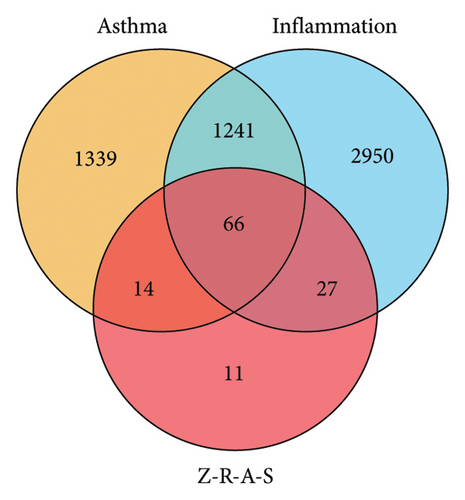
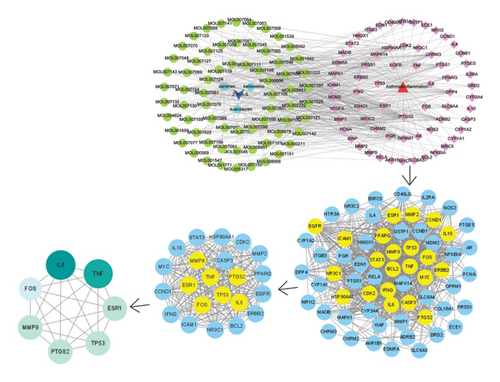
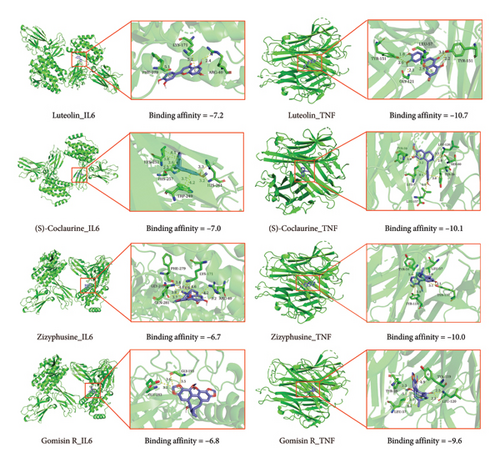
From TCMIP and TCMSP database, we selected 72 compounds in ZRAS. Screening criteria included oral bioavailability thresholds ≥ 0.3 and druglikeness ≥ 0.18. Seven compounds were identified in Beiwuweizi, 58 in Danshen, and 7 in Suanzaoren (Table S1). A total of 118 targets associated with compound were obtained from the two aforementioned databases (Table S2). In addition, 2660 asthma-related targets (Table S3) and 4284 inflammation-related targets (Table S4) were obtained from TTD, PharmGKB, DrugBank, OMIM, and GeneCards database. Then, we identified the candidate targets through which ZRAS exerts its anti-inflammatory effects by extracting the overlap among the three datasets (66 targets, Figure 1(a) and Table S5). Connecting 67 candidate compounds and 66 candidate targets, we constructed the candidate compound–candidate target network as well as PPI network (Figure 1(b)). Assigning roles based on the hierarchical relationships of monarch, ministers, assistants, and messengers in TCM, (S)-Coclaurine, zizyphusine, luteolin, and Gomisin R showed the most relevance, while IL6, TNF, MMP9, PTGS2, TP53, FOS, and ESR1 were the seven most relevant candidate targets. Among these 7 targets, IL6, TNF, and MMP9 were the key targets.
