Assessing Left Ventricular Dysfunction in Pediatric Chronic Kidney Disease Patients: A 2D Echocardiography Study From Ethiopia
Abstract
Background: Although chronic kidney disease (CKD) is less prevalent among the pediatric population in comparison to adults, it remains a significant contributor to morbidity and mortality in this age group. As the disease advances, it gives rise to diverse complications, with cardiovascular issues emerging as a primary cause of morbidity and mortality. Remarkably, the intricate association between CKD and left ventricular dysfunction (LVD) has not been extensively investigated in the context of African pediatric populations. This study attempts to close this gap.
Method: This cross-sectional study aimed to evaluate the prevalence of LVD using 2D echocardiography in pediatric patients diagnosed with CKD and identify associated factors. The study enrolled 95 CKD patients, all under 18, receiving care at the kidney follow-up clinic of Tikur Anbessa Specialized Hospital, Ethiopia.
Results: Analysis indicated that 55.8% of participants were male, while 44.2% were female. The leading cause of CKD was congenital anomalies of the kidney and urinary tract (CAKUT), accounting for 68.4% of cases. Among CKD cases, 42% were at Stages 2 and 3. Systolic and diastolic hypertension were present in 23.2% and 31.6% of the patients, respectively. Concentric remodeling was observed in 64%, while 10.6% showed concentric hypertrophy. Diastolic dysfunction was found in 20.4%, and only 3.2% had a low ejection fraction. Anemia was the sole factor significantly associated with cardiac dysfunction. The mean relative wall thickness was lower in those with anemia. The left ventricular mass index (LVMI) is strongly correlated (Pearson correlation coefficient of 0.764) with diastolic dysfunction.
Conclusion: Most of the children with CKD had evidence of cardiac remodeling. Diastolic dysfunction was the most common type of functional cardiac impairment, as opposed to systolic dysfunction. Anemia was associated with a higher relative incidence of diastolic dysfunction. LVMI was the echocardiography parameter that was strongly correlated with diastolic dysfunction.
1. Introduction
Although research on chronic kidney disease (CKD) in children is generally limited, earlier studies suggest that the prevalence varies between 15 and 74.7 cases per million of the age-related population (PMARP) [1]. After the introduction of the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, the previously observed wide variability in prevalence stabilized [2]. Subsequently, there has been a notable increase in prevalence due to advancements in CKD survival and treatment [2, 3]. However, this trend does not apply to resource-limited settings such as Africa, where there is a lack of sufficient studies addressing this matter [4].
CKD leads to a spectrum of complications, encompassing but not limited to anemia, cardiovascular diseases (CVDs), hypertension, dyslipidemia, mineral bone disease, and ultimately culminating in kidney failure [5, 6]. Childhood CKD also presents clinical features that are specific and peculiar to the pediatric age, such as the impact of the disease on growth, development, and psychosocial adjustment, which severely impact the quality of life [2–4, 7].
CVD is prevalent in individuals with CKD, irrespective of age or disease stage. It is attributable to the interconnected hemodynamic and regulatory functions between the heart and kidneys [8]. Furthermore, CVD stands as a notable factor contributing to mortality in children with CKD [9–12].
The predominant CV abnormalities observed in pediatric patients with CKD encompass left ventricular hypertrophy (LVH) and both left ventricular (LV) systolic and diastolic dysfunctions. These abnormalities manifest in the early stages of CKD and exhibit progression concurrent with the deterioration of kidney function [13]. The pathophysiology of these changes is multifactorial and includes chronic volume overload, anemia, inflammation, activation of the renin–angiotensin–aldosterone system (RAAS), and disturbances in calcium–phosphate metabolism. These factors promote increased cardiac workload and myocardial remodeling, ultimately leading to LVH [14]. In addition, uremic toxins and oxidative stress contribute to endothelial dysfunction and myocardial remodeling, further exacerbating cardiac structural and functional impairments [15].
Nonetheless, comprehensive data on the prevalence of left ventricular dysfunction (LVD) in pediatric patients with CKD remain scarce, particularly in our specific setting. Our research is motivated by the need to address this knowledge gap. The primary objective is to employ echocardiography for the evaluation of LVD and the identification of contributing factors among pediatric CKD patients. Additionally, we aim to assess the risk of LVD development based on the severity of CKD in the pediatric population. Ultimately, the study seeks to identify specific subsets of CKD patients warranting cardiac assessment via echocardiography to ensures timely intervention and effective management of modifiable risk factors to preclude the onset of cardiovascular abnormalities and the progression of CKD.
2. Methods
2.1. Study Setting
The research was carried out at Tikur Anbessa Specialized Hospital (TASH), the Department of Pediatrics and Child Health, in Addis Ababa, Ethiopia. It is the teaching hospital of Addis Ababa University. It is also the largest referral hospital in Ethiopia.
The Department of Pediatrics and Child Health has a pediatric kidney follow-up clinic twice weekly, where all kidney patients are followed. At the time of data collection, kidney replacement therapy (hemodialysis) was available only to patients with acute kidney injury (AKI) and kidney transplantation services had not yet been started.
