The Caucasian Dwarf Goby (Knipowitschia caucasica)—Population Dynamics and Feeding Ecology in the Lower Rhine With a Special Focus on Winter
Abstract
Artificial expansion of shipping routes in the course of international trade opens up fish migration routes for numerous species. Since 2006 migration of four different goby species took place from the Ponto–Caspian region to the Lower Rhine. Neogobius fluviatilis, Neogobius melanostomus, Proterorhinus semilunaris and Ponticola kessleri were able to establish in the local fish community of the Lower Rhine. Besides the four other goby species, Knipowitschia caucasica migrated from the Ponto–Caspian region to the Lower Rhine as well in 2019. Due to increasing abundance in the last years and lack of information regarding population dynamics, feeding activity and prey preferences of the dwarf goby, the length of 1218 Caucasian dwarf gobies was analysed as well as the diet of 519 individuals in the course of May 2021 to February 2022. The populations of three different locations along the Lower Rhine were examined in winter on differences in population dynamics and feeding ecology. Analysis of the population over time suggests that K. caucasica in the Lower Rhine is an annual species with spawning during summer. The juvenile individuals of the Caucasian dwarf goby increased in total length until winter, while the adults vanished after spawning. Both juveniles and adults mainly fed on zooplankton and insect larvae. The feeding activity of the dwarf goby was higher in summer than in winter and started to increase as early as February. No difference in winter prey was recorded between populations at different sites, as all populations fed mostly on copepods and chironomids.
1. Introduction
With the completion of the Main-Danube Canal in 1992, a commercial waterway for shipping a wide variety of goods between the Rhine Delta at Rotterdam in the Netherlands and the Danube Delta in south-western Ukraine and south-eastern Romania was created [1]. As manmade waterways and shipping are the most important dissemination channels, the Main-Danube Canal enables many species, such as gobies, to invade new habitats across Europe [2, 3].
This resulted in the migration of the monkey goby Neogobius fluviatilis, the round goby Neogobius melanostomus, the tubenose goby Proterorhinus semilunaris and the big head goby Ponticola kessleri, which all have their native range in the Ponto–Caspian Region and now are establishing in the local fish community of the Lower Rhine since 2006 [4, 5]. For these species, it is documented that the most preferred prey are fish, crustaceans, chironomids and molluscs [5, 6]. Especially, for the small individuals of the invasive gobies, chironomids are an important food resource [5]. In 2019, a new record of another invasive goby in the Lower Rhine near the city of Wesel-Bislich was made. A specimen was caught with high similarities to individuals from the Knipowitschia genus, which was classified belonging to the Knipowitschia caucasica–complex [7]. Besides the Lower Rhine, K. caucasica already populates in numerous areas in the Ponto–Caspian region, like the other invasive gobies, such as the Aral Sea, the North Aegean Sea, the Adriatic Sea, the Evros Delta [8, 9] and freshwaters in the Ukraine such as the Stugna River and the Dnieper Reservoir [10]. The euryhaline character of K. caucasica enables it to live in saltwater habitats such as the Mediterranean Sea and freshwater habitats such as the Lower Rhine [8]. Few studies on feeding and population dynamics of K. caucasica exist, studying fish in its native range and in Ukrainian or Balkan water bodies [8, 10]. Data about the Caucasian dwarf goby population in the Lower Rhine in Germany is, however, very limited. To find out more about the seasonal changes in population dynamics and feeding of K. caucasica in the Lower Rhine throughout the year, this study was performed. Because the presence of the K. caucasica has been documented in multiple oxbows of the Lower Rhine, such as the floodplain in Bislich [7] and the Bienener Altrhein [11], the population of K. caucasica was additionally examined on feeding differences due to location disparities during winter.
