Geographic Origin Tracing and Variety Authentication of Vietnamese Mangoes (Mangifera indica L.) Using ICP–MS and Statistical Analysis
Abstract
Food traceability, which involves verifying the history and location of a product throughout its production, processing, and distribution, is increasingly vital for consumers and the food industry. As a major exporter of mangoes and the 13th largest supplier to the US, Vietnam faces challenges from rising trade volumes, leading to more instances of low-quality and fraudulent products. This study investigates the traceability of Vietnamese mangoes (Mangifera indica L.), particularly the high-value Hoa Loc variety, using an integrated approach with principal component analysis (PCA) and inductively coupled plasma–mass spectrometry (ICP–MS). Mango samples from Tien Giang, Vinh Long, Dong Thap, and Can Tho provinces were analyzed for their elemental composition. PCA revealed significant regional differences, with key elements for distinguishing mango samples including magnesium (Mg), cesium (Cs), iron (Fe), silver (Ag), molybdenum (Mo), strontium (Sr), antimony (Sb), rubidium (Rb), zinc (Zn), and copper (Cu). Notably, Can Tho samples had the highest Mg concentration (575.52 ± 42.29 μg/g), while Tien Giang samples had the lowest (80.06 ± 10.24 μg/g). Significant regional variations were also observed in Fe, Co, and Zn. These findings demonstrate the efficacy of advanced analytical techniques in verifying traceability and ensuring mango product quality. The study underscores the importance of robust analytical methods in combating food fraud and maintaining consumer trust.
1. Introduction
Ensuring food traceability is essential for safeguarding the safety, quality, and authenticity of food products in the global supply chain [1]. It provides consumers and regulatory bodies with the ability to verify the origin and handling history of food items, thereby increasing confidence and trust in the system [2]. In the context of international trade, where food products often traverse complex distribution networks, robust traceability frameworks are critical in mitigating food fraud, enhancing transparency, and ensuring compliance with both national and international food safety standards. Food traceability has become a central concern of government agencies, international organizations, and the scientific community, particularly in the face of rising incidents of adulteration, mislabeling, and fraudulent branding [3].
Among modern analytical techniques, inductively coupled plasma–mass spectrometry (ICP–MS) has emerged as a powerful tool in food authentication due to its ability to detect and quantify multielemental compositions at ultratrace levels with high sensitivity and reproducibility. When combined with multivariate statistical techniques such as principal component analysis (PCA) and linear discriminant analysis (LDA), ICP–MS enables effective classification of agricultural products based on their elemental fingerprints, which often reflect their geochemical origin and environmental growth conditions. Several studies have validated the application of this integrated approach across different fruit types. For example, a microwave-assisted ICP–MS method was developed for the simultaneous determination of 27 elements (sodium [Na], magnesium [Mg], iron [Fe], zinc [Zn], strontium [Sr], lead [Pb], etc.) in fruit matrices, demonstrating accuracy and environmental compatibility in multielemental analysis [4]. PCA was employed to differentiate the geographical origins of prickly pear (Opuntia ficus-indica L. Miller) from various volcanic zones in Sicily, where two principal components accounted for 72.03% of the total variance [5]. In a separate study, ICP–MS combined with machine learning techniques such as support vector machines achieved 89.18% accuracy in classifying organic and conventional grape juice samples based on their mineral composition [6, 7]. These studies highlight the robustness and flexibility of ICP–MS-based multielemental analysis for tracing origin and verifying authenticity in agricultural products.
In Vietnam, such technologies are especially relevant given the economic significance of mango production, particularly of the Hoa Loc variety, which holds considerable value in both domestic and export markets. While a range of traceability systems exists, utilizing barcoding, RFID, and blockchain technologies, many are limited by their reliance on labeling, which can be falsified. In contrast, elemental profiling reflects inherent characteristics of the product, unaffected by packaging or postharvest processes. Thus, incorporating scientific markers such as elemental composition into traceability protocols enhances accuracy and reduces the risk of fraud [8]. PCA is widely used to reduce complex datasets into meaningful components by transforming correlated variables into a smaller set of orthogonal axes, preserving the most significant variability. LDA, by contrast, maximizes the separation between predefined categories, making it ideal for verifying sample identity. When used in conjunction with ICP–MS, these tools offer a reliable analytical framework for differentiating agricultural commodities such as fruit based on their compositional profiles. This combination has been applied successfully in previous studies to identify geographic origins of rice, beetroot, and cashew nuts, among others [9, 10].
Vietnam ranks among the top ten mango-producing countries, with over 115,000 ha under cultivation, yielding approximately 969,000 tons annually [11, 12]. In 2023, the country produced 1.4 million tons, making it the 13th largest mango exporter to the U.S. market [13, 14]. Among the 46 mango varieties grown in Vietnam, Hoa Loc mangoes are particularly prized for their aromatic profile, texture, and sweetness [15]. Originally in Tien Giang province, this variety is now grown across several provinces, including Vinh Long, Dong Thap, and Can Tho. It has secured intellectual property protection due to its economic importance and unique sensory characteristics [16]. However, the growing market demand has led to an increase in mislabeling and fraudulent substitution, threatening consumer confidence and undermining legitimate producers. Therefore, robust analytical traceability is necessary to authenticate genuine Hoa Loc mangoes and protect the reputation of Vietnamese exports [17].