3.2. Target Enrichment Analysis
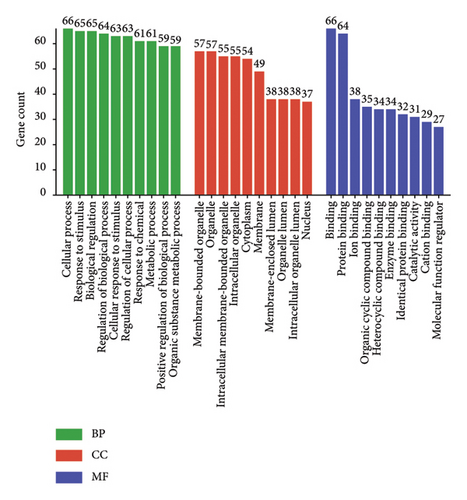
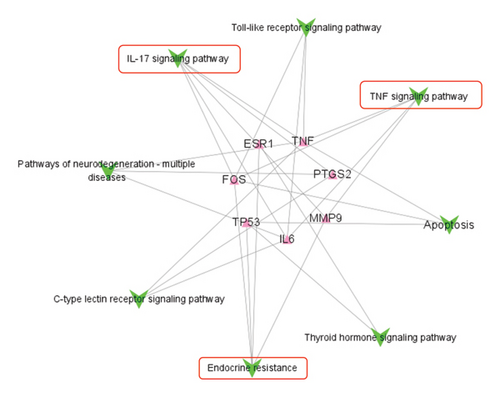
Then, the R package “cluster profiler” was used to conduct functional enrichment analysis on the 66 candidate targets, which was employed to perform GO and KEGG analyses. The GO analyses obtained enriched results of those candidate target genes, which showed that the top items were cellular process, response to stimulus, biological regulation, cellular response to stimulus, and response to chemical were closely related to the activities of ZRAS in anti-inflammation and antiasthma (Figure 2(a)). Figure 2(b) shows the top 20 of KEGG analysis. Among these, there are only three signaling pathways with three or more of the seven core genes (Figure 2(c)). They are endocrine resistance, IL-17 signaling pathway, and TNF signaling pathway.

3.3. Molecular Docking Between Candidate Compounds and Targets
With the aid of molecular docking simulation technology, the four most relevant candidate compounds (S)-Coclaurine, zizyphusine, luteolin, and Gomisin R were docked with the most relevant candidate targets (Figure 3). The results indicated that (S)-Coclaurine, zizyphusine, luteolin, and Gomisin R had good binding activities to IL6 and TNF.
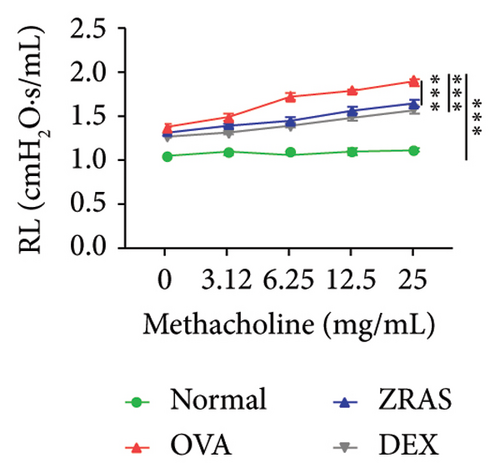
3.4. ZRAS Alleviated Airway Hyper Reactivity in an OVA Mouse Model
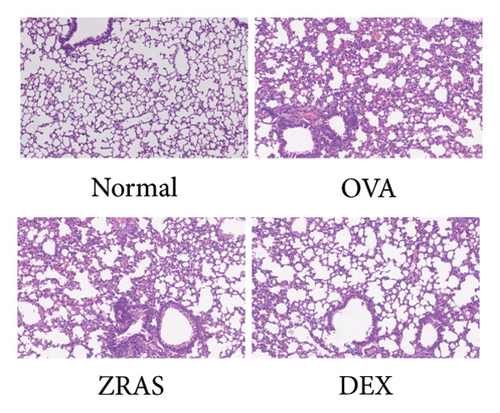
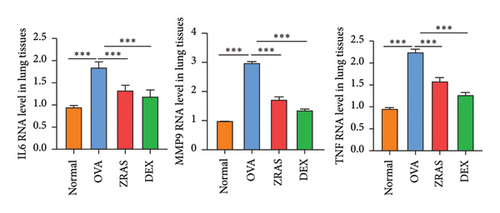
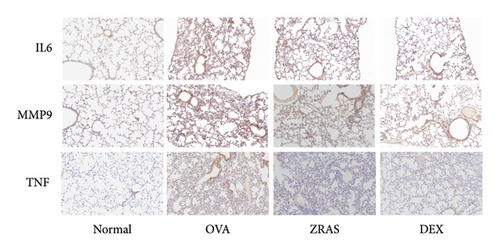
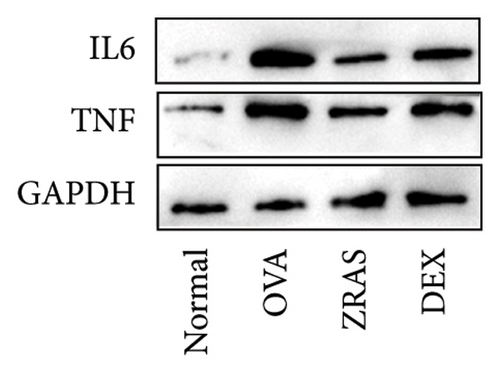
Compared with the control group, the OVA group showed increases in RL at five concentrations of methacholine (Mch) provocation, indicating that we successfully established an asthma model with AHR. In addition, when treated with ZRAS and DEX, respectively, RL showed significant decreases in compared with the OVA group (Figure 4(a)). HE staining results showed that OVA induced abundant infiltration of peritracheal and perivascular inflammatory cells, as well as mucosal edema, which were alleviated by treatment with both ZRAS and DEX (Figure 4(b)). We then investigated the expression of the three hub targets (IL6, TNF, and MMP9) in lung tissues on mRNA and protein level. The qRT-PCR results showed significantly increased mRNA expression of IL6, TNF, and MMP9 in the OVA group and significant reductions in the ZRAS and DEX treatment groups (Figure 4(c)). Similar changes of the three hub target proteins were observed in those groups through IHC analyses (Figure 4(d)). Meanwhile, western blot results also showed that the protein expressions of IL6 and TNF were increased in the OVA group and decreased after ZRAS and DEX treatment (Figure 4(e)).