Children diagnosed with CKD were managed through conservative treatment approaches due to the unavailability of kidney replacement therapy for this population. Conservative management encompassed a range of interventions designed to slow the progression of the disease and reduce associated complications. These measures included stringent blood pressure (BP) control, primarily through the use of antihypertensive agents; dietary counseling to ensure adequate caloric intake and maintain electrolyte balance; treatment of anemia using iron supplementation and/or erythropoiesis-stimulating agents; administration of phosphate binders and vitamin D to address mineral bone disorders; infection prevention strategies; and routine monitoring of renal function.
2.2. Study Population
All pediatric CKD patients aged < 18 years and attending the pediatric renal clinic at TASH.
2.3. Study Design and Study Period
A hospital-based cross-sectional study was conducted from May through September 2019. The study realization took place from November 2019 to March 2020, encompassing data cleaning, analysis, and manuscript draft preparation.
2.3.1. Sample Size Determination
- •
n = required sample size
- •
Z = Z-value corresponding to the confidence level (1.96 for 95% confidence)
- •
P = estimated proportion (0.50 in this case)
- •
d = margin of error
- •
n′ = revised sample size with finite population correction
- •
N = population size (207 in this case)
- •
Z = Z-value corresponding to the confidence level
- •
P = expected proportion
- •
d = margin of error (precision)
With this correction, the revised sample size is 135.
2.3.2. Sampling Method
All patients with CKD coming for follow-up during the study period and fulfilling the inclusion criteria were included.
2.4. Inclusion and Exclusion Criteria
2.4.1. Inclusion Criteria
All pediatric patients aged < 18 years with confirmed and documented CKD, irrespective of the underlying cause. The inclusion criteria encompassed all stages of CKD, as defined by the KDIGO 2013 guidelines [17].
2.4.2. Exclusion Criteria
- •
Those patients with previously confirmed congenital or acquired cardiac illnesses not associated with CKD
- •
Patients′ parents or guardians
2.5. Operational Definitions
- •
Normal: less than or equal to the 90th percentile
- •
Prehypertension: between 90th and 95th percentiles
- •
Stage 1: between 95th and 99th + 5 mmHg
- •
Stage 2: above 99th + 5 mmHg
- •
Overweight: > +1SD
- •
Obesity: > +2SD
- •
Thinness: < −2SD and > −3SD
- •
Severe thinness: < −3SD
Severe acute malnutrition: according to the WHO 2006 growth chart, it is defined as a weight-for-length/height (WFL/H) < −3z score or mid-upper arm circumference (MUAC) < 11.5 cm. WFL/H compares a child’s weight to that of a healthy peer with similar length/height and sex, while MUAC measures the circumference of a child’s arm midway between the shoulder and elbow.
Stunting: according to the WHO 2006 growth chart, it is defined as a length/height-for-age (L/HFA) < −3z score. L/HFA compares a child’s height (or length, if aged 0–24 months) to the expected height/length of a healthy child of the same age and sex.
Anemia: According to KDIGO clinical practice guidelines for anemia in CKD, the definition of anemia varies with age, that is, hemoglobin (Hb) concentration < 11.0 g/dL in children 0.5–5 years, < 11.5 g/dL in children 5–12 years, < 12.0 g/dL in children 12–15 years, and < 13.0 g/dL in males and < 12.0 g/dL in females > 15 years of age.
- 1.
Serum calcium (mg/dL)
- ○
Age 1–10 years: 8.8–10.8
- ○
Age > 10 years: 8.4–10.2
- ○
- 2.
Serum phosphorus (mg/dL)
- ○
Age 1–3 years: 3.8–6.5
- ○
Age 4–11 years: 3.7–5.6
- ○
Age 12–15 years: 2.9–5.4
- ○
Age 16–19 years: 2.7–4.7
- ○
- 3.
Calcium–phosphorus product
- ○
< 55 mg2/dL2
- ○
- •
Left ventricular mass index (LVMI): is calculated as the ratio of left ventricular mass (LVM) to body surface area (BSA), expressed in g/kg/m2.
- •
Left ventricular hypertrophy (LVH): LVM index greater than the 95th percentile for normal children and adolescents.
- •
Relative wall thickness (RWT): a measure of concentricity, was calculated as the average thickness of the posterior and septal wall divided by the LV diastolic diameter. A value of 0.375 was used as the cutoff to define concentricity.
- •
Concentric LVH: patients with increased LVMI and elevated RWT (> 0.41).
- •
Eccentric LVH: patients with increased LVMI and normal RWT (< 0.41).
- •
Concentric remodeling: patients with elevated RWT but with a normal LVMI.
- •
Early diastole: assessed using indices of LV relaxation and reported as the ratio of maximal early (E wave) and late (A wave) diastolic flow velocities (E/A) obtained from pulsed-wave transmitral doppler.