This substantial price gap incentivizes fraudulent practices, such as passing off lower-grade or different varieties as Type 1 mangoes. Such fraud not only deceives consumers but also creates economic distortion and damages brand integrity [21]. In response to these challenges, the current study applies ICP–MS-based elemental profiling, combined with PCA and LDA, to determine the geographic origin and verify the authenticity of Hoa Loc mangoes from different provinces. This approach has proven effective in other agricultural products such as citrus, sausages, and leafy vegetables, establishing regional and varietal differentiation through multielemental analysis [22–29].
Therefore, the objective of this study is to develop a reliable, science-based method for traceability and authenticity verification of Vietnamese mangoes using ICP–MS and multivariate statistical analysis. By identifying region-specific elemental markers, we aim to provide a foundation for building a reference database that supports transparent labeling, fraud detection, and quality assurance in domestic and international markets. Ultimately, this research contributes to sustainable agricultural development and promotes consumer confidence in Vietnamese mangoes.
2. Materials and Methods
2.1. Chemicals and Reagents
The chemicals employed in this research comprised 65% nitric acid (HNO3) and 30% hydrogen peroxide (H2O2), both sourced from Merck in Darmstadt, Germany. Ultrapure deionized water, exhibiting a resistivity of 18.2 MΩ·cm, was generated using a Milli-Q Plus water purification system from Millipore, Bedford, MA, USA. The standard solution containing 21 elements such as Mg, aluminum (Al), vanadium (V), chromium (Cr), manganese (Mn), Fe, cobalt (Co), nickel (Ni), copper (Cu), Zn, arsenic (As), rubidium (Rb), strontium (Sr), molybdenum (Mo), silver (Ag), cadmium (Cd), antinomy (Sb), cesium (Cs), barium (Ba), mercury (Hg), and Pb (TraceCERT, Merck, Darmstadt, Germany) was supplied by Sigma-Aldrich, based in Missouri, USA.
2.2. Sample Collection and Preparation
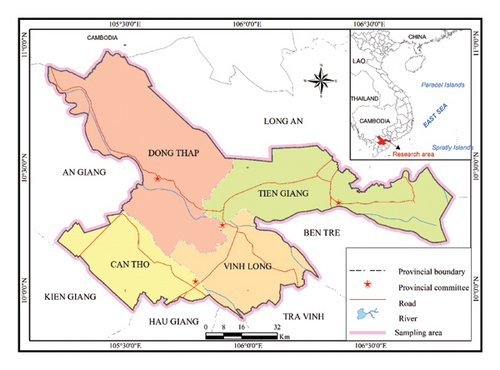
Samples of Hoa Loc mango were collected from orchards in four different provinces: Tien Giang, Vinh Long, Dong Thap, and Can Tho, with each region providing 100 samples (Figures 1 and 2). Careful selection criteria were applied to ensure uniformity in the age of the mango trees and the ripening period after harvesting. The preparation process included systematically removing the seeds and peels from each mango. Following this, the samples were homogenized using a Seka SK200 grinder to achieve a consistent mixture. The homogenized samples were then stored at 4°C until they were ready for further analysis. This method ensured that the samples maintained uniformity and integrity throughout the preparation and storage phases.




Approximately 0.2 g of each processed sample were accurately weighed and placed into individual Teflon digestion tubes. To each tube, 4 mL of 65% nitric acid and 2 mL of 30% H2O2 were added. The tubes were allowed to sit overnight to enhance the reaction. The following day, the samples were digested using a MARS6 microwave oven (CEM Corporation, Matthews, NC, USA) at 190°C for 30 min. After cooling to room temperature, the digested samples were diluted with deionized water to a final volume of 50 mL in volumetric flasks. The resulting solutions were filtered through 11 μm filter paper to eliminate any particulate matter or impurities before subsequent analysis.
2.3. Phytochemical Analysis
2.3.1. Vitamin C Determination
The total ascorbic acid content was quantified using a UV-vis spectrophotometer. Thirty grams of the sample were accurately weighed and placed into a 50 mL Falcon tube. The mixture was centrifuged at 4000 rpm for 15 min, and the supernatant was collected. To 4 mL of this sample solution, 0.23 mL of 3% bromine was added, followed by 0.13 mL of 10% thiourea to neutralize the excess bromine. Subsequently, 1 mL of 2,4-dinitrophenylhydrazine was added to the mixture. The solution was incubated in a water bath at 30°C for 3 h. After cooling for 30 min, 5 mL of 85% sulfuric acid (H2SO4) was added. The absorbance of the resulting colored solution was measured at 521 nm. The vitamin C content was determined using an ascorbic acid standard curve [30].
2.3.2. DPPH Radical Scavenging Activity
2.3.3. Total Acidity
2.3.4. Polyphenol Content
The polyphenol concentration was determined using the Folin–Ciocalteu assay. The extract was diluted to the required concentration, and 0.5 mL of this diluted extract was transferred to a test tube. Next, 5 mL of Folin–Ciocalteu reagent was added to the tube. The mixture was thoroughly homogenized using a Vortex mixer, followed by the addition of 4 mL of 7.5% sodium carbonate (Na2CO3). The reaction mixture was then allowed to stand at room temperature for 30 min. Subsequently, the absorbance was measured at 765 nm using a spectrophotometer. The polyphenol content was quantified and expressed as milligrams of gallic acid equivalent (mg GAE) per gram of dry matter. This method ensures accurate quantification of polyphenol concentrations, reflecting the antioxidant capacity of the sample [34].