2. Materials and Methods
2.1. Sampling Area
To compare data from K. caucasica individuals from different locations, fishing was carried out at three different sites at the Lower Rhine in North Rhine-Westphalia, Germany (Supporting Figure 5). The first location Rheinaue Bislich (BIS)–Vahnum (decimal degree: 51.6708061, 6.4851405), in the following always referred to as Bislich (BIS), is a branch of the River Rhine between Rhine-kilometre 823 and 827. A meadow with a few trees borders the shore of the side channel, and at low water levels, the shore zone has a slight slope. The riverbed consists of sand and small stones. The gradient of the shoreline increases and the habitat changes, with rising water levels. The near meadow with trees was flooded on the second date (January 12th) of sampling in winter.
The second location Grietherorther Altrhein (GAR) (decimal degree: 51.7924325, 6.3361441) is a backwater of the Rhine between Rhine-kilometre 845 and 848. The shoreline of GAR has reeds as vegetation and trees grow nearby. The habitat changes with rising water, and at low water levels, the shore zone has a decent slope, and the riverbed consists of mud, sand and stones. At the second sampling date (January 11th), the high-water level resulted in a flooded meadow with trees and grasses building the habitat additionally.
The third location Bienener Altrhein (BAR) (decimal degree: 51.8050588, 6.3629595) is a backwater of the Rhine between Rhine-kilometre 845 and 848. The shoreline of the sampling site has lots of reeds as vegetation. The riverbed is very muddy, and the shore zone has almost no gradient.
2.2. Water Level and Temperature
Data for the water temperature of the sampling area BIS were provided by the State Office for Nature, Environment and Consumer Protection of North Rhine-Westfalia, measured in Bimmen in the main stream of the River Rhine at 865 km. Measurements of the daily water levels are obtained by the electronic waterway information service (ELWIS) from the monitoring station in Emmerich (Rhine main stream at 852 km) and are used in the following as a proxy for BIS. Bimmen is about 40 km from connection of BIS oxbow to the Rhine, and Emmerich is about 27 km away. As BIS is highly dependent on the Rhine in temperature and water level, no differences were found for both parameters between the two measuring station in the Rhine main stream and the oxbow of BIS (own studies, not published). Water level and temperature data for the location BAR were provided by the Nature Conservation Centre, Kleve, and measured multiple times a day at the BAR oxbow (decimal degree: 51.80598, 6.36319). No data could be provided for GAR as no measuring stations are situated there or nearby.
2.3. Fish Sampling
Beach seining was performed at GAR, BAR and BIS. The sampling always took place between 1 a.m. and 4 p.m., once per month per location. For the beach seining, a net with the length of 10 m and the width of 1.5 m was used. The net had a mesh size < 1 mm. For each stretch, the net was dragged by two persons, one inside the water, the other one at the waterline of the shore for 20 m per stretch (for details see also [12, 13]). The net was dragged against the current as a current was visible, but always parallel to the shoreline. Depending on the area, the total numbers of stretches sampled differed (1–14 stretches). Sampling in GAR and BAR was performed only during winter months, without success in December in BAR.
After each stretch, the net was controlled for any fish to be caught. All fish were counted, if possible, determined to the species level by their external characteristics and documented. Gobies were killed by a knock or flick on their head and put into 96% ethanol to be fixed. All other fish were measured to the nearest millimetre and put back to the river.
2.4. Laboratory
In the laboratory, the weight of all individuals of K. caucasica was documented with a digital scale to the nearest 0.001 g. Total length, the head width at the broadest point of the head, eye distance and mouth width were measured to the nearest 0.01 mm with a digital calliper. After cutting the individual ventral from the anus to the cranium and opening the body, all intestines were removed, and the gills were cut off at the oesophagus. The liver and the stomach (liver and stomach full) were weighed separately with a digital scale to the nearest 0.001 g. The stomach was gently emptied on a Petri dish and weighed again (stomach empty). All prey items were defined as accurate as possible, and the percentages of the diet items were estimated, for further details see [5, 13].
2.5. Data Analysis
The ISF-values of the individual months were statistically compared using the unpaired Mann–Whitney U test of python and for significant differences in size, one-way ANOVA was performed with Python (Python version 3.11).
