Bacterial Diversity in Aquacultured African Catfish and Source Pond Water in Buea, Cameroon
Abstract
The catfish is a prominent freshwater fish species farmed in Cameroon to meet the escalating demand for fish products. Despite considerable growth potential, there are concerns about the occurrence of bacteria pathogenic to both fish and humans within aquaculture systems. Research on the microbiome of catfish and their habitats remains largely unexplored. Given the critical importance of understanding the microbial composition within aquaculture systems to ensure food safety and protect public health, this study aimed to generate vital preliminary data by investigating the bacteriome of catfish gills and intestines and pond water environment in Cameroon using 16S rRNA gene amplicon sequencing. The findings revealed a diverse bacterial community (30 phyla, 678 genera, and 1056 species), with Fusobacteria, Bacteroidetes, Proteobacteria, Firmicutes, and Verrucomicrobia collectively representing over 93% of the bacterial community observed. Notably, Fusobacteria emerged as the dominant phylum in catfish gills (49.98%) and intestines (65.3%), while Proteobacteria predominated in the pond water environment (40.24%). Bacteria of genus Cetobacterium dominated all three samples (gills, 49.93%; intestines, 65.19%; and pond water, 23.85%). Furthermore, this study identified many bacterial genera, including potential fish pathogens such as Edwardsiella, Aeromonas, Plesiomonas, and Flavobacterium, and human gut bacteria such as Clostridium and Bacteroides, alongside potential beneficial probiotic bacteria such as Lactococcus spp. The coexistence of both potentially pathogenic and probiotic species underscores ecological complex dynamics within freshwater fish aquaculture and highlights the need for thorough microbial management strategies. This study provides insights into the bacterial landscape of Cameroonian aquaculture, revealing potential risks and benefits of catfish farming.
1. Introduction
Catfish ranks among the most cultivated freshwater fish species in Cameroon, where aquaculture as an emerging practice holds great potential to bridge the huge gap that exists between fish demand and local production. The high demand for fish imposes a significant economic burden on the country, resulting in large-scale importation of frozen fish. In 2019 alone, the Cameroonian government imported around 185,000 tonnes of fish, costing an estimated 294 million USD [1]. To alleviate the significant economic burden associated with the importation of frozen fish, the Cameroonian government has prioritized the development of local aquaculture to bolster local fish output. Even though aquaculture is an expensive economic activity (due to initial high startup costs including the high cost of fish feeds, fingerlings, and land acquisition) [2], it has proven to be a lucrative venture in Cameroon [3].
The aquaculture production model is the most rapidly growing animal production sector, currently accounting for approximately 50% of global fish production for food [4]. This rapid transition has been driven by the realization that the capture fisheries model was unsustainable due to rapidly increasing demand, which led to overfishing, increased marine pollution, and global climate change [5]. While the practice of intensive freshwater fish aquaculture addresses the huge demand, it is characterized by several risk factors that lead to microbial contamination of fish [6, 7]. The manipulation of breeding cycles, high stocking density of fish in the pond, and the feeding or fertilization of ponds with agricultural by-products, including animal droppings, impose a certain amount of stress on the fish, thus increasing the predisposition of fish to microbial contamination.
Bacteria (Aeromonas hydrophila, Bacillus spp., and Vibrio cholerae), viruses (tilapia lake virus), parasites (like Myxobolus and Henneguya), and fungi (Aspergilllus spp. and Trichosporon spp.) are among the common contaminants found in fish, often originating from the aquatic environment [8–10]. Given numerous reports on the occurrence of pathogenic bacterial species in aquaculture fish [10–15] and humans [10, 16, 17], it is critical to deploy precautionary measures to enhance disease control and prevention. The use of antibiotics is one of such method, and there is no discrimination between the classes of therapeutic antimicrobials used in humans and food-producing animals [18]. It has been reported that approximately 70%–80% of antibiotics fed to fish are excreted and persist in the aquaculture environment as fish are unable to effectively metabolize these antibiotics [19, 20]. As a consequence, these residual unmetabolized antibiotics exert selective pressure on the microbiota of aquaculture ecosystems [21, 22].
