Theoretical Design of H2S Fluorescent Probes Based on π Expansion and a Strong Electron-Withdrawing Group
Abstract
Hydrogen sulfide (H2S) regulates crucial physiological and pathological processes, and its dysregulation is linked to serious diseases. A sensitive and selective imaging method is essential for detecting endogenous H2S in complex biological systems. The probe benzo1-DCM-O-NBD and its product benzo1-DCM-OH, derived from π expansion of the DCM-O-NBD probe, have been studied for their luminescence mechanisms. Investigations into the effects of various geometric modifications on their optical properties have been conducted. Results of calculation reveal that the introduction of strong electron-withdrawing substituent groups (F, Cl, Br, and CN) into the benzopyran position of benzo1-DCM-OH effectively suppresses molecular skeleton scissoring vibrations and significant geometric relaxations associated with line-type π expansion, thereby enhancing fluorescence emission. This approach also enhances two-photon absorption (TPA) ability and spectral resolution. CN-benzo1-DCM-OH particularly exhibits excellent two-photon fluorescence properties. These studies have led to the identification of potential materials for biological imaging and H2S detection that provide easily distinguishable spectra (with a Stokes shift of up to 85.43 nm), improved luminous efficiency (14.1%), and a larger effective TPA cross section (295 GM at 950 nm). This research provides a more detailed theoretical framework for the design of novel two-photon excited fluorescent probes.
1. Introduction
Hydrogen sulfide (H2S) is an important endogenous gaseous signaling molecule [1–3]. It regulates many important physiological functions, including cardiovascular, gastrointestinal, immune, neuromodulatory, and endocrine systems, and regulates many crucial physiological and pathological processes [4–6]. However, when H2S levels become imbalanced in living systems, it would disrupt normal physiological processes, which would result in a variety of human diseases, including diabetes, Alzheimer’s disease, cirrhosis of the liver, and Down syndrome [7–11]. Therefore, the development of a sensitive and highly selective imaging method to detect endogenous H2S levels in complex living systems is important for the study of relevant biological processes.
Currently, there are severe major methods for H2S detection, including electrochemical analysis, gas chromatographic methods, colorimetric methods, and sulfide precipitation methods [12]. However, it is difficult for these traditional detection methods to overcome the challenge of achieving rapid and precise real-time measurement of H2S levels in organisms from the rigorous demands of sample preparation and the complexities involved in extracting H2S from organisms. [13]. To date, various fluorescent probes have been deeply investigated and developed as effective imaging tools for the real-time detection of H2S levels in organisms, which is contributed to their simplicity of operation, excellent selectivity, noninvasive nature, high sensitivity, real-time imaging capability, and high spatial resolution [9, 10, 14–19]. However, most fluorescent probes are designed based on one-photon excitation with relatively short excitation wavelengths, and these excited fluorescent probes have the following limitations: (1) Many of the reported H2S probes require long response times, which might be a disadvantage for real-time tracking and bio-imaging of reactive and transient H2S in vivo. (2) Most of these fluorescent probes have excitation and emission wavelengths in the ultraviolet to visible region, which greatly restricts their practical application in biological imaging. (3) Unavoidable interference of background fluorescence and the shallow depth of tissue penetration. These limitations make it difficult to realize further applications of one-photon excitation fluorescence (OPEF) probes in living systems [15, 16, 20–22]. In contrast to these drawbacks of OPEF probes, two-photon excited fluorescent (TPEF) probes are capable of absorbing two photons with low energy simultaneously and thus have the advantages of deep tissue penetration, less photodamage to tissues, and high spatial-temporal resolution [23–25], which makes it attract more attention. However, the relatively small two-photon absorption (TPA) cross-section of TPEF probes, along with the still poorly understood internal mechanisms, deeply limits their practical application [25–27]. Consequently, further research is needed to develop novel TPEF probes with larger TPA cross-sections and higher luminescence efficiency.