2.3.5. Soluble Solids Content (SSC)
The SSC of the mango samples was measured using a handheld refractometer, which provides a direct reading in degrees Brix (°Brix). The °Brix value represents the percentage of total soluble solids (TSS), primarily sugars, in the mango juice, and is commonly used as an indicator of fruit sweetness and ripeness. Measurements were taken at 20°C, and the refractometer was calibrated with distilled water before each use to ensure accuracy [35].
2.3.6. pH Measurement
The pH of the samples was determined using a benchtop pH meter calibrated with standard buffers, ensuring accurate acidity assessment [36].
2.4. Elemental Analysis and Statistical Methods
The elemental composition of the mango samples was analyzed using an iCap TQ ICP–MS (Agilent Technologies, Bremen, Germany), targeting Mg, Al, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Rb, Sr, Mo, Ag, Cd, Sb, Cs, Ba, Hg, and Pb. The ICP–MS was operated under the following conditions: radio frequency (RF) power of 1400 W, plasma gas flow rate of 15 L/min, carrier gas flow rate of 1.05 L/min, and makeup gas flow rate of 0.9 L/min. The spray chamber temperature was maintained at 2°C, and spectral analysis was performed with three points per spectral peak and ten sweeps per reading, targeting the isotopes 24Mg, 27Al, 51V, 52Cr, 55Mn, 56Fe, 59Co, 60Ni, 63Cu, 66Zn, 75As, 85Rb, 88Sr, 98Mo, 107Ag, 111Cd, 121Sb, 133Cs, 137Ba, 202Hg, and 208Pb (Figure S1).
To enhance clarity and provide a comprehensive reference, Table 1 summarizes the selected metals, their atomic masses, and the isotopes measured using ICP–MS.
| Element | Atomic mass (amu) | Measured isotope |
|---|---|---|
| Magnesium (Mg) | 24.31 | 24Mg |
| Aluminum (Al) | 26.98 | 27Al |
| Vanadium (V) | 50.94 | 51V |
| Chromium (Cr) | 52 | 52Cr |
| Manganese (Mn) | 54.94 | 55Mn |
| Iron (Fe) | 55.85 | 56Fe |
| Cobalt (Co) | 58.93 | 59Co |
| Nickel (Ni) | 58.69 | 60Ni |
| Copper (Cu) | 63.55 | 63Cu |
| Zinc (Zn) | 65.38 | 66Zn |
| Arsenic (As) | 74.92 | 75As |
| Rubidium (Rb) | 85.47 | 85Rb |
| Strontium (Sr) | 87.62 | 88Sr |
| Molybdenum (Mo) | 95.94 | 98Mo |
| Silver (Ag) | 107.87 | 107Ag |
| Cadmium (Cd) | 112.41 | 111Cd |
| Antimony (Sb) | 121.76 | 121Sb |
| Cesium (Cs) | 132.91 | 133Cs |
| Barium (Ba) | 137.33 | 137Ba |
| Mercury (Hg) | 200.59 | 202Hg |
| Lead (Pb) | 207.2 | 208Pb |
Each experiment was performed in triplicate, with results presented as mean values and standard deviations. For the purpose of sample classification and discrimination, the data underwent statistical analysis using XLSTAT (Lumivero, Denver, USA) and STATISTICA 12 (Dell Software, USA). Elements with concentrations below the LOD in all four regions were excluded from the analysis whereas those below the LOD in some regions were set to zero for analytical purposes. PCA was applied to the data for the 21 elements (Mg, Al, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Rb, Sr, Mo, Ag, Cd, Sb, Cs, Ba, and Pb). The multivariate statistical analysis yielded several key outcomes: a screening histogram delineating the contribution of principal components to the PCA model, a score scatter plot illustrating the differentiation of the four distinct groups, a loading scatter plot elucidating the impact of factors on clustering, and a moving range graph showing the means and overall distribution of variables across the cases.
These analytical methods allowed for precise characterization and verification of the geographical origin and authenticity of the mango samples. The PCA provided insights into the elemental composition of mangoes from different regions, enabling the differentiation between the samples based on their elemental profiles. This rigorous analytical approach ensures that the data provide a solid foundation for distinguishing between different sources of mangoes, enhancing the traceability and authenticity of the products. This methodology not only protects consumer health by verifying the authenticity of the mangoes but also safeguards the reputation of genuine producers by preventing fraud and ensuring the integrity of the supply chain. By employing advanced elemental analysis and robust statistical techniques, the study offers a comprehensive approach to the authentication and traceability of agricultural products, providing a valuable tool for both producers and regulatory agencies.
3. Results
3.1. Comparative Nutritional and Phytochemical Profiles of Mango From Plantation From Different Regions
The nutritional and chemical composition of Vietnamese mangoes from different cultivation areas is summarized in Table 2, providing a comparative assessment of Vitamin C, antioxidant activity, acidity, polyphenols, dry matter content, and pH. These parameters are essential indicators of fruit quality, nutritional value, and postharvest stability. The Vitamin C content varied slightly among the regions, with the highest levels observed in Tien Giang (36.2 ± 1.8 mg AA/100 g), followed by Vinh Long (35.7 ± 1.1 mg AA/100 g), Can Tho (35.1 ± 1.5 mg AA/100 g), and Dong Thap (34.6 ± 1.3 mg AA/100 g). The antioxidant activity, measured using DPPH scavenging percentage, ranged from 49.6 ± 2.1% (Can Tho) to 51.4 ± 2.2% (Dong Thap), with Tien Giang and Vinh Long exhibiting intermediate values of 50.5 ± 2.5% and 49.9 ± 2.1%, respectively. These variations in antioxidant potential are likely influenced by differences in climatic conditions, soil composition, and agricultural practices, which impact the biosynthesis of antioxidant compounds in mangoes.