Infectious disease remains one of the major challenges for the aquaculture industry [23, 24], leading to high mortality rates in fish and substantial economic losses for fish farmers. The problem of the disease in the aquaculture setting is further worsened by antibiotic resistance wherein resistance patterns observed in inland animal husbandry have also been observed in aquaculture [25]. The widespread dissemination of resistant organisms and genes across humans, farmed animals, fish, and the environment underscores the threat posed by antimicrobial resistance (AMR) to human and animal health [10], highlighting the urgent need for the controlled use of antibiotics in animal husbandry and aquaculture to preserve available antimicrobials [26].
Fish vaccination and breeding for disease resistance have shown great promise for fish health management, but a sustainable solution also should involve continuous disease monitoring and surveillance, rapid diagnosis, and strengthened biosecurity in hatcheries and breeding centers [27]. Detailed knowledge of the organisms circulating within aquaculture setups will enable better management of fish farms and serve as a baseline for evaluating interventions aimed at improving output, and most importantly, ensuring the health and safety of fish products for human consumption. With the continuous rise and spread of antibiotic resistance and knowledge of the strong influence of gut microbiota on fish health, information on the dominant populations in the fish gut can provide a better insight into obtaining good bacterial species that can serve as probiotic candidates for feed formulations that can contribute to improving fish health, nutrient uptake, and disease resistance within the sub-Saharan African (SSA) aquaculture ecosystem [28]. Moreover, knowledge of human pathogenic genera circulating within aquaculture systems can help identify hazards or potential risks for both the manipulating and consuming human population.
Few studies, with none reported in SSA, have explored the bacteriome of the African catfish, Clarias gariepinus [29], and microbiome investigations in other fish species have focused primarily on factors influencing their growth, survival, and yield. However, there is a notable gap in such detailed studies within the SSA context. Given the importance of locally generated data for the regional aquaculture industry, this study focused on generating preliminary data by characterizing the microbiome of aquacultured African catfish gills, intestines, and the surrounding pond water environments.
2. Methods
2.1. Collection of Samples
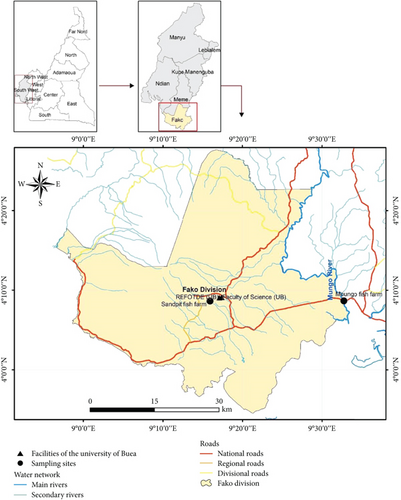
Catfish and pond water samples were purposively collected from the main commercial fish farm in Sandpit-Buea (Figure 1) which serves Buea’s community with table catfish. Samples were collected from a concrete pond, rectangular in shape, and adequately covered by roofing sheets, with access to the farm restricted from the general population. The feed was supplemented with antibiotics (oxytetracycline is spread on fish feed at the rate of 50 g/10 kg), and fish were reared in a flow-through system that uses protected underground clean spring water. Samples were collected between January and March of 2023 in two visits. Then, 10 fish samples comprising five females and five males were caught using a scoop net, while pond water was collected in four sterile 50-mL tubes as water was emptied through an escape nozzle in preparation for fishing. Fish samples were transported in large open-mouth transparent disinfected buckets containing fish pond water, while water samples were transported on icepacks in small cooler flasks to the laboratory within 1 h of collection. Water quality analysis was not performed.
2.2. Sample Processing and Total DNA Extraction
Upon arrival at the laboratory, fish were euthanized by decapitation. The skin of each fish was passively washed by pouring sterile water over the skin surface of the fish before processing. Prior to dissection, the skin surface of the fish was cleaned with 70% alcohol, and 1 g of the gills and intestines from individual fish were separately dissected out and ground in a disinfected mortar. Samples from five individual fishes of the same gender were pooled to form a single sample. Five samples, including separate pools of gills and intestines from both sexes and pooled source water samples, were processed for extraction during each field visit. Subsequently, 250 mg of each homogenous ground subsample of gills and intestines were transferred to a ZR BashingBead lysis tube (0.1 and 0.5 mm) and 750-μL ZymoBIOMICS lysis solution was added to the tube for processing as per the optimized