Since the first H2S probe construction based on the fluorescence resonance energy transfer (FRET) strategy using 7-nitrobenzofurazan (NBD) by Wei et al. in 2013 [28], many H2S detection probes based on NBD have been designed and synthesized. To date, Xin et al. [29] appropriately designed and synthesized three H2S probes with NBD-piperazine at different positions of BODIPY using BODIPY and NBD as fluorophore and quenching abilities and systematically investigated their fluorescence properties for H2S detection. The position of the NBD moiety in the probe showed different fluorescence-quenching abilities. They found that the distance between NBD and the core of BODIPY affected the performance of the three probes. The probe with the smallest distance (o-BNP) has the best selectivity and the lowest detection limit (105 nM). The experiments of Ju et al. [14] described a near-infrared fluorescent probe DCM-NBD that employs DCM-OH as a fluorophore and NBD as a recognition group. This probe showed ultrafast response and high selectivity to H2S with a response time as low as 5 s. A large Stokes shift (120 nm) and near-infrared emission (690 nm) of DCM-NBD is suitable for biological imaging. In addition, Liu et al. [19] developed a new type fluorescent probe (DCM-O-NBD) for monitoring the endogenous H2S levels in complicated living systems, which is designed based on dicyanomethylene-4H-pyran (DCM) and incorporates a nitrobenzoxadiazole (NBD) ether moiety as a recognition group. This design allows for highly sensitive detection of H2S, with a detection limit as low as 3.89 nM and a response time less than 1 s. Furthermore, the reaction product, DCM-OH, also exhibits TPA properties. Zhou’s experiments [25] developed a series of DCM-based compounds to explore how different geometrical modifications influence their optical properties, which was achieved by introducing electron-withdrawing/electron-donating substituent groups (F, Cl, Br, CN, and NMe2) into the benzopyran and by incorporating various π-expanding modes in the molecular skeleton, respectively. This work was based on the study conducted by Liu et al. [19]. Among them, the line-type π-expanding compound benzo1-DCM-OH has a large Stokes shift (80.53 nm) and a high TPA cross section (1752.71 GM), which make it a potential material with excellent two-photon properties in the near-infrared (NIR) region. However, the enhanced molecular skeleton vibration and large geometrical relaxation in the low-frequency region of benzo1-DCM-OH lead to an increase in nonradiative decay processes (1.28 × 1010 s−1), which caused lower fluorescence quantum yields (1.9%) and smaller effective TPA cross sections (39.85 GM). In addition, the literature [25] also mentioned that the introduction of strong electron-withdrawing substituent groups into benzopyrans not only effectively suppresses molecular backbone vibrations but also enhances the TPA properties of the compounds. Meanwhile, Sun’s study [30] also pointed out that the introduction of strong electron-withdrawing substituent groups can suppress ICT phenomena, resulting in a relatively stronger fluorescence compared to electron-donating substituent groups. In order to gain a deeper understanding of these two geometrical modifications, we combined them and analyzed in depth the relationship between their geometrical structures and optical properties and fluorescence quantum yields by studying the intrinsic mechanisms to provide more detailed theoretical guidance for the design of novel two-photon fluorescent probes.
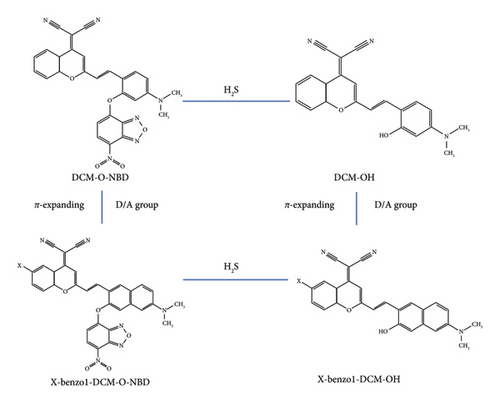
In this work, the internal mechanism of the interaction between π expansion and substituent groups is deeply investigated by analyzing the excited-state electronic structures. In order to provide theoretical guidance for the design and synthesis of TPEF probes, the effects of the interbinding of different geometrical modifications on the optical properties were further investigated. By introducing electron-withdrawing/electron-donating substituent groups (F, Cl, Br, CN, and NMe2) into benzo1-DCM-OH benzopyrans, we have designed a series of compounds based on benzo1-DCM-OH (see Figure 1 and Figure S1 in the Supporting Information), and we theoretically analyzed the changes in their optical properties from the perspectives of one-photon absorption (OPA), TPA, fluorescence emission characteristics, and fluorescence quantum yield. In order to deeply investigate the changes in the optical properties and fluorescence efficiency of the compounds resulting from the combination of two geometrical modifications (π expanding in molecular skeletons and the introduction of substituent groups), as well as to explore the potential mechanisms of the abovementioned geometrical modifications, we have conducted theoretical simulations of the photophysical processes between the ground and excited states of all product compounds. This work summarized a new approach to the geometric modifications of probes and provided theoretical guidance for the design of efficient TPEF compounds for the detection of H2S levels in organisms.
2. Computational Details
In this study, the optimization and frequency calculations of the ground-state and first excited-state geometric structures for all the investigated compounds were performed at the B3LYP/6–31G (d) level using density functional theory (DFT) in the Gaussian 16 program package [31, 32]. Time-dependent density functional theory (TD-DFT) was also applied at the same level for the first excited-state calculations [33], with no imaginary frequencies observed. The resulting stable molecular structures are shown in Figure 1. The one-photon absorption spectra, emission spectra, and electronic structures were simulated using TD-DFT based on the B3LYP/6–311 + G (d) method [33]. Meanwhile, the solvent effect was considered using the polarizable continuum model (PCM) [34]. Additionally, TPA cross-sections were calculated using the few-state model [35–37] and quadratic response theory in the DALON program [38]. The two-photon absorption spectra in water were simulated using TD-DFT with the CAM-B3LYP/6–311 + G (d) method [33, 39–41], as the range-separated CAM-B3LYP functional is more suitable for simulating nonlinear optical properties. For photophysical processes, radiative decay rates were determined using the Einstein spontaneous emission equation [42], while nonradiative decay rates were calculated using the MOMAP program in Device Studio, incorporating normal mode displacements and the Duschinsky rotation effect with the DUSHIN program [43–48]. The electron transition characteristics were analyzed using the Multiwfn program [49], and the contour surfaces of frontier molecular orbitals for all the investigated compounds were visualized using Multiwfn and VMD programs [49, 50]. The spin–orbit couplings were calculated at the B3LYP/def2-TZVP level using the ORCA program [51]. As shown in Table S1, the maximum one-photon absorption peak (λOPA), maximum emission peaks (λems), maximum two-photon absorption peaks (λTPA), and the corresponding two-photon absorption cross sections (σ) of the experimental molecules in water are in good agreement with the theoretical literature [25] and experimental literature [19]. The data above validate the reliability of the calculations in this study.