In total, the dataset contains biometrical data of 1218 individuals and information about stomach analysis from 519 individuals of K. caucasica. General analysis of the stomach content and the ISF was performed regardless the size of the individual. For further analysis on the differences between individuals < 26 mm and ≥ 26 mm of K. caucasica, the sample got divided into two groups of different lengths’ classes.
3. Results
3.1. Water Level and Temperature
Over the three winter months, the water levels strongly fluctuated in all three sampling locations with the amplitude of changes differing between locations. The level measured in BAR was between 12.4 m minimum and 13.0 m maximum, whilst in BIS, the minimum of 0.82 m and maximum of 6.13 m was reached (Supporting Figures 3 and 4). Both maximum values were measured in January, in BAR on the third of January and in BIS on the eighth of January. Concerning the temperature, BIS showed fluctuations between 4.7°C and 8.5°C. Water temperatures measured in BAR were between 1.7°C and 9.9°C (Supporting Figures 1 and 2). Fluctuations of water levels also appeared in GAR throughout the winter, and we assume that the temperature in GAR is similar to that in the Rhine (BIS), as the data were collected in winter, there was no major warming and therefore no major temperature fluctuations between the oxbow and the main stream.
3.2. Population Dynamics and Feeding Over the Seasons in Bislich
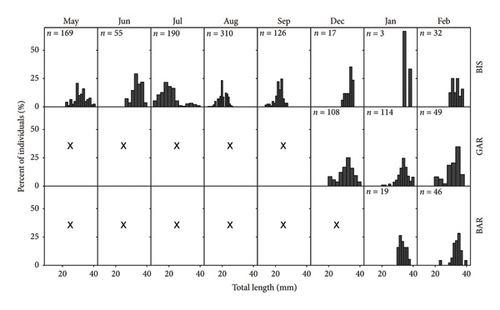
In total, 902 K. caucasica were caught in BIS. The number of individuals caught per sample differed over the seasons, with a maximum in August (n = 310) and a minimum in January (n = 3) (Figure 1). Individuals of K. caucasica caught in BAR between May and February were between 13 mm and 41 mm in size (mean 24.2 ± 6.62 mm) and 0.02 g–0.59 g in weight (mean 0.12 ± 0.09 g). In May and in June, only one size cohort of large individuals was visible in the studied population (Figure 1). In July, two size cohorts could be documented with a high number of smaller individuals appearing. The average length decreased to a minimum of 20 ± 6 mm until July. In August, however, only one size cohort was visible again with the largest individuals having disappeared. The following month, the size-distribution stayed unimodal and the average total length continuously increased until December (mean 33.19 ± 2.28 mm). From December until February, there was no change in the average total length (one-way ANOVA, p = 0.322), while the sample from January was disregarded due to a small sample size.
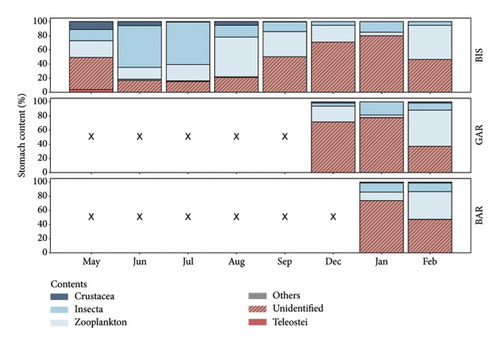
The stomach content of the BIS individuals changed between summer and winter months (Figure 2). In May, the stomachs contained a majority of zooplankton (23.8%) and insects (15.8%). Nevertheless, the proportion of unidentifiable items (45.1%), that were too digested to identify, was the largest. In June and July, K. caucasica mainly fed on insects, (59.0% and 60.0%, respectively) then shifting to zooplankton again as a preferred food resource from August onwards (56.0%). The amount of unidentified food items increased from August until December steadily up to 70.9%. The highest amount of unidentified food items was found in January (80.0%). In February, the main stomach content was zooplankton (48.2%), and a decline in the unidentifiable items (46.5%) was noticeable (Figure 2).