- •
Late diastole: determined using the index of LV compliance. A ratio of peak transmitral E velocity to early diastolic mitral annular velocity (E/Em).
- •
LV systolic function: assessed using the ejection fraction (EF) and the fractional shortening.
- •
LV diastolic dysfunction (LVDD): assessed using the E/A ratio compared to decreased EF, LVH, and increased LAV, according to the American Society of Echocardiography.
- •
G1: > 90, indicating normal or high kidney function.
- •
G2: 60–89, suggesting mildly decreased kidney function.
- •
G3a: 45–59, indicating mild to moderately decreased kidney function.
- •
G3b: 30–44, pointing to moderately to severely decreased kidney function.
- •
G4: 15–29, signifying severely decreased kidney function.
- •
G5: < 15, representing kidney failure.
2.6. Data Collection
A structured checklist was used to collect data on the sociodemographic characteristics and health (CKD)-related characteristics of participant children, that is, all included patients had a short demographic and clinical history taken along with a physical examination. Recent serum creatinine, urea, calcium, phosphorus, hemoglobin, red blood cell (RBC) indices, and urinalysis (for proteinuria) results were taken, and for those who did not have the above-stated investigation, blood samples were taken as a standard of care for CKD patients.
The data were gathered by two trained nurses working at the follow-up clinic during the study period. BP measurements were conducted by the nurses adhering to a standardized protocol, employing a manual BP cuff. Echocardiographic assessments were carried out in the cardiology department by a pediatric cardiologist. The cardiologist was blinded to the CKD patient’s status, which included information about the native kidney disease, BP levels, CKD stage, and biochemical profiles. The echocardiographic examination utilized M-mode and two-dimensional images, along with color and pulsed-wave Doppler measurements.
An assessment of LVD was made in M-mode, and linear measurements of the LV cavity were obtained. Key parameters such as left ventricle end-diastolic diameter (LVEDD), left ventricle end-systolic diameter (LVESD), interventricular septum thickness (IVST), posterior wall thickness (PWT), and the calculation of LV systolic function (EF) for all subjects were determined following the guidelines provided by the American Society of Echocardiography [20]. Transmitral inflow velocities were acquired using pulsed-wave Doppler at the leaflet tips, including the early diastolic inflow velocity (E) and velocity during active atrial contraction (A). The E/A ratio was also calculated as part of the assessment.
The initial target sample size of 135 respondents was determined using Cochran’s formula; however, only 95 respondents were successful participants, as only this number was available for follow-up during the study period. Although the original plan was to enroll all eligible participants until the target was reached, recruitment was limited by the number of patients actively in follow-up.
2.7. Data Quality Assurance
The principal investigator, Elham Shemsu, supervised the data collection on a regular and periodic basis for quality and completeness.
2.8. Data Analysis and Interpretation
After data cleaning, the data were entered manually into SPSS Version 21. For continuous variables, a descriptive summary of the results was performed using means and standard deviations (SDs). Frequency and percentages were used to summarize the results of categorical variables with tables and graphic demonstrations. Associations were determined by the chi-square test for categorical outcome variables. One-way ANOVA and an independent sample t-test were used for continuous outcome variables. Multivariate analysis was done by using binary and multinomial logistic regression for categorical variables and a linear regression model for continuous variables. Associations were considered statistically significant when the p was < 0.05 at a CI of 95%.
2.9. Ethics Approval and Consent to Participate
Ethical approval for this study was granted by the Research and Publications Ethics Committee of the Department of Pediatrics and Child Health, Addis Ababa University, College of Health Sciences. Given that the study involved minors, written informed consent was obtained from the children’s parents or legal guardians. In addition, age-appropriate verbal assent was obtained from all participating children to ensure their voluntary involvement in the study.
3. Results
The study encompassed 95 CKD patients, among whom 53 (55.8%) were male and 42 (44.2%) were female. The age range of study participants spanned from 1 month to 17 years, encompassing pediatric patients. Specifically, 31 (32.6%) of the participants were below 5 years old, 38 (40%) fell within the age range of 5–10 years, and 26 (27.4%) were above 10 years old. Among the study subjects, 10 (10.5%) were classified as underweight (defined as weight-for-age Z-score < −2SD), while 11 (11.6%) were categorized as stunted (height-for-age Z-score < −3SD). Severe acute malnutrition (weight-for-height Z-score < −3SD or MUAC < 11.5 cm) was present in only 3 (3.1%) of patients, whereas 11 (11.5%) exhibited moderate acute malnutrition. Notably, no patients were classified as obese or overweight (see Table 1).
| Variable | Frequency | Percent | |
|---|---|---|---|
| Gender | M | 53 | 55.8 |
| F | 42 | 44.2 | |
| Age | < 5 | 31 | 32.6 |
| 5–10 | 38 | 40 | |
| > 10 | 26 | 27.4 | |
| Underweight | Yes (WAZ < −2SD) | 10 | 10.5 |
| No | 85 | 89.5 | |
| Stunting | Yes (HAZ < −3SD) | 11 | 11.6 |
| No | 84 | 88.4 | |
| Acute malnutrition | No | 82 | 86.3 |
| Moderate (WHZ < −2SD) | 11 | 11.5 | |
| Severe (WHZ < −3SD) | 3 | 3.1 | |
- Abbreviations: HAZ, height-for-age Z-score; WAZ, weight-for-age Z-score; WHZ, weight-for-height Z-score.