3. Results and Discussion
3.1. Electron Structures and Optical Properties
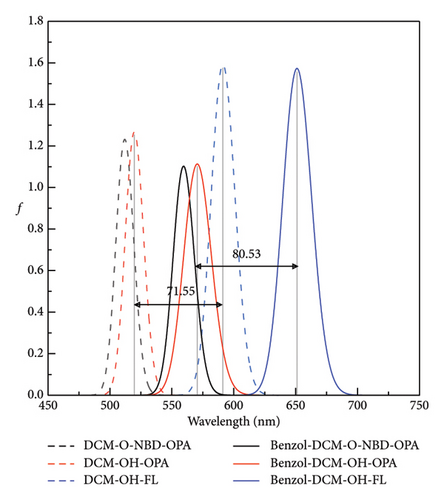
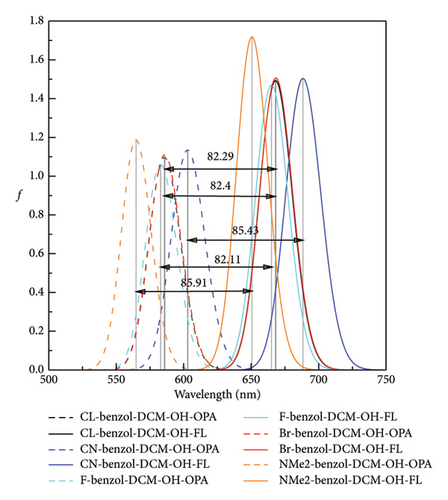
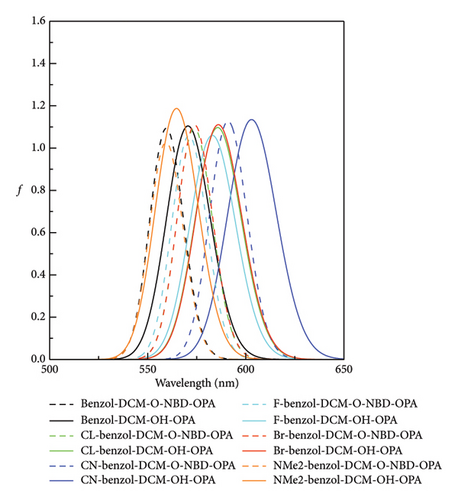
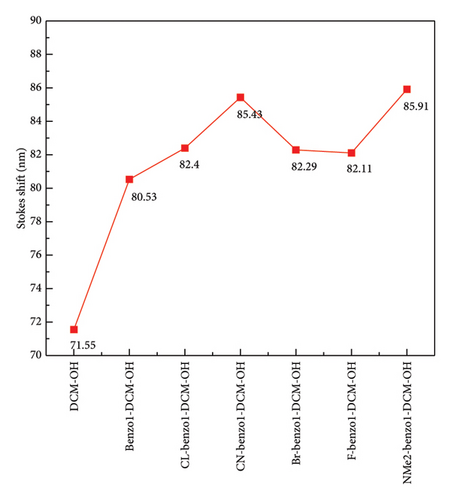
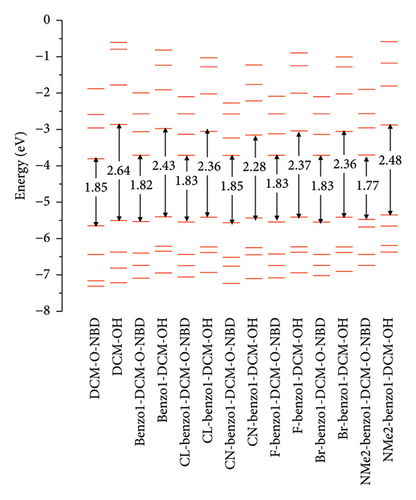
Spectra are an important and indispensable part of analyzing the connection between the electron structures and the optical properties. As shown in Figure 2 and Tables S2 and S3 in Supporting information, for probe benzo1-DCM-O-NBD, the maximum OPA peak is at about 559 nm, which is mainly derived from S0⟶S2 electron transition with major contribution (98.9%) of H ⟶ L + 1 transition. After reaction with H2S, the maximum OPA peak of the product benzo1-DCM-OH (570 nm) is connected with the electron transition process S0⟶S1, which has electron excitation configuration of H ⟶ L (99.4%) with local excitation (LE) characteristics (see Figure S2 in Supporting information). The absorption and emission spectra of the product benzo1-DCM-OH displayed a significant red shift compared with the product DCM-OH, and the Stokes shift increased from 71.55 to 80.53 nm, which made the product benzo1-DCM-OH possess easier distinguished spectra. Compared with benzo1-DCM-OH, the absorption and emission spectra of the designed compounds with electron-withdrawing groups (F, Cl, Br, and CN) in benzopyran were slightly red-shifted, and the Stokes shifts were significantly increased (more than 80 nm). Especially for CN-benzo1-DCM-OH, the maximum OPA peak was located at about 602 nm, and the fluorescence emission peak was located at about 688 nm, and the spectra were significantly red-shifted, with the Stokes shift as high as 85.43 nm, and the spectral resolution was significantly improved. In contrast, the spectra of NMe2-benzo1-DCM-OH (λOPA = 564 nm and λems = 651 nm) are blue-shifted, which is still rλemsed-shifted compared to DCM-OH (λOPA = 520 nm and = 591 nm). Therefore, in order to investigate the connection between the frontier molecular orbit energy levels of the molecules and their spectra, the frontier molecular orbit energy levels of all the compounds were analyzed. From Figure 3, compared to DCM-OH (2.69 eV), the π-expanding compound benzo1-DCM-OH reduces the energy gap to 2.43 eV by decreasing the LUMO energy and increasing the HOMO energy, which promotes large red shifts of the absorption and emission spectra. The introduction of strong electron-withdrawing groups in benzopyran mainly contributes to spectral red shift by decreasing the energy of LUMO. Among them, CN-benzo1-DCM-OH (2.38 eV) has the smallest energy gap, which makes it have distinct shifts in