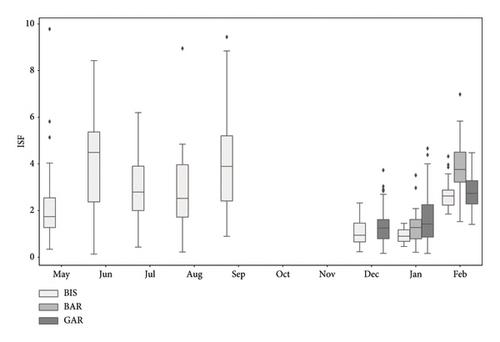
The ISF values in summer (June–September) were on average always higher than in December and January (Figure 3, Wilcoxon rank sum test: p < 0.05, for details, see Supporting Table 1). The highest mean ISF (4.23 ± 2.13) was found in September, while the lowest ISF (0.94 ± 0.5) appeared in January. During summer, the ISF values changed significantly between May and June (Wilcoxon rank sum exact test: p = 0.009) and August and September (Wilcoxon rank sum exact test: p = 0.005). A significant decrease in ISF was shown between September and December (Wilcoxon rank sum exact test: p < 0.001) and a significant increase of ISF from February compared to December (Wilcoxon rank sum exact test: p < 0.001) and January respectively (Wilcoxon rank sum exact test: p = 0.005). No significant changes were noticeable between December and January (Wilcoxon rank sum exact test: p = 0.560).
3.3. Population Dynamics and Feeding in Winter
Since the three sites, BIS, GAR and BAR have different characteristics that may affect the population and feeding of K. caucasica, the samples from the three sites were compared to each other, for variations in abundance, total length and feeding. The number of individuals caught on the different dates and locations fluctuated (Figure 1). At the GAR and in BIS on all three sampling dates, individuals of K. caucasica were caught. On the sampling in December in BAR, no K. caucasica were caught, and on the sampling day in January in BIS, only three specimens were captured. The size of all individuals caught in winter ranged from 19 to 41 mm total length. A major part of each population, regardless of the location and month caught, was between 30 and 36 mm of total length. The average length of K. caucasica did not deviate substantially from December until February in GAR (one-way ANOVA: p = 0.644) and from January until February in BAR (one-way ANOVA, p = 0.647). Besides two individuals from BAR, only in GAR individuals with a length below 26 mm appeared constantly in the population throughout the winter.
To test for site-specific differences in feeding, the diet of individuals of all three water bodies was compared, regardless of their size. No differences appeared in comparing the stomach contents of the individuals from the different locations to another. The stomachs of the samples from December and February from all locations contained in particular zooplankton, especially copepods. In January, the identifiable food items in the analysed stomachs from all sites were mostly chironomids. During December, individuals from BIS and GAR had a majority of unidentified food items in their stomachs. The individuals from all locations showed a decrease of unidentifiable food items in the stomachs from January to February. To test for size-specific differences in feeding, the stomach contents of the two size classes, < 26 mm and ≥ 26 mm were compared with each other. Both size classes had similar stomach contents.
The average of the ISF from each sampled location during winter was displayed and showed significant alterations (Figure 3). The samples of all locations showed an increase in average ISF from January to February (Wilcoxon rank sum exact test: p = 0.005, p < 0.001 and p < 0.001, respectively, for BIS, GAR and BAR).
4. Discussion
4.1. Population Dynamics and Feeding Over the Seasons in Bislich
The results of the 8-month examination of the Caucasian dwarf goby population of BIS showed that reproduction of K. caucasica probably takes place between May and June. Evidence for this was the missing juvenile-sized individuals in the months May and June in combination with the small-sized appearing in the samples of July. As the larvae of K. caucasica need about 10 days to reach the total length of 5 mm [15], and the smallest individual in the sample of July had already a length of 10 mm, spawning must have taken place in the months before. In July, a bimodality can be seen in the population, which is due to the simultaneous appearance of juveniles and adults. Until August, the juveniles make up most of the population and the adults vanish. This could be due to the migration of the adult fish, which retreat to deeper waters after spawning. Migrations specially to avoid cold temperatures in winter have already been documented for other goby species such as the common goby Pomatoschistus microps and for other populations of K. caucasica as well [10, 16]. Another approach would be the death of the adults after spawning, which is already documented for the dwarf goby population in the Stugna River and is therefore more likely, since at the time of the disappearance of the adults there were no clear differences in the water temperature [10]. Thus, it can be assumed that K. caucasica is also an annual species in the Lower Rhine, such as in its native region and in water bodies in the Ukraine where it is non-native [8, 10].