CAKUT constituted the predominant cause of CKD, accounting for 65 (68.4%) cases. Among the acquired etiologies of CKD, 14 (14.7%) cases were attributed to nephrotic syndrome, while 7 (7.4%) cases were due to glomerulonephritis.
The majority of the children included in the study were diagnosed with Stage 2 and Stage 3 CKD. Specifically, 40 (42.1%) of the CKD patients were classified as being at Stage 2, while Stage 3 accounted for 38 (40%) of the total CKD cases. Additionally, there were 5 (5.3%) patients identified with Stage 5 CKD. Regarding BP status, systolic hypertension was observed in 22 (23.4%) of the children, with 9 (9.5%) falling within the prehypertension range. Furthermore, 30 (31.6%) exhibited Stage 1 diastolic hypertension, while 12 (12.6%) were classified as having diastolic prehypertension.
Among the study participants, 20 (21.5%) were diagnosed with anemia, with 10.5% classified as having mild anemia and 3 (3.2%) presenting with severe anemia. Of the cases of anemia, 37.2% were characterized as microcytic, while 56.4% exhibited normocytic anemia. Hypocalcemia was identified in 22 (26.8%) of the children, with 5 (6.1%) demonstrating hypercalcemia, while the remaining participants had normal calcium levels. Additionally, hyperphosphatemia was observed in 29 (35.4%) children, and 9 (11%) presented with hypophosphatemia. Calcium–phosphorus product levels were elevated in 28.04% of the children, while the remainder exhibited normal levels. Proteinuria was found to be negative in 47.4% of patients, with +2 proteinuria detected in 16.8% of cases.
The mean LVMI value derived from the echocardiographic assessment of the 95 patients was 71.723, with an SD of 33.06. Additionally, a median left ventricular end-diastolic diameter (LVEDD) of 31 was obtained, accompanied by an SD of 6.28. The skewness of the LVEDD data was calculated to be 0.032 (see Table 2).
| E/A | LAD | LVM | LVMI | LVEDD | IVST | PWT | RWT | LVSD | |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 1.71 | 2.33 | 59.3 | 71.72 | 30.93 | 7.10 | 7.60 | 0.50 | 19.88 |
| Std. deviation | 0.54 | 0.53 | 31.27 | 33.06 | 6.28 | 1.93 | 1.85 | 0.12 | 4.43 |
| Skewness | 1.63 | 0.63 | 0.86 | 1.40 | 0.03a | 0.83 | 0.37a | 0.43 | 0.14a |
- Note: IVST, interventricular septal thickness; E/A, the ratio of maximal early (E wave) and late (A wave) diastolic flow velocities.
- Abbreviations: LAD, left atrial diameter; LVEDD, left ventricular end-diastolic dimension; LVM, left ventricular mass; LVMI, left ventricular mass index; LVSD, left ventricular systolic dimension; PWT, posterior wall thickness; RWT, relative wall thickness.
- aindicates mean is the better estimator of a statistical summary of the population.
Cardiac remodeling, indicative of abnormal cardiac geometry, was evident in 71 (75.5%) of the patients. Specifically, 62.8% exhibited concentric remodeling, 9.6% demonstrated concentric hypertrophy, and 3.2% presented with eccentric hypertrophy. Diastolic dysfunction was identified in 19 (20.4%) of the children. Notably, a low EF was detected in only 3.2% of the children, with the remaining participants displaying a normal EF.
Among the 63 patients with normal systolic blood pressure (SBP), 41 (65.1%) exhibited concentric remodeling. In patients with Stage 1 hypertension, 57.9% displayed concentric remodeling, while 21.1% presented with normal geometry. Regarding patients diagnosed with anemia, 40% demonstrated concentric remodeling, whereas 20% exhibited concentric hypertrophy (see Table 3).
| Variable | Types of geometric change | ||||
|---|---|---|---|---|---|
| CH | EH | CR | NG | ||
| SBP | Prehypertension | 2 (22.2%) | 1 (11.1%) | 5 (55.6%) | 1 (11.1%) |
| Stage 1 | 3 (15.8%) | 1 (5.3%) | 11 (57.9%) | 4 (21.1%) | |
| Stage 2 | 00.0% | 00.0% | 2 (66.7%) | 1 (33.3%) | |
| Normal | 4 (6.3%) | 1 (1.6%) | 41 (65.1%) | 17 (27.0%) | |
| Total | 9 (9.6%) | 3 (3.2%) | 59 (62.8%) | 23 (24.5%) | |
| Anemia | Yes | 4 (20.0%) | 3 (15.0%) | 8 (40.0%) | 5 (25.0%) |
| No | 4 (5.6%) | 00.0% | 50 (69.4%) | 18 (25.0%) | |
| Total | 8 (8.7%) | 3 (3.3%) | 58 (63.0%) | 23 (25.0%) | |
- Abbreviations: CH, concentric hypertrophy; CR, concentric remodeling; EH, eccentric hypertrophy; NG, normal geometry; SBP, systolic blood pressure.