both absorption and emission processes. In contrast, the compound NMe2-benzo1-DCM-OH with a strong donor increases the energy gap to 2.48 eV by increasing the energy of the LUMO, which causes a blue shift of the spectrum [52]. However, its energy gap still shows a decreasing trend compared to that of DCM-OH (2.69 eV), thus resulting in a spectrum that is still red-shifted with respect to DCM-OH. In summary, in benzo1-DCM-OH, π expansion changes the energy gap and thus the spectral shifts by changing the energies of HOMO and LUMO, while electron-donating/electron-withdrawing substituent groups change the energy gap and thus the spectral shifts mainly by changing the energy of the LUMO, and π-expansion dominates when both interact simultaneously.
3.2. Two-Photon Absorption
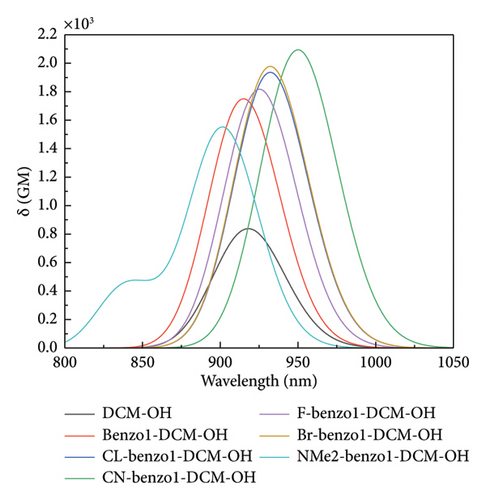
In biological imaging and detection, TPA ability is another important factor that should be considered when designing two-photon fluorescence excitation materials. As shown in Figure 4 and Table S4 in the Supporting information, though the TPA cross section of the π-expanding compound benzo1-DCM-OH is up to 1752 GM at 915 nm, which is more than twice as large as that of DCM-OH (845 GM at 915 nm), the TPA peak is almost the same as that of DCM-OH. The compounds with strong electron-withdrawing substituent groups in benzopyran have red-shifted TPA spectra and increased TPA cross sections (> 1800 GM). Especially, the TPA cross section of the compound CN-benzo1-DCM-OH is up to 952 GM at 950 nm in water, which has significantly improved TPA properties and contributed to imaging in living organisms. In contrast, the product NMe2-benzo1-DCM-OH with the electron-donating substituent group in benzopyran has a blue-shifted TPA spectrum to 901 nm and a TPA cross-section that has decreased to 1548 GM but still displays an increasing trend in the TPA cross-section compared to that of DCM-OH (915 nm at 845 GM). In addition, there is another TPA peak at 840 nm in NMe2-benzo1-DCM-OH, which originates from the H-1 ⟶ L electron transition and has obvious CT characteristic [53] (see Figure S2). In a word, in benzo1-DCM-OH, the position of the maximum TPA peak is mainly determined by the substituent group type when π expansion and substituent groups work together. Both geometrical modifications contribute to changes in the TPA cross section, and π expansion dominates when they both interact simultaneously. In particular, the compound CN-benzo1-DCM-OH possesses not only a significantly red-shifted emission spectrum but also a large TPA cross-section, making it a potential fluorescent material with excellent TPA properties in NIR.