| Mango cultivation Areas | Vitamin C (mg AA/100 g) | Antioxidant activity DPPH (%) | Acidity (g/L) | Polyphenols (mg GAE/100 g) | Dry matter content (°Bx) | pH |
|---|---|---|---|---|---|---|
| Tien Giang | 36.2 ± 1.8a | 50.5 ± 2.5b | 0.4 ± 0.03b | 60.0 ± 3.0c | 16.0 ± 0.9a | 4.2 ± 0.2b |
| Vinh Long | 35.7 ± 1.1b | 49.9 ± 2.1c | 0.4 ± 0.06b | 62.0 ± 2.1a | 15.5 ± 0.8b | 4.1 ± 0.4c |
| Dong Thap | 34.6 ± 1.3c | 51.4 ± 2.2a | 0.5 ± 0.04a | 59.0 ± 2.6d | 14.8 ± 0.7c | 4.3 ± 0.2a |
| Can Tho | 35.1 ± 1.5b | 49.6 ± 2.1c | 0.4 ± 0.07b | 61.0 ± 3.1b | 14.2 ± 0.9d | 4.2 ± 0.1b |
- Note: The values are expressed as the mean ± standard deviation. Statistical groupings are indicated by letters (a, b, c, and d) next to each mean value, representing groups that are not significantly different from each other at the 0.05 level, as determined by a post hoc test.
Among the chemical parameters, total acidity was highest in Dong Thap (0.5 ± 0.04 g/L) whereas the other three regions exhibited similar values (0.4 ± 0.03–0.07 g/L). Acidity is a critical factor affecting mango flavor, postharvest storage, and processing suitability, with higher acidity levels contributing to a longer shelf life and enhanced sensory attributes. The polyphenol content, expressed in mg GAE per 100 g, was highest in Vinh Long (62.0 ± 2.1 mg GAE/100 g), followed by Can Tho (61.0 ± 3.1 mg GAE/100 g), Tien Giang (60.0 ± 3.0 mg GAE/100 g), and Dong Thap (59.0 ± 2.6 mg GAE/100 g). As polyphenols contribute to antioxidant activity and fruit bioactivity, these values align with the observed DPPH scavenging trends. The dry matter content, an indicator of fruit ripeness and sugar accumulation, ranged from 14.2 ± 0.9°Brix (Can Tho) to 16.0 ± 0.9°Brix (Tien Giang), suggesting differences in fruit moisture levels and carbohydrate content among regions. The pH values remained relatively uniform, with slight variations observed: 4.3 ± 0.2 (Dong Thap), 4.2 ± 0.2 (Tien Giang and Can Tho), and 4.1 ± 0.4 (Vinh Long). These results indicate that mangoes from different regions maintain a stable pH, contributing to consistent sensory attributes and processing properties.
3.2. Elemental Composition of Hoa Loc Mangoes Across Four Provinces
The elemental concentrations in Hoa Loc mango samples (Table 3) from four provinces, namely, Tien Giang, Vinh Long, Dong Thap, and Can Tho illustrate the variations in elemental composition across different regions.
| Element (μg/g) | Tien Giang | Vinh Long | Dong Thap | Can Tho |
|---|---|---|---|---|
| Mg | 80.06 ± 4.24 | 281.13 ± 15.75 | 374.22 ± 18.88 | 575.52 ± 42.29 |
| Al | < LOD∗ | 17.66 ± 0.84 | < LOD∗ | < LOD∗ |
| V | 0.05 ± 0.01 | 0.08 ± 0.01 | 0.11 ± 0.01 | < LOD∗ |
| Cr | 0.73 ± 0.06 | 2.89 ± 0.25 | 2.57 ± 0.28 | 0.01 ± 0.01 |
| Mn | 7.61 ± 0.71 | 6.38 ± 0.80 | 12.38 ± 1.58 | 7.83 ± 0.78 |
| Fe | < LOD∗ | 55.72 ± 2.81 | 25.70 ± 3.32 | < LOD∗ |
| Co | 0.06 ± 0.01 | 0.26 ± 0.03 | 16.79 ± 1.89 | 0.06 ± 0.01 |
| Ni | 1.39 ± 0.17 | 6.48 ± 0.63 | 1.95 ± 0.16 | 6.78 ± 0.82 |
| Cu | 0.06 ± 0.01 | 8.14 ± 1.05 | 3.52 ± 0.42 | 9.14 ± 0.53 |
| Zn | 5.36 ± 0.64 | 20.99 ± 1.51 | 13.28 ± 1.81 | 22.86 ± 2.08 |
| As | < LOD∗ | < LOD∗ | 0.03 ± 0.01 | < LOD∗ |
| Rb | 2.43 ± 0.16 | 3.50 ± 1.38 | 9.23 ± 3.06 | 18.98 ± 5.33 |
| Sr | < LOD∗ | < LOD∗ | < LOD∗ | 1.98 ± 0.20 |
| Mo | 0.10 ± 0.01 | 0.35 ± 0.04 | 0.33 ± 0.04 | 0.31 ± 0.03 |
| Ag | < LOD∗ | 1.22 ± 0.12 | 0.14 ± 0.01 | < LOD∗ |
| Cd | 0.07 ± 0.01 | 0.79 ± 0.05 | 0.19 ± 0.02 | < LOD∗ |
| Sb | < LOD∗ | < LOD∗ | < LOD∗ | 0.10 ± 0.01 |
| Cs | < LOD∗ | 0.02 ± 0.01 | 0.06 ± 0.01 | 0.15 ± 0.01 |
| Ba | 0.09 ± 0.01 | 1.19 ± 0.08 | 0.24 ± 0.02 | 0.88 ± 0.06+ |
| Hg (μg/kg) | < LOD∗ | < LOD∗ | < LOD∗ | < LOD∗ |
| Pb | 0.03 ± 0.01 | 0.63 ± 0.05 | 0.22 ± 0.01 | < LOD∗ |
- ∗LOD: limit of detection (3x standard deviation = 0.003 μg/kg). Mean concentrations of elements in mango samples (μg/g) from four provinces. Values represent the mean ± standard deviation of triplicate measurements for each of the samples per region. Triplicate measurements were conducted to ensure analytical robustness.