From July until December, the small individuals from BIS grew into adult-sized gobies, as already reported from K. caucasica in other fresh waters [10]. Over the period from December to February, a phase of no growth is estimated since the population from December (33 ± 2 mm) has nearly the same total length as the population in February (34 ± 3 mm).
The stomach analyses of the eight samples show an alteration of prey consumed between summer and winter by K. caucasica. The proportion of unidentified food items in the analysed stomachs varied in between the months. From May to June, the proportion of unidentifiable diet items in the stomachs decreased, then remained relatively unchanged over the summer months and increased again substantially from September onwards, with a maximum during winter. The high quantity of unidentifiable food items in winter can possibly be explained by the fact that the fish sampled have not eaten for a longer period, therefore food was more digested. Another reason could be a higher digestion rate, as is already assumed for the shimofuri goby, Tridentiger bifasciatus [17], to more effectively utilise nutrients when food supply is low. The change of the average ISF over the year was the opposite, with a maximum in the summer months (September: 4.23) and a minimum in winter (December: 1.09, January: 0.94). Together the results indicate an increased consumption of prey in summer and show a decrease in feeding activity in winter. Comparable seasonal changes in consumed prey and an increase in stomach fullness towards summer have also been documented for other goby species such as the bay goby Lepidogobius lepidus and the tubenose goby P. semilunaris [18, 19] and fits the seasonal dynamics of K. caucasica in the Stugna River [10].
In the lower Stugna River, the dwarf goby feeds on copepods, Cladocera and larvae of chironomids, while in the Evros Delta benthic amphipods and polychaetes are its main prey [8, 10]. In the Lower Rhine in May, the analysed dwarf gobies were most likely to eat zooplankton with a total probability of 24%, split into 19% copepods and 5% Cladocera. The preferred prey in June and July were insects with the likelihood of at least 59% to be found in the analysed stomachs. Nearly all items of insects being detected through stomach analysis of the gobies belong to chironomids (59% in June and 57% in July). In August and September, the proportions alter again, so zooplankton was the preferred prey of the population sampled in August and September. As well as in May, most of the consumed zooplanktons were copepods. A preference of copepods over Cladocera as prey for freshwater dwarf gobies in the Ukraine has been documented before [10]. An explanation for the shifting ratios of consumed prey can be the abundance of each prey in the water. Individuals of K. caucasica may consume what is most abundant and are not focussed on one certain type of prey. Nonselective eating but consuming on what is most abundant is already known for the round goby, which feeds during summer more on chironomids as well [20, 21]. To confirm the hypothesis of feeding on what is most abundant, further information about the quantity of the individual prey categories in the Lower Rhine is necessary.
4.2. Population Dynamics and Feeding in Winter
Comparing the sample sizes of the winter with the ones from summer in general, a difference in abundance is noticeable. The highest amount of K. caucasica caught in summer in BIS (n = 310) exceeded the biggest sample of the winter by a factor of nearly 10 (n = 32). An explanation for this could be that most of the juveniles may be eaten by their predators during autumn. Another reason for the decrease of the population during winter might be again attributed to the migration into deeper waters as it is documented for other populations of K. caucasica [8] and is believed to reduce the risk of predation of fish in general [22, 23]. The latter explains the regrowth of the sample in February. The small sample in January can therefore also be attributed to the lack of success in sampling, instead of a lack of K. caucasica. The low success in sampling was due to an elevated water level in January (Supporting Figures 3 and 4). The high-water level made it difficult to complete an effective beach sein unlike the other months.