In order to gain a deep understanding of the relationship between the molecular structures and TPA properties, the TPA process was analyzed through a few-state model (see Tables S5 and S6 in the Supporting information). TPA cross section (σTPA) and TPA transition tensors (Sif) are determined by excitation energy (ω), transition dipole moment (μ), and the difference between excited-state and ground-state dipole moment (Δμ) (see Table 1).
| Molecules | f | n | ΔEn | Δμx | Δμy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DCM-OH | 1 | 1 | 2.72 | 4.73 | −0.70 | 4.61 | 0.13 | 6.55 | 11.16 | −4.86 | −4.73 |
| Benzo1-DCM-OH | 1 | 1 | 2.72 | 4.92 | 0.57 | 6.20 | 0.10 | 5.58 | 11.78 | 4.86 | 4.96 |
| CL-benzo1-DCM-OH | 1 | 1 | 2.67 | 4.97 | 0.73 | 6.45 | 0.33 | 6.55 | 13.00 | 4.97 | 5.30 |
| CN-benzo1-DCM-OH | 1 | 1 | 2.60 | −5.05 | 0.74 | −6.68 | 0.36 | −8.30 | −14.98 | 4.80 | 5.16 |
| F-benzo1-DCM-OH | 1 | 1 | 2.68 | −4.92 | 0.67 | −6.36 | 0.21 | −6.53 | −12.89 | 4.93 | 5.14 |
| Br-benzo1-DCM-OH | 1 | 1 | 2.67 | 4.98 | 0.84 | 6.46 | 0.48 | 6.40 | 12.86 | 5.09 | 5.57 |
| NMe2-benzo1-DCM-OH | 1 | 1 | 2.76 | 5.04 | 0.31 | 6.13 | 0.45 | 3.87 | 10.00 | 5.34 | 5.79 |
From the description in Table S6, it can be concluded that the main contribution to the TPA cross-section is made by Sxx, in addition to a small contribution by Sxy and Syy , and the other TPA transition tensors play a negligible role. As shown in Table 1 and Table S5 in the Supporting information, for the compound benzo1-DCM-OH, the line-type π-expanding mode increases Δμx (6.20 D for benzo1-DCM-OH and 4.61 D for DCM-OH) mainly by decreasing the ground-state dipole moment (6.55 D for DCM-OH and 5.58 D for benzo1-DCM-OH), which leads to an increase of Sxx and Sxy, resulting in an increased TPA cross section. In addition to the significant increased Δμx, the π-expanding compound benzo1-DCM-OH has a distinct increased μx (4.73 D for DCM-OH and 4.92 D for benzo1-DCM-OH), which leads to the enhanced TPA properties. As for compounds with strong acceptors, electron-withdrawing substituent groups not only increased the transition dipole moment and decreased the excitation energy difference but also increased both ground-state and excited-state dipole moments of investigated molecules. And by analyzing the data of Table 1, it can be seen that electron-withdrawing substituent groups mainly act to make the increase of the excited-state dipole moment larger so as to gain an increased difference between excited-state and ground-state dipole moment, thus improving the TPA cross sections. Compared to the compound benzo1-DCM-OH, CN-benzo1-DCM-OH exhibits an increase in by 2.72 D and an increase in by 3.20 D, resulting in an enhanced transition dipole moment up to −6.68 D, which leads to an increase of Sxx and Sxy, thus improving TPA cross section. In contrast, for the compound NMe2-benzo1-DCM-OH, the electron-donating substituent group can lead to the decrease of the first excited-state and ground-state dipole moments, as well as the reduction in the difference between them. Though the μx of NMe2-benzo1-DCM-OH increases to 5.04 D and its Δμx (6.13 D) is almost the same as that of benzo1-DCM-OH, the excitation energy increases significantly (2.76 eV), resulting in the decrease of TPA transition tensors and the TPA cross section. In summary, π expansion and electron-withdrawing substituent groups not only increase the difference between the ground-state and excited-state dipole moments as well as the transition dipole moment but also reduce the excitation energy, thus increasing TPA cross-section.
3.3. Fluorescence Quantum Yield
Apart from good spectral resolution and excellent TPA properties, fluorescence detection also requires high fluorescence luminescence efficiency, and fluorescence quantum yield (Φ) is an important index to measure it. The fluorescence quantum yield depends on the contribution of spontaneous radiative decay to the total excited-state electron transition process, determined by both radiative decay (Kr) and nonradiative decay (Knr). On the other hand, nonradiative decay usually refers to the internal conversion (IC) process when spin–orbit coupling is very weak [43, 54] (see Table S7 in the Supporting information). As shown in Table 2, the fluorescence quenching of the probe molecules was derived from the small radiative rate (4.98 × 104 s−1 for benzo1-DCM-O-NBD and 2.55 × 104 s−1 for DCM-O-NBD), which was mainly due to quite small electron transition dipole moment μ (0.25 D for benzo1-DCM-O-NBD and 0.55 D for DCM-O-NBD) and very low excitation energy Evt (0.76 eV for benzo1-DCM-O-NBD and 0.81 eV for DCM-O-NBD). After reaction with H2S, DCM-OH produced large fluorescence in water due to the increased excitation energy (2.16 eV) and electron transition dipole moment (13.72 D), with a large Φ increased to 16.1%. In the case of benzo1-DCM-OH, despite the increase in Evt (1.96 eV) and μ (14.19 D), the fluorescence remained weak (1.9%) due to the high nonradiative decay rate, resulting in a low effective TPA cross-section (Φσ) of 33.3 GM.