The elemental composition of Hoa Loc mangoes exhibited significant regional variations. Mg concentrations were highest in Can Tho (575.52 ± 42.29 μg/g), followed by Dong Thap (374.22 ± 18.88 μg/g), Vinh Long (281.13 ± 15.75 μg/g), and Tien Giang (80.06 ± 4.24 μg/g). Similarly, Zn levels were most elevated in Can Tho (22.86 ± 2.08 μg/g) and Vinh Long (20.99 ± 1.51 μg/g), with lower concentrations in Dong Thap (13.28 ± 1.81 μg/g) and Tien Giang (5.36 ± 0.64 μg/g). A comparable trend was observed for Cu, which was highest in Can Tho (9.14 ± 0.53 μg/g) and Vinh Long (8.14 ± 1.05 μg/g) whereas Dong Thap (3.52 ± 0.42 μg/g) and Tien Giang (0.06 ± 0.01 μg/g) had lower levels.
Fe concentrations were notably high in Vinh Long (55.72 ± 2.81 μg/g) and Dong Thap (25.70 ± 3.32 μg/g) but remained below the LOD in Tien Giang and Can Tho. Co levels followed a similar pattern, with Dong Thap (16.79 ± 1.89 μg/g) showing the highest concentration, while levels in the other regions were ≤ 0.06 μg/g. V was most concentrated in Dong Thap (0.10 ± 0.01 μg/g). Rb levels were highest in Can Tho (18.98 ± 5.33 μg/g), followed by Dong Thap (9.23 ± 3.06 μg/g), Vinh Long (3.50 ± 1.38 μg/g), and Tien Giang (2.43 ± 0.16 μg/g). Cr concentrations were greater in Vinh Long (2.89 ± 0.25 μg/g) and Dong Thap (2.57 ± 0.28 μg/g), while Tien Giang (0.73 ± 0.06 μg/g) and Can Tho (0.01 ± 0.01 μg/g) had significantly lower levels.
Regarding heavy metals, Cd was highest in Vinh Long (0.79 ± 0.05 μg/g), with other regions showing concentrations ≤ 0.19 μg/g. Pb was detected in Vinh Long (0.63 ± 0.05 μg/g) and Dong Thap (0.22 ± 0.01 μg/g) but remained below LOD in the other regions. As was only detected in Dong Thap (0.03 ± 0.01 μg/g), while Hg was below LOD in all samples. These results highlight the influence of regional soil composition and environmental conditions on the elemental profile of Hoa Loc mangoes.
3.3. Multivariate Statistical Analysis: PCA and LDA
The PCA and LDA analyses confirmed the variations observed in key nutritional parameters, such as polyphenol content and acidity, across different regions (as shown in Table 3). Notable variables include Mg, Al, Cr, Mn, Zn, Cu, V, Fe, Co, Ni, As, Rb, Sr, Sb, Ag, Cd, Mo, Cs, Ba, and Pb. PCA was conducted on the average concentrations of 21 elements using STATISTICA software. The PCA results are illustrated in Figures 3(a) and 3(b). In the two-dimensional scatter plot based on PC1 and PC2, mango samples from the four regions were successfully distinguished, demonstrating the PCA method’s effectiveness in differentiating the geographical origin of mango samples based on elemental composition.
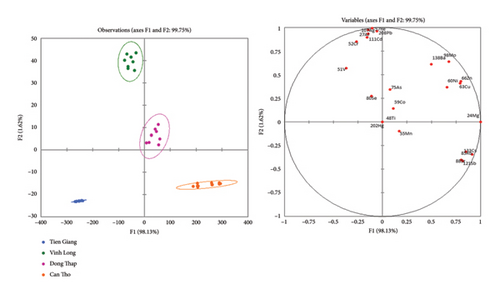
The PCA loading scatterplot in Figure 3(b) illustrates the contribution of various elemental variables to the differentiation of mango samples from different regions. The variables are categorized into three distinct groups based on their correlations: Group 1 (Pb, Fe, V, Al, and Ag), Group 2 (Zn, Cu, Mg, and Mo), and Group 3 (Rb, Sr, Sb, and Cs). Notably, elements from Groups 1 and 3 exhibit an inverse proportional relationship, indicating contrasting influences on sample differentiation. Among these elements, Mg, Cs, Fe, Ag, Mo, Sr, Sb, Rb, Zn, and Cu are identified as the most significant in distinguishing each group, with Mg, Cs, Fe, and Ag showing a strong positive correlation and substantial influence on the first principal component (PC1). These results highlight the distinct geochemical characteristics that contribute to the classification of Hoa Loc mangoes based on their elemental composition.