The variable significantly associated with LVMI was the presence of anemia (p = 0.018). The mean LVMI among those with anemia was 85.9, compared to 66.7 in those without anemia. Although there was no statistically significant association between the stage of CKD and LVMI, patients with CKD Stage 3 and above exhibited greater LVMI compared to those in stages below 3, with a p value of 0.286 (see Table 4).
| LVEDD | RWT | LVMI | |||||
|---|---|---|---|---|---|---|---|
| Mean | p | Mean | p | Mean | p | ||
| Age | < 5 | 26.24 | ≤ 0.001 | 0.50 | 0.93 | 71.17 | 0.99 |
| 5–10 | 31.42 | 0.49 | 72.05 | ||||
| > 10 | 35.64 | 0.50 | 71.88 | ||||
| Sex | Male | 29.83 | 0.06 | 0.50 | 0.85 | 72.88 | 0.71 |
| Female | 32.29 | 0.49 | 70.29 | ||||
| Cause | Congenital | 29.01 | ≤ 0.001 | 0.50 | 0.94 | 69.87 | 0.43 |
| Acquired | 35.04 | 0.50 | 75.67 | ||||
| Stage | < 3 | 32.98 | ≤ 0.001 | 0.49 | 0.37 | 68.06 | 0.29 |
| 3 and above | 28.88 | 0.51 | 75.38 | ||||
| Anemia | Yes | 32.59 | 0.19 | 0.45 | 0.04 | 85.85 | 0.02 |
| No | 30.51 | 0.51 | 66.72 | ||||
| BP | Pre HTN | 30.92 | 0.63 | 0.51 | 0.84 | 94.44 | 0.19 |
| Stage 1 | 30.06 | 0.50 | 69.42 | ||||
| Stage 2 | 27.17 | 0.55 | 72.33 | ||||
| Normal | 31.38 | 0.49 | 69.14 | ||||
| Calcium | Low | 33.37 | 0.11 | 0.47 | 0.26 | 80.43 | 0.683 |
| Normal | 30.32 | 0.52 | 72.25 | ||||
| High | 28.40 | 0.46 | 48.00 | ||||
| P04 | Low | 32.22 | 0.79 | 0.49 | 0.66 | 77.22 | 0.89 |
| Normal | 30.65 | 0.51 | 73.14 | ||||
| High | 31.15 | 0.49 | 71.07 | ||||
| Ca_P04 | Normal | 31.34 | 0.45 | 0.50 | 0.5 | 75.02 | 0.38 |
| High | 30.11 | 0.49 | 67.27 | ||||
| Acute malnutrition | No | 30.45 | 0.15 | 0.49 | 0.45 | 69.46 | 0.46 |
| Moderate | 30.95 | 0.50 | 71.74 | ||||
| Severe | 31.05 | 0.51 | 72.89 | ||||
- Note: Ca_Po4, calcium–phosphorus product; Po4, phosphate; HTN, hypertension.
- Abbreviations: LVEDD, left ventricular end-diastolic diameter; LVMI, left ventricular mass index; RWT, relative wall thickness; SBP, systolic blood pressure.
In the crude association analysis, age, native cause of CKD, and stages of CKD were found to be significantly associated with LVEDD. Children with CKD attributed to an acquired cause exhibited a larger LVEDD compared to those with congenital kidney anomalies, suggesting the potential impact of acute volume overload on cardiac function. Moreover, children diagnosed with CKD Stages 3 and above demonstrated a lower mean LVEDD than those in lower CKD stages. However, only age remained statistically significant in the multivariate analysis incorporating all three variables. As age increased, the mean LVEDD tended to increase, suggesting a potential increase in LVEDD as part of the normal developmental process (see Table 4).
Concerning the mean RWT, it was found to be significantly associated with the presence of anemia, with a p value of 0.043. Patients with anemia exhibited a lower mean RWT compared to those without anemia. Although not statistically significant (p = 0.840), the mean RWT was higher among patients with Stage 2 hypertension than those without hypertension (see Table 4).