| Molecules | Ead/eV | f | μ/D | Evt/eV | Kr/s−1 | Kic/s−1 | Φ (%) |
|---|---|---|---|---|---|---|---|
| DCM-O-NBD | 1.13 | 0.00 | 0.55 | 0.81 | 2.55 × 104s−1 | 1.74 × 108s−1 | 0.02 |
| DCM-OH | 2.20 | 1.54 | 13.72 | 2.16 | 3.08 × 108s−1 | 1.60 × 109s−1 | 16.1 |
| Benzo1-DCM-O-NBD | 1.17 | 0.00 | 0.25 | 0.76 | 4.98 × 103s−1 | 2.04 × 104s−1 | 19.7 |
| Benzo1-DCM-OH | 2.02 | 1.50 | 14.19 | 1.96 | 2.47 × 108s−1 | 1.28 × 1010s−1 | 1.9 |
| CL-benzo1-DCM-OH | 1.96 | 1.43 | 14.06 | 1.91 | 2.22 × 108s−1 | 1.21 × 109s−1 | 15.5 |
| Br-benzo1-DCM-OH | 1.96 | 1.44 | 14.12 | 1.91 | 2.24 × 108s−1 | 1.21 × 109s−1 | 15.6 |
| F-benzo1-DCM-OH | 1.98 | 1.41 | 13.91 | 1.93 | 2.24 × 108s−1 | 1.14 × 109s−1 | 16.4 |
| CN-benzo1-DCM-OH | 1.90 | 1.43 | 14.28 | 1.85 | 2.11 × 108s−1 | 1.29 × 109s−1 | 14.1 |
| NMe2-benzo1-DCM-OH | 2.04 | 1.68 | 15.00 | 1.97 | 2.78 × 108s−1 | 1.13 × 1011s−1 | 0.25 |
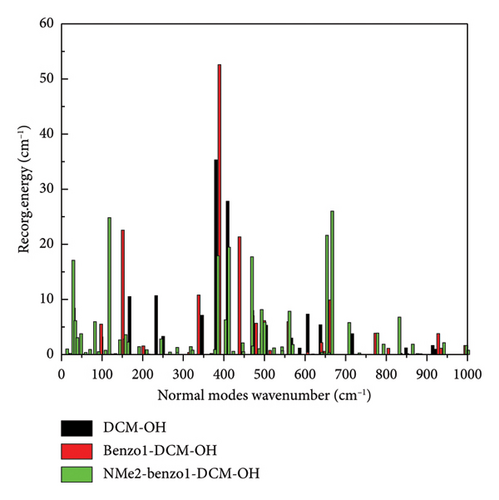
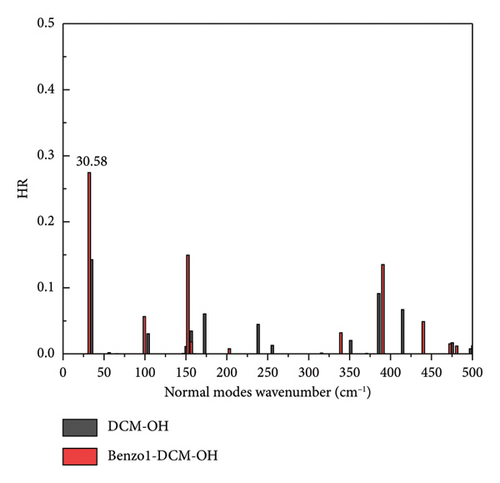
In addition, the introduction of the electron-withdrawing group in benzopyran of benzo1-DCM-OH leads to the increase of fluorescence quantum yield by decreasing the nonradiative decay rate (see Table 2). The compounds with electron-withdrawing substituents (CL-, CN-, F-, and Br-benzo1-DCM-OH) showed fluorescence quantum yields (Φ) more than 10%, and the effective TPA cross sections (Φσ) were all over 250 GM, which were significantly improved compared to DCM-OH (136 GM) and benzo1-DCM-OH (33.3 GM), and could be beneficial for in vivo imaging and detection. Nonradiative decay is relatively complex, and the internal transition process is related to the geometrical relaxation between the excited and ground states. Therefore, we have analyzed the Huang Rhys-factors (HR) and the reorganization energy(λ) of all compounds (see Figures 5 and 6 and Table 3).