The differentiation of these variables into three groups is primarily driven by geochemical and environmental factors. Group 1 elements are commonly associated with industrial contamination, soil composition, and potential anthropogenic influences, indicating environmental factors affecting mango production. Group 2 consists of essential plant nutrients, whose concentrations are likely influenced by fertilization practices, natural soil mineral content, and plant uptake efficiency. Group 3 includes geo-tracers, which are strongly associated with regional soil characteristics and geological differences. The clear separation of these groups in the PCA plot underscores the role of elemental profiling as a reliable indicator of geographic origin, reinforcing the effectiveness of multivariate analysis in food traceability and authentication.
The elemental composition of mango samples from different provinces further supports the geographical classification observed in Figure 3. Can Tho mangoes are characterized by higher concentrations of Mg, Cr, and Cu, distinguishing them from other regions. Vinh Long samples exhibit significantly higher Fe content, making it a key differentiating factor. Dong Thap mangoes are primarily distinguished by elevated Co and Ni levels whereas the combination of Zn and Rb contributes significantly to the differentiation of Vinh Long samples. Tien Giang samples do not exhibit exceptionally high concentrations of any single element; however, they are notable for their higher Mn and Ba levels, which may reflect distinct soil compositions in the region. These findings demonstrate how elemental composition analysis can effectively distinguish mango samples and serve as a robust marker for geographic origin verification.
The inverse proportional relationship observed between Group 1 elements and Group 3 elements can be explained by differences in geochemical behavior and plant uptake mechanisms. Group 1 elements are often associated with anthropogenic influences, including industrial contamination and agricultural inputs, whereas Group 3 elements function as geo-tracers, primarily reflecting natural soil composition. The correlation analysis in Figure 3 suggests that in regions with higher Pb and Fe concentrations, Sr and Rb levels tend to be lower, indicating possible competitive interactions during root absorption. Studies have shown that Pb and Fe can interfere with the uptake of alkali and alkaline earth metals, such as Rb and Sr, due to similar transport pathways in plant root systems. In addition, soil properties such as pH and cation exchange capacity (CEC) influence element mobility, where acidic conditions enhance Fe solubility but restrict Sr uptake. These findings underscore the importance of considering both environmental factors and elemental interactions in food traceability studies. Integrating elemental fingerprinting with soil composition analysis enhances the accuracy of geographic origin differentiation, providing a more robust framework for authentication and quality control in agricultural products.
To further validate these findings, Figure S2 presents the moving range chart for elemental concentrations in Hoa Loc mango samples from Tien Giang, Vinh Long, Dong Thap, and Can Tho. This visualization provides a clear representation of elemental variation across regions, supporting the application of PCA and LDA in food authentication. The effectiveness of these statistical approaches in tracing geographic origin and ensuring quality control in agricultural products is evident in the distinct clustering of elemental variables.
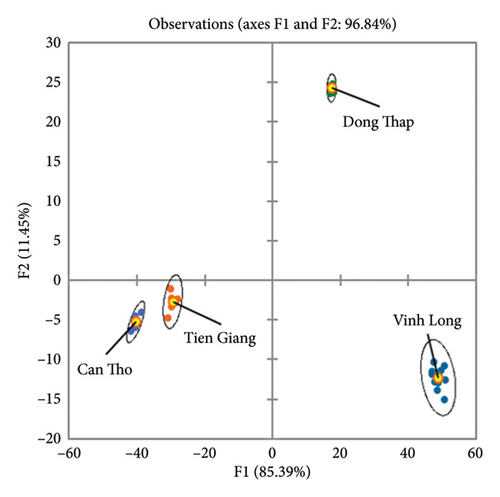
The LDA results in Figure 4 further demonstrate the ability of elemental profiling to distinguish mango samples by geographic origin. The LDA model effectively separates the samples into distinct clusters, with the first two linear discriminants accounting for 96.84% of the total variance. Can Tho samples, characterized by higher Mg and Cr levels, form a separate cluster, whereas Vinh Long samples, with elevated Fe concentrations, are clearly distinguished. Dong Thap samples are defined by Co and Ni content, while Tien Giang mangoes, despite lacking extreme values for any specific isotope, show notable Mn and Ba levels. The LDA clustering confirms the role of elemental composition as a reliable marker for traceability, ensuring robust authentication and quality control in food production.
The integration of PCA and LDA into food traceability systems significantly enhances authentication accuracy, helping to prevent food fraud and maintain consumer trust. These statistical techniques provide scientifically validated methods for verifying geographic origin, supporting market integrity and product differentiation. By leveraging unique elemental profiles, stakeholders can implement effective quality control measures, ensuring the authenticity and premium quality of Hoa Loc mangoes. This study underscores the importance of precise analytical techniques in safeguarding regional agricultural products, benefiting both producers and consumers by reinforcing trust and reliability in the global market.