Among the factors tested for possible association with geometric changes, no factor was identified as having a significant association. Anemia was the only factor with a statistically significant association with cardiac dysfunction in this study. The proportion of diastolic dysfunction among anemic children was 45% in comparison to 12.7% among children without anemia. The remaining variables analyzed were not significantly associated with cardiac dysfunction (see Tables 5 and 6).
| Diastolic dysfunction | p | AOR | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| Anemia | Yes | Count | 11 | 9 | 0.01 | 7.45 | 1.57–35.18 |
| % | 55.0% | 45.0% | |||||
| No | Count | 62 | 9 | Ref | |||
| % | 87.3% | 12.7% | |||||
| Variable | p value | AOR | 95% CI for AOR |
|---|---|---|---|
| Anemia (ref: no) | |||
| Yes | 0.01 | 7.45 | 1.57–35.18 |
| SBP (ref: normal) | |||
| Prehypertension | 0.72 | 1.47 | 0.16–12.38 |
| Stage 1 hypertension | 0.89 | 0.88 | 0.13–5.97 |
| Stage 2 hypertension | 0.57 | 2.78 | 0.08–92.24 |
| DBP (ref: normal) | |||
| Prehypertension | 0.67 | 1.58 | 0.19–12.71 |
| Stage 1 hypertension | 0.08 | 6.07 | 0.82–45.07 |
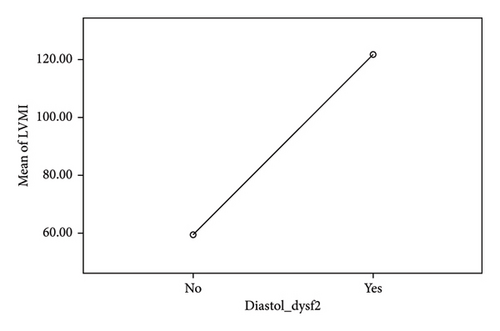
Among the echocardiographic parameters, the parameter exhibiting the strongest correlation with diastolic dysfunction was the LVMI, with a Pearson correlation coefficient of 0.764. The correlations of the remaining parameters were not found to be statistically significant. A one-way ANOVA test was conducted to assess the mean difference in LVMI between those with and without diastolic dysfunction. The mean LVMI among individuals with diastolic dysfunction was 59.2, whereas it was 121 among those without diastolic dysfunction (see Table 7 and Figure 1).
| N | Mean | Std. deviation | Std. error | 95% confidence interval for mean | |||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| LVMI | No DD | 74 | 59.22 | 17.08 | 1.99 | 55.26 | 63.17 |
| DD | 19 | 121.68 | 33.96 | 7.79 | 105.31 | 138.05 | |
- Abbreviations: DD, diastolic dysfunction; LVMI, left ventricular mass index.
4. Discussion
CVDs are prevalent among pediatric patients diagnosed with CKD at a higher rate compared to the general population. Furthermore, these conditions represent the primary cause of mortality within this patient demographic [21]. This trend has become increasingly conspicuous in recent times, largely attributable to advancements in CKD therapy and the subsequent extension of life expectancy among affected individuals [13].
LV dysfunction, observable even in the early stages of CKD, arises from various mechanisms. Key factors associated with CKD contributing to LV dysfunction include hypertension, atherosclerosis, inflammation, anemia, albuminuria, and malnutrition [22–25].
Our study revealed that 20.4% of the participants demonstrated diastolic dysfunction, whereas a diminished EF was detected in 3.2% of cases. Previous research studies have reported varying prevalence rates of LVDD, ranging from 4.5% to 14.5%, and systolic dysfunction, approximately 11.3% [26–29].
The elevated prevalence of LVDD observed in our study, in contrast to findings in other studies, may be attributed to variations in patient demographics. Notably, our study reported a higher incidence of malnutrition among participants, a factor known to contribute to LVDD [24]. Another plausible factor meriting investigation is genetic predisposition. Certain individuals with CKD may possess a genetic susceptibility to cardiac-related events, as suggested by studies [30]. It is pertinent to note that the majority of research on this topic originates from non-African regions, prompting further exploration into whether individuals of black ethnicity exhibit heightened susceptibility compared to other racial groups.
In our study, a relatively lower prevalence of systolic dysfunction was observed compared to findings from other studies. This variance may be attributed to the possibility that children with CKD could harbor subclinical systolic dysfunction, which may not manifest through a reduced EF. Systolic dysfunction can manifest through abnormal strain analysis and measurements of endocardial and mid-wall fractional shortening [31–33], parameters that were not assessed in our study for diagnosing systolic dysfunction. Furthermore, a significant proportion of patients in our study were classified as having Stage 2 CKD, a noteworthy detail as the severity of CKD stages correlates with an increased prevalence of systolic dysfunction. Additionally, the fact that diastolic dysfunction typically precedes systolic dysfunction may contribute to the higher prevalence of diastolic dysfunction and the lower prevalence of systolic dysfunction observed in our study [34].