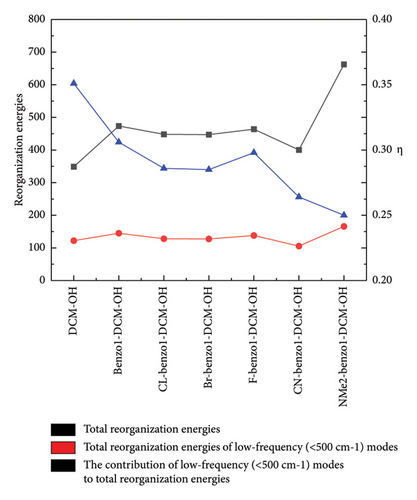
| Molecules | (cm−1) | (< 500 cm−1) modes | η (%) |
|---|---|---|---|
| DCM-O-NBD | 45,697.68 | 124.30 | 0.3 |
| DCM-OH | 348.83 | 122.12 | 35.0 |
| Benzo1-DCM-O-NBD | 666,971.56 | 46,016.74 | 6.9 |
| Benzo1-DCM-OH | 473.62 | 144.99 | 30.6 |
| CL-benzo1-DCM-OH | 448.04 | 128.18 | 28.6 |
| Br-benzo1-DCM-OH | 447.12 | 127.54 | 28.5 |
| F-benzo1-DCM-OH | 463.82 | 138.09 | 29.8 |
| CN-benzo1-DCM-OH | 400.35 | 105.59 | 26.4 |
| NMe2-benzo1-DCM-OH | 662.44 | 165.89 | 25.04 |
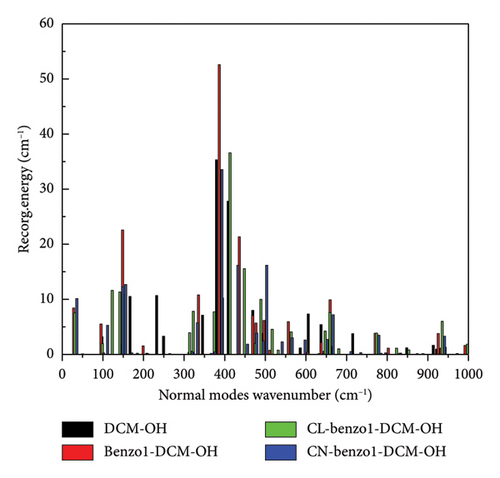
For example, the total reorganization energies are 348.83 and 473.62 cm−1 for the compounds DCM-OH and benzo1-DCM-OH, respectively. The molecular skeleton scissoring vibration mode at 348.83 cm−1 possesses large reorganization energy up to 52.58 cm−1 for benzo1-DCM-OH, which strongly enhances its nonradiative process. And the introduction of a strong electron-withdrawing substituent group in benzopyran not only reduces the total reorganization energies but also effectively suppresses this reorganization energy peak. The low-frequency modes (< 500 cm−1) of compounds with strong electron-withdrawing groups contribute less than 30% to the total reorganization energy. In particular, the peak reorganization energies of CN-benzo1-DCM-OH and CL-benzo1-DCM-OH were even reduced to 33.54 cm−1 and 36.58 cm−1, respectively, weakening the molecular skeleton scissoring vibration mode and reducing nonradiative decay rates (for CN-benzo1-DCM-OH, λtotal = 400.35 cm−1, kic = 1.29 × 109 s−1; and for CL-benzo1-DCM-OH, λtotal = 448.04 cm−1 and kic = 1.21 ×109 s−1), which improves the fluorescence quantum yield.
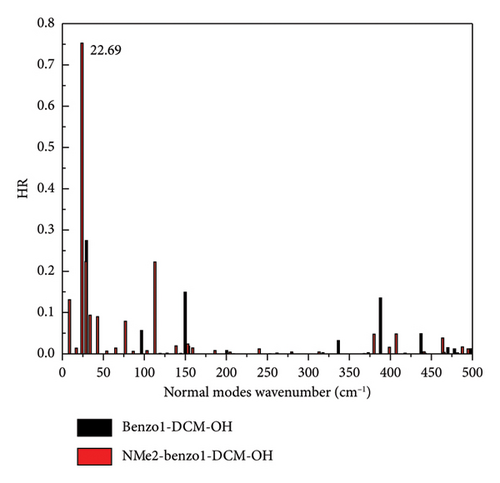
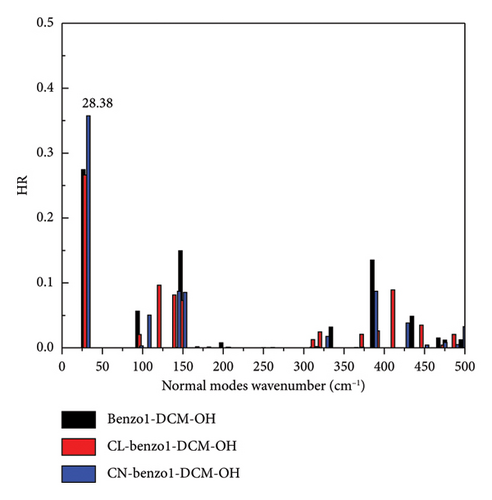
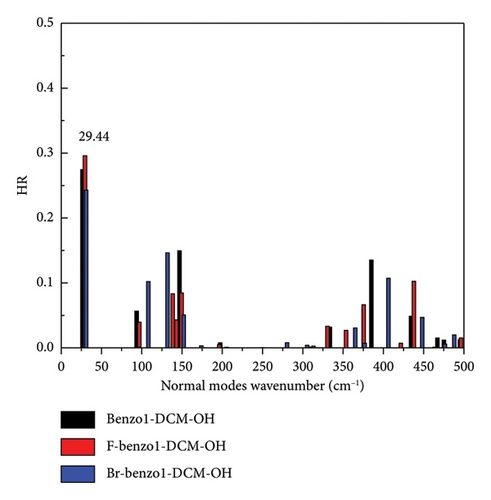
However, for the compound NMe2-benzo1-DCM-OH with the electron-donating substituent group, although the radiative decay rate is increased by the increase in excitation energy and electron transition dipole moment (Evt = 1.97 eV, μ = 15.00 D, kr = 2.78 × 108 s−1), the strong nonradiative decay process (kic = 1.13 × 1011 s−1) leads to a decrease of the fluorescence quantum yield (0.25%), which is unfavorable for fluorescence emission. From Figure 5(c), though the molecular skeleton scissoring vibration mode at 348.83 cm−1 possesses large reorganization energy up to 52.58 cm−1 for benzo1-DCM-OH, which is suppressed in NMe2-benzo1-DCM-OH, there are also some large reorganization energy peaks in the low-frequency region (< 500 cm−1). To further explore the effect of the substituent groups and the line-type π-expanding mode on the IC rate when acting together, we investigated the HR factors of all compounds. From Figure 6, the HR factors of NMe2-benzo1-DCM-OH (with a maximum value of 0.75) is significantly higher than those of benzo1-DCM-OH (with a maximum value of 0.27). In the low-frequency region (< 500 cm−1), NMe2-benzo1-DCM-OH exhibits multiple molecular vibrational modes with large HR factors, which promotes nonradiative decay between the excited state and the ground state. In addition, the molecular skeleton twisting vibration at 22.69 cm−1 possesses obviously large HR factors of 0.75, attributed to electron-vibration coupling, thus causing an increased IC decay rate and decreased Φ in comparison with those of the compound benzo1-DCM-OH. In contrast, although CN-benzo1-DCM-OH reduces the peak reorganization energy of benzo1-DCM-OH, it has the molecular skeleton scissoring vibration mode at 28.38 cm−1 with large HR factors up to 0.36, and thus its nonradiative decay rate remains high compared to other compounds with electron-withdrawing substituent groups.