4. Discussion
PCA is an essential statistical tool for analyzing complex datasets that require the simultaneous examination of multiple variables to identify patterns and trends [37]. By reducing high-dimensional data into a smaller set of uncorrelated principal components, PCA facilitates a clearer interpretation of relationships among variables while preserving the most significant variance. However, handling large datasets in food authentication research presents computational challenges, including the need for significant processing power, memory resources, and optimization techniques to maintain analytical speed, precision, and reliability. The extensive volume of data generated by ICP–MS and other high-throughput techniques requires sophisticated statistical modeling to distinguish geographic origins and ensure food traceability. To address these challenges, advanced computational approaches, such as parallel processing, high-performance computing (HPC), and cloud-based solutions, can significantly enhance data analysis efficiency [38–40]. These methods allow for the effective management of large-scale authentication datasets while ensuring the reproducibility of results. In addition, feature selection algorithms and dimensionality reduction techniques help maintain interpretability without compromising statistical accuracy. These computational improvements are particularly relevant in elemental fingerprinting studies, where distinguishing between intrinsic and extrinsic sources of variation is essential for accurately identifying geographical markers in agricultural products.
The elemental composition of mangoes is influenced by both natural and anthropogenic factors, with soil characteristics, climate, and agricultural practices playing central roles [41–43]. Variations in Mg, Fe, and Zn concentrations can be directly linked to the geochemical properties of the soil, shaped by geological formations, weathering processes, and nutrient bioavailability. For instance, Mg is crucial for chlorophyll synthesis and photosynthetic efficiency, while Fe and Zn serve as cofactors for enzymatic processes that regulate fruit development and ripening. Agricultural inputs, including fertilization, irrigation, and pest control strategies, significantly influence mineral uptake in plants. The application of phosphate fertilizers, for example, can increase phosphorus levels in mango tissues whereas pesticide use may alter the bioavailability of trace elements such as Cu and Zn. In addition, organic versus conventional farming practices may result in distinct elemental profiles, as organic systems rely on natural soil amendments and composting, leading to variations in soil microbiota and nutrient cycling [44–46]. These findings highlight the necessity of integrating elemental profiling with agronomic data to ensure that authentication methods account for both natural soil mineral composition and external agricultural influences on food authenticity.
Beyond soil composition, environmental conditions such as rainfall patterns, temperature fluctuations, and atmospheric deposition influence the bioaccumulation of elements in mango tissues. Studies have demonstrated that high Fe and Mn concentrations in soil contribute to elevated levels in plant tissues whereas alkaline soils limit the uptake of Zn and Cu, affecting fruit quality and mineral density. Precipitation and irrigation regimes also play a crucial role in nutrient mobility, with high rainfall regions experiencing greater leaching of mobile elements such as potassium (K) and Mg, leading to reduced availability in fruit. Conversely, low-rainfall areas often accumulate salts and minerals, resulting in higher concentrations of certain trace elements. The use of fertilizers and pesticides further modulates elemental uptake, as phosphorus-based fertilizers can enhance Cd absorption, while Cu-based fungicides may elevate Cu concentrations in fruit tissues. These findings reinforce the importance of integrating soil analysis with elemental fingerprinting techniques to enhance food traceability and geographic authentication in agricultural commodities.
The integration of ICP–MS with phytochemical profiling provides a comprehensive approach to characterizing Hoa Loc mangoes, improving traceability, quality assessment, and authentication accuracy. While ICP–MS excels in detecting elemental signatures, its true value lies in its ability to correlate elemental findings with nutritional and phytochemical properties. Mangoes are rich in polyphenols, flavonoids, carotenoids, and vitamin C, all of which contribute to antioxidant potential and health benefits. Several studies suggest that mineral composition influences metabolic pathways linked to these bioactive compounds, affecting fruit ripening, flavor development, and overall nutritional quality. For example, Mg and Zn, which were key differentiating elements in this study, regulate enzyme activation, oxidative stress resistance, and sugar metabolism, directly impacting fruit sweetness and sensory appeal. These findings suggest that understanding the interactions between specific elements and bioactive compounds could further refine authentication models and inform agricultural best practices to enhance fruit quality.
While PCA and LDA effectively classify mango samples based on elemental composition, it is important to recognize that bulk analysis alone cannot fully define food quality or authenticity. Elemental profiling should be considered one component of a broader analytical framework, incorporating physicochemical parameters (pH, total soluble solids, and titratable acidity), sensory attributes, and nutritional analyses. Integrating multiple analytical techniques improves accuracy and reliability, ensuring that findings are applicable in both scientific research and agricultural quality control. This multidimensional approach enhances supply chain traceability, helping Vietnamese mango producers meet international export standards while maintaining product integrity.
The classification of Hoa Loc mangoes into Type 1 (export quality) and Type 2 (domestic consumption) is traditionally based on sensory and morphological attributes, but our findings suggest that elemental profiling may serve as an additional quality indicator. The higher concentrations of Mg, Cr, and Cu in Can Tho mangoes may enhance postharvest longevity, texture, and resistance to spoilage, making them more suitable for export markets. Similarly, higher Fe levels in Vinh Long mangoes could influence flesh pigmentation and oxidative stability, affecting consumer perception and storage potential. Further research is needed to establish specific elemental thresholds for mango classification, but these results highlight the potential role of elemental analysis in refining quality grading systems and optimizing cultivation strategies for market segmentation.