In our study, we found that 23.2% of patients exhibited systolic hypertension, while 31.6% displayed diastolic hypertension. These figures are notably lower compared to the prevalence reported in various other studies, where hypertension rates generally range from 48% to 70% [35–38]. The lower prevalence observed in our study may indeed be attributed to the presence of masked hypertension, given that we solely relied on office BP measurements without incorporating home or a 24-h BP monitoring. This hypothesis is supported by a previous study that reported that 35% of CKD patients had masked hypertension, indicating that relying solely on office measurements may underestimate true hypertension prevalence [39]. Additionally, it is important to consider the impact of changes in diagnostic criteria. The 4th report raised the BP thresholds for diagnosing hypertension compared to previous guidelines. Consequently, some children who would have been diagnosed under older guidelines might now go undetected using the 4th Report criteria. This change in diagnostic criteria may have contributed to the lower prevalence of hypertension observed in our study cohort. Furthermore, the presence of malnutrition and loss of muscle mass in CKD patients can complicate BP measurement, potentially contributing to the lower prevalence observed in our study. Additionally, our study primarily included milder cases of CKD, which may also contribute to the lower prevalence of hypertension compared to other studies.
The higher mean LVMI observed in the prehypertension group compared to the hypertension groups in our study suggests a potential underdiagnosis of hypertension. This discrepancy implies that individuals classified as prehypertensive based on office BP measurements may actually have elevated BP levels that were not accurately captured during the assessment [39]. As LVMI is positively associated with hypertension [40], the elevated LVMI in the prehypertension group may also indicate that these individuals may be experiencing hemodynamic changes characteristic of hypertension, despite not meeting the diagnostic criteria based on office measurements alone. Therefore, this finding underscores the importance of utilizing comprehensive BP monitoring techniques, such as home or 24-h BP monitoring, to ensure accurate diagnosis and appropriate management of hypertension in pediatric CKD patients. It is noteworthy that stringent management of hypertension has been shown to ameliorate LVH and subclinical systolic dysfunction, leading to improved cardiovascular outcomes [41].
In our study, a significant portion, comprising 74.5% of participants, exhibited cardiac remodeling, indicating abnormal cardiac geometry. Among these, 63.8% displayed concentric remodeling, 10.6% manifested concentric hypertrophy, and only 3.2% presented with eccentric hypertrophy. The progression of LVH correlates with advancing stages of CKD in a dose-dependent relationship. LVH is associated with factors such as hyperparathyroidism, vitamin D deficiency, hypertension, and anemia [42, 43]. However, our study did not find any significant association between native kidney disease, CKD stage, hypertension, anemia, electrolyte abnormality, and geometric changes.
These findings align with previous reports from Europe, Turkey, and Nigeria, which have documented LVH as the most prevalent cardiovascular abnormality in children with CKD, showcasing various types of geometric changes [34, 42, 44–47]. A study in Turkey revealed that concentric LVH was the predominant abnormal geometric pattern, observed in 30.2% of cases [48]. However, this proportion differed from the European study, where abnormal LV geometry was detected in 43.3% of patients, with concentric LV remodeling, concentric LVH, and eccentric LVH observed in 10.2%, 12.1%, and 21% of patients, respectively. Importantly, the distribution of LV geometry was found to be independent of arterial hypertension and the nature of the underlying kidney disease [47].
Additionally, findings from Nigeria diverged from our study, indicating that LVH was the most common abnormality, with 12 patients (50.0%) exhibiting LVH, among whom 8 (66.6%) had eccentric and 4 (33.3%) had concentric hypertrophy [44].
In our study, even though we did not assess the use of antihypertensives, it might be that the antihypertensive treatment masked an underlying association between BP and concentric LV geometry. Therefore, it needs further extension in this line of study. The lower percentage of eccentric hypertrophy might be associated with the number of patients (10.2%) with Stages 4 and 5 CKD (where volume overload becomes significant). Their number was smaller compared to Stages 2 and 3, which accounted for 82.1% of the study population.
Anemia represents a significant complication of chronic kidney insufficiency, exerting a potentially profound influence on LV remodeling. In our study, hemoglobin levels emerged as independent negative correlates of both LVMI and RWT, with respective p values of 0.018 and 0.043. This implies that anemia was associated with heightened circulating volume and preload. However, hemoglobin levels did not serve as predictors for the geometry of cardiac remodeling. Consequently, kidney-related anemia appears to contribute moderately to the elevated prevalence of LVH in children with CKD.
In our assessment of LVDD, we utilized the E/A ratio, comparing it with decreased EF, LVH, and increased left atrial volume (LAV) per guidelines from the American Society of Echocardiography. Our study revealed a notable trend, indicating a higher prevalence of LVDD (20.4%) compared to LV systolic dysfunction (3.2%). Among the factors examined for potential association with cardiac dysfunction, anemia emerged as the sole factor demonstrating a statistically significant correlation in this study. Specifically, the proportion of diastolic dysfunction among anemic children was 45%, contrasting with a 12.7% prevalence of diastolic dysfunction among children without anemia (p = 0.011). Factors analyzed in this context included age, sex, BMI, SBP, diastolic blood pressure (DBP), stage of CKD, and calcium–phosphorus product.