4. Discussion
Asthma is a chronic inflammatory disease, and airway immune inflammation plays a key role in airway inflammation, airway hyper-responsiveness, reversible airway limitation, and airway remodeling of asthma [19, 20]. In Asia, western medicine and TCM are often combined to treat asthma since multicompound, multitarget, and multipathway effects are exerted through TCM [21]. ZRAS have a distinct role in improving sleep rhythms and are considered safe for use. Moreover, they possess notable anti-inflammatory properties, with key ingredients shown to have significant anti-inflammatory effects in conditions such as acute lung injury [22], myocardial fibrosis [23], gastrointestinal diseases [24], and skeletal muscle atrophy [25]. Since inflammation and circadian rhythm disruption are two major characteristics of asthma, ZRAS may represent a promising potential adjunctive treatment for asthma management. In the current study, a compound asthma-anti-inflammatory target network was constructed. We also demonstrated ZRAS’s anti-inflammatory mechanism in asthma treatment through GO pathway enrichment analysis and molecular docking. A total of 67 compounds with satisfactory and druglikeness and bioavailability (druglikeness ≥ 0.18 and bioavailability ≥ 30%) were obtained from ZRAS, among which 3 showed outstanding pharmacological effects. Through the compound–target network, 4 key compounds (luteolin, (S)-Coclaurine, zizyphusine, and Gomisin R) were obtained. Luteolin can inhibit MAPK and NF-κB pathway activation and thereafter reduce the production of inflammatory factors [26]. (S)-Coclaurine and zizyphusine are the main chemical constituents of suanzaoren and can effectively improve insomnia via inhibiting dopamine from accessing the D1 and D2 receptors [27]. Furthermore, previous studies have demonstrated that coclaurine and zizyphusine possess anticancer, anti-inflammatory, and antiarrhythmic effects [28–30]. Gomisin R can decrease inflammatory cytokine production in human [31].