The potential to incorporate elemental profiling into a traceability and quality control database represents a major advancement in food authentication research. As demonstrated in Figure S2, the distinct elemental fingerprints of mango samples suggest that a standardized reference database could support geographic authentication and fraud prevention in the mango industry. This database could be integrated into blockchain-based traceability systems, allowing stakeholders (farmers, exporters, regulatory agencies, and consumers) to verify mango authenticity with high confidence. By combining ICP–MS, advanced statistical modeling, and digital traceability technologies, this research enhances transparency, strengthens regulatory compliance, and protects the reputation of Vietnamese mango exports in global markets.
The growing importance of food authentication has led to the development of alternative analytical methods, including microbial fingerprinting, metabolomics, and sensor-based detection technologies [47–49]. Microbial fingerprinting, based on the principle that microbial communities are unique to their geographical environment, has been successfully applied to organic food authentication and fermented product verification [50, 51]. Metabolomics, utilizing NMR, GC-MS, and LC-MS, provides comprehensive chemical profiling, particularly for flavor and bioactive compound authentication [52]. In addition, sensory analysis incorporating electronic tongue, nose, and eye technologies enhances consumer-oriented quality control and authentication [53, 54]. However, ICP–MS remains the most robust and stable method for elemental fingerprinting, as it is unaffected by processing, storage conditions, or external contamination, making it ideal for long-term traceability and market verification.
Integrating multiple authentication methods strengthens food traceability systems, ensuring fraud prevention and consumer protection [55]. For example, combining ICP–MS with chromatography-based separation techniques (HPLC and GC) allows for detailed characterization of molecular species, enhancing the precision of food authentication models. Geographic Information Systems (GISs) and climate data analytics further improve food origin prediction, while portable ICP–MS technologies offer real-time authentication capabilities [56]. As food authentication technology evolves, integrating high-performance computing, blockchain, and big data analytics will reshape global food traceability frameworks, ensuring the integrity and authenticity of agricultural products in international markets [57]. This will ultimately contribute to the broader field of food science research, ensuring the authenticity and quality of food products in the global market.
Future research should focus on expanding the scope of elemental profiling by incorporating a broader range of environmental variables, such as humidity, light exposure, and soil microbial interactions, to better understand their influence on mineral uptake and mango quality. In addition, longitudinal studies tracking elemental composition across multiple harvest seasons would help assess the stability of geographic markers and the impact of climate variability on food authentication. Advancements in machine learning and artificial intelligence should also be leveraged to develop predictive models for traceability, integrating multielemental data with physicochemical and sensory properties for more robust authentication frameworks. Furthermore, the standardization of global traceability databases, coupled with the implementation of portable and field-adapted ICP–MS systems, could enhance real-time verification and fraud prevention in the mango supply chain. These efforts will contribute to strengthening food safety, regulatory compliance, and consumer confidence in high-value agricultural commodities.
5. Conclusion
The use of ICP–MS provided significant insights into the elemental composition of mangoes from various regions, enabling clear distinctions among them. Elements such as Mg, Cs, Fe, Ag, Mo, Sr, Sb, Rb, Zn, and Cu were identified as key contributors to these distinctions, emphasizing the role of elemental composition in food product characterization. PCA effectively managed multidimensional datasets, transforming the data into principal components that revealed significant differences in the metal element profiles of mango samples from different regions. Six variables, namely, Mg, Cr, Mn, Zn, Cu, and Pb were critical in distinguishing the samples, demonstrating PCA’s effectiveness in dimensionality reduction and variable identification. In addition, LDA further refined the classification, with the first two linear discriminants accounting for 96.84% of the total variance. This approach highlighted high levels of Mg and Cr in Can Tho samples and elevated Fe content in Vinh Long samples as distinguishing factors. These findings illustrate the complexity of mango data and the benefits of combining ICP–MS with PCA and LDA to uncover patterns and regional differences. Integrating these advanced statistical methods underscores their importance in managing and interpreting complex datasets, contributing to food science research, quality control, and safety evaluation.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
T.N.M. and T.H.M.D.: conceptualization; B.Q.M., N.Q.T., and N.T.D.: methodology; T.H.M.D., H.M.T., and N.V.K.: software; L.V.N., L.V.A., and N.N.T.: validation; L.V.N., N.B.N., and N.V.K.: formal analysis; L.V.A., H.M.T., and N.H.K.: investigation; B.Q.M.: resources; L.V.A. and N.B.N.: data curation; T.H.M.D., N.T.D., and N.Q.T.: writing–original draft preparation; T.N.M.: writing–review and editing and project administration; L.V.N. and N.N.T.: supervision; B.Q.M: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Vietnam Academy of Science and Technology under grant number CN4000.02/22-25.
Acknowledgments
The authors acknowledge the Center for High Technology Research and Development, Vietnam Academy of Science and Technology, for supporting this research. We also express our gratitude to Dr. Nguyen Van Hong from the Institute of Geography for his assistance in providing geographical mapping of the sampling locations.
Supporting Information
The following supporting information is Figure S2: A moving range chart of (a) Mg, (b) Al, (c) V, (d) Cr, (e) Mn, (f) Fe, (g) Co, (h) Ni, (i) Cu, (j) Zn, (k) As, (l) Se, (m) Rb, (n) Sr, (o) Mo, (p) Ag, (q) Cd, (r) Sb, (s) Cs, (t) Ba, and (u) Pb. The vertical axis represents the content (μg/g, dried weight basis) of each element in the sample, while the horizontal axis indicates the names of the regions.
Open Research
Data Availability Statement
The data used to support the findings of this study are available on request from the corresponding author.