In our study, among the echocardiographic parameters examined, the one exhibiting the strongest correlation with diastolic dysfunction was the LVMI, demonstrating a Pearson correlation coefficient of 0.764. Similarly, a Nigerian study reported LVDD in 37.5% of patients, compared to only 8.3% with systolic dysfunction [44]. This finding resonates with a Turkish study utilizing pulsed wave Doppler and tissue wave Doppler echocardiography, which highlighted LVDD through an elevated E/E′ ratio, positively correlated with LVMI and negatively correlated with low hemoglobin levels [48]. Moreover, a European study revealed that low hemoglobin levels, reduced GFR, younger age, and higher BMI were independent correlates of LV mass index (0.005 < p < 0.05) [47]. Notably, children undergoing hemodialysis exhibited poorer diastolic LV function, characterized by a lower E/A ratio in conventional echocardiography and Em, as well as a higher E/Em in tissue Doppler imaging, when compared to their healthy counterparts [21].
4.1. Limitations of the Study
The study aimed to evaluate LVD in pediatric CKD patients. However, several limitations should be acknowledged. Firstly, the study was conducted at a single tertiary hospital, which may limit the generalizability of the findings to a broader population. This could potentially result in an underestimation or overestimation of the true burden of LVD in pediatric CKD patients within the wider community. Secondly, the final sample size did not meet the initially calculated target, as only 95 respondents were available for inclusion. Nevertheless, the study proceeded with this sample, which still provides valuable insights. Thirdly, the absence of a comparison group, such as a control group, complicates the interpretation of the results and hinders the ability to draw definitive conclusions about the observed cardiac abnormalities. Fourthly, the study did not include an analysis of the use of antihypertensive medications, which could have provided valuable insights into the relationship between medication use and cardiac geometry, particularly regarding concentric changes. Finally, the study could have benefited from a larger representation of patients with Stages 4 and 5 CKD to better understand the association with eccentric hypertrophy. Lastly, the failure to reach the predetermined sample size may have impacted the robustness of the results and should be considered when interpreting the findings. These limitations underscore the need for future research endeavors to address these gaps and provide a more comprehensive understanding of LVD in pediatric CKD patients.
5. Conclusion
This study highlights the critical interplay between CKD and cardiovascular complications in children, emphasizing the need for early and targeted interventions. The findings support the central role of anemia as a modifiable risk factor associated with cardiac dysfunction in pediatric CKD, underscoring the importance of timely diagnosis and management. Strengthening efforts to monitor and address such risk factors may help curb the burden of cardiovascular morbidity in this vulnerable population.
5.1. Recommendation
Pediatric CKD patients stand to gain significant benefits from regular follow-up appointments aimed at identifying cardiovascular risk factors before the onset of geometric changes and diastolic dysfunction. For those patients diagnosed with cardiac dysfunction, particularly diastolic dysfunction, prompt and aggressive treatment of anemia is essential to mitigate the risk of progression to symptomatic failure. An extended study is warranted to investigate the impact of hypertension, volume overload, and additional risk factors such as inflammatory markers (e.g., CRP), PTH levels, and dyslipidemia on the development of cardiac remodeling with varying geometries. This expanded research could provide valuable insights into optimizing the management and care of pediatric CKD patients, ultimately enhancing their long-term health outcomes.
Nomenclature
-
- BMI
-
- Body mass index
-
- BP
-
- Blood pressure
-
- BUN
-
- Blood urea nitrogen
-
- CAKUT
-
- Congenital anomaly of the kidney and urinary tract
-
- CKD
-
- Chronic kidney disease
-
- CKiD
-
- Chronic kidney disease in children
-
- Cr
-
- Creatinine
-
- CrCl
-
- Creatinine clearance
-
- CRP
-
- C-reactive protein
-
- CVRF
-
- Cardiovascular risk factor
-
- ESKD
-
- End-stage kidney disease
-
- GFR
-
- Glomerular filtration rate
-
- eGFR
-
- estimated glomerular filtration rate
-
- Hb
-
- Hemoglobin
-
- KDIGO
-
- Kidney Disease: Improving Global Outcomes
-
- KDOQI
-
- Kidney Disease Outcomes Quality Initiative
-
- LVH
-
- Left ventricular hypertrophy
-
- LVM
-
- Left ventricular mass
-
- RAAS
-
- Renin–angiotensin–aldosterone system
-
- TASH
-
- Tikur Anbessa specialized hospital
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
The study was conceptualized and designed by E.S.S. Data analysis and the initial draft of the manuscript were conducted collaboratively by E.S.S. and B.A.B. Echocardiography was performed by E.G.B. Subsequent revisions and improvements to the drafts were made by Y.T.K. and B.D.M. All authors critically reviewed and approved the final version of the manuscript.
Funding
No funding was received for this research.
Acknowledgments
We extend our sincere gratitude to the dedicated staff members of the pediatric, cardiac, and renal clinics at TASH for their invaluable assistance throughout our research endeavor. Additionally, we express our profound appreciation to the primary caregivers of the study participants’ children at the renal clinic, whose consent and cooperation were essential for the realization of this study.
Open Research
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.