Using the R package “cluster profiler”, we conducted functional enrichment analysis on 66 candidate targets. Enriched candidate target genes were identified through GO analyses, in which the top items were cellular process, response to stimulus, biological regulation, cellular response to stimulus, and response to chemical were closely related to the activities of ZRAS in anti-inflammation and antiasthma. Among the top 20 pathways identified in the KEGG enrichment analysis, the majority of the seven relevant candidate targets (IL6, TNF, MMP9, PTGS2, TP53, FOS, and ESR1) were significantly enriched in the context of endocrine resistance. Notably, the IL-17 signaling pathway is crucial for promoting inflammation and airway hyper-responsiveness, which are key features of asthma. In parallel, the TNF signaling pathway plays a vital role in the recruitment of inflammatory cells and the maintenance of chronic inflammation. Together, these pathways not only highlight the intricate interplay between inflammatory processes in asthma but also suggest that they may significantly contribute to the underlying mechanisms of endocrine resistance [32–38]. With the aid of molecular docking simulation technology, we also found that (S)-Coclaurine, zizyphusine, luteolin, and Gomisin R had good binding activities to IL6 and TNF. Then, we established an asthma model and found that IL6, TNF, and MMP9 were increased in the OVA group, and ZRAS treatment significantly decreased the AHR and level of three genes.
Despite the study’s deep relevance, it is important to acknowledge some limitations. First, there was no group established for the combination of ZRAS and corticosteroid treatment, which limits our ability to elucidate the synergistic effects of ZRAS and corticosteroids in asthma management. This aspect could be further validated in future research. Second, this study utilized existing dosage guidelines without establishing new concentration gradient groups for the drug, which restricts our understanding of the relationship between efficacy and dosage. Future studies could strengthen this validation.
5. Conclusion
In conclusion, ZRAS could improve the prognosis of asthma through anti-inflammatory measures. Moreover, in the treatment of asthma, the regulation of the IL-17/TNF/Endocrine resistance signaling pathways is likely to be crucial for ZRAS to exert its protective effects.
Nomenclature
-
- ZRAS
-
- Zao Ren An Shen capsule
-
- TCM
-
- Traditional Chinese Medicine
-
- OVA
-
- Ovalbumin
-
- TCMSP
-
- Traditional Chinese Medicine Systems Pharmacology Database
-
- TCMIP
-
- Pharmacology-based Research Platform of Traditional Chinese Medicine
-
- GO
-
- Gene Ontology
-
- KEGG
-
- Kyoto Encyclopedia of Genes and Genomes analyses
-
- MF
-
- Molecular function
-
- CC
-
- Cellular component
-
- BP
-
- Biological process
-
- DEX
-
- Dexamethasone
-
- HE
-
- Hematoxylin and eosin
-
- qRT-PCR
-
- Quantitative real-time-PCR
Ethics Statement
The study protocol was approved by the Ethics Committee of Huai’an First People’s Hospital (no.: DW-P-2023-001-22) and in conformity with the Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Baolan Wang and Xiuqin Zhang: conceptualization; Baolan Wang: data curation; Baolan Wang and Zhe Zhang: formal analysis; Zhe Zhang and Yi Wang: methodology; Baolan Wang and Rong Zhu: writing the original draft; Xiuqin Zhang: reviewing and editing. Baolan Wang, Zhe Zhang, and Yi Wang contributed equally to the work and should be considered co-first authors.
Funding
This work was partly supported by Jiangsu Provincial Medical Key Discipline (grant no. ZDXK202201) and Jiangsu Science and Technology Association research topic (grant no. JSKXKT2023029).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. All relevant data are included within the manuscript and its supporting information.