In summary, in benzo1-DCM-OH, introducting strong electron-withdrawing substituent groups can reduce the reorganization energies of the compounds and their peaks, thus suppressing the enhancement of scissoring vibration mode due to the line-type π-expanding mode of the molecular skeleton, which weakens the nonradiative decay process and improves the luminescence efficiency. On the other hand, the introduction of the strong electron-donating substituent group significantly increases the HR factors and its peak value, which changes the main contribution mode of molecular vibration in the low-frequency region from the scissoring vibration of molecular skeleton to the twisting vibration, thus increasing the nonradiative decay rate and weakening the fluorescence.
4. Conclusion
In this paper, the changes in OPA properties, fluorescence emission properties, TPA properties, and fluorescence quantum yields of the π-expanding compound benzo1-DCM-OH after the introduction of electron-donating/electron-withdrawing substituent groups are theoretically investigated in detail, and the intrinsic mechanism of the interaction of the two geometrical modifications of the π expansion and the electron-donating/electron-withdrawing substituent groups is thoroughly explored. It is found that in the studied molecules, in OPA properties and fluorescence emission properties, π expansion changes the energy gap and thus the spectral shifts by changing the energies of HOMO and LUMO, while electron-donating/electron-withdrawing substituent groups change the energy gap and thus the spectral shifts mainly by changing the energy of the LUMO, and that π expansion dominates when both interact simultaneously. In TPA properties, π expansion has a little effect on the position of the TPA peak in benzo1-DCM-OH, although it benefits the increase in TPA cross section and improves the TPA capacity. When π expansion and substituents groups work together, the shifts in TPA spectra are mainly determined by the type of substituent groups, and π expansion has a little effect. The TPA cross section is affected by both π expansion and substituent groups, with π expansion dominating. Meanwhile, the introduction of strong electron-withdrawing substituents groups (F, CL, Br, and CN) in benzopyran can effectively suppress molecular skeleton scissoring vibration caused by π expansion to weaken nonradiative decay processes, which enables these compounds to have superior TPA properties in NIR and higher fluorescence quantum yields than benzo1-DCM-OH in NIR. Especially, the compound CN-benzo1-DCM-OH simultaneously possesses the largest effective TPA cross section (Φσ) up to 295 GM at 950 nm and easily distinguishes spectra with a Stokes shift as large as 85.43 nm. Therefore, it may be one of the potential materials with excellent two-photon excited fluorescent properties in the application of biological imaging and H2S detection.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
JBW was supported by the National Natural Science Foundation of China (no. 11047164), the National Key Laboratory of Infrared Detection Technologies (no. IRDT-23-S01), and the Shanghai Explorer Program (no. 24TS1403400).
Acknowledgments
The authors gratefully acknowledge HZWTECH for providing computation facilities.
Supporting Information
Theoretical methods of two-photon absorption simulation, fluorescence quantum yield and excited-state dynamic processes, and reorganization energy and Huang-Rhys factor; Figure S1: Stable molecule structures in S0 and S1; Figure S2: Contour surfaces of frontier molecular orbits; Table S1: Comparison between this work with theoretical and experimental literature; Table S2: OPA properties of all compounds in water; Table S3: Fluorescence properties of all compounds in water; Table S4: TPA properties in water were calculated at CAM-B3LYP/6–311 + G (d) level using DALTON programs; Table S5: TPA tensor elements (Sab) and TPA cross section () of all compounds in water; Table S6: TPA tensor elements (Sab) and TPA cross section () of all compounds in water; Table S7: Spin-orbital coupling matrix elements Hsoc between T1 and S1 of all compounds; Table S8: Optimized ground-state structures of all investigated compounds were obtained with PCM//B3LYP/6–31G (d) in water.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