The analysis of K. caucasica shows commonalities and differences between the populations of the different sites at the Lower Rhine. No growth in total length was observed for the individuals from all locations during winter. Nevertheless, when comparing the length frequency distribution, only in GAR juvenile-sized individuals appeared throughout the winter. The appearance of small individuals on each of the three dates in GAR can have several reasons. A cause could be a different type of preferred prey between adults and juveniles, which enables the juveniles to survive during winter despite little food resources. Such differences have already been found for other gobies such as the round goby and the racer goby [24, 25] but would not explain why only in GAR juvenile-sized specimen were sampled continuously during winter and not in BAR and BIS as well. In addition, for K. caucasica at GAR, no differences in prey regarding the size of the individual were seen. The nonappearance of the small-sized individuals in BAR and BIS is rather due to the migration of individuals away from the shallow into deeper waters [16, 22, 23]. This would attribute the absence of small-sized individuals to a methodological error where individuals did not enter the net during sampling the shallow water because they were in deeper waters of BAR and BIS. For the two small-sized specimen of K. caucasica in the February sample of BAR, it is likely that they migrated back to the littoral zone for feeding, as the shallow coastal zone represents a preferred habitat for juvenile round gobies in terms of feeding and growing as well [26].
Diet shifts within one species due to different settled habitats have already been documented for the round goby N. melanostomus and the common goby Pomatoschistus microps and may be a possible explanation for K. caucasica as well [24, 27]. But in contrast to differences in length distribution, there were no differences between the prey consumed in the different locations. Although the sites form different habitats with varying amounts of vegetation and differing sediments, in all sampled areas K. caucasica fed on macrozoobenthic organisms such as larvae from chironomids and on zooplankton such as Cladocera and copepods. Compared to already documented data of K. caucasica mainly feeding of benthic amphipods in its native range of the Evros Delta [8], K. caucasica additionally feeds on free-swimming zooplankton in the backwaters of the Lower Rhine.
The water temperatures in BIS did not differ distinctively between the 12th of January (5.91°C daily average) and 15th of February (6.14°C daily average). Nevertheless, the feeding activity, interpreted by the ISF, rose significantly from January to February. Usually, the feeding activity of fish is temperature-dependent, like for the round goby, which usually migrates back to the littoral for feeding, as the water temperature rises to 10°C after winter [26, 28, 29]. The fact that the populations of all three locations show a distinctive increase in mean ISF, regardless the water temperature, leads to the assumption that it is species-specific activity. This property might be useful to gain enough energy to successfully develop gonads for reproduction in May and June, as the reproductive performance of fish is highly dependent on the nutrition [30].
5. Conclusion
K. caucasica is an annual species in the Lower Rhine with a narrow spawning period once a year in early summer. No habitat-specific diet was observed as K. caucasica fed on zooplankton and macrozoobenthos in all three locations to a similar extent. The feeding activity was clearly higher in summer than in winter and already increased significantly again in February.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was funded by Universität zu Köln.
Supporting Information
Supporting Figure 1: Water temperature (°C) in BAR, measured by nature conservation centre, Kleve, and State Office for Nature, Environment and Consumer protection.
Supporting Figure 2: Water temperature (°C) in Bimmen, measured by nature conservation centre, Kleve and State Office for Nature, Environment and Consumer protection.
Supporting Figure 3: Water level (m) in BAR, measured by nature conservation centre, Kleve, and federal waterways and shipping administration during winter.
Supporting Figure 4: Water level (m) in Emmerich, measured by nature conservation centre Kleve and federal waterways and shipping administration during winter.
Supporting Table 1: Wilcoxon rank sum exact test p values of ISF, sampled in the months June–September and December–February in BIS.
Supporting Figure 5: Map of Lower Rhine, close up between Rhine km 815 Wesel and 854 Emmerich. Rhine and oxbows are shown in light blue, and other waterbodies shown in dark blue. Sources: GeoBasis-DE/BKG. Geoportal.nrw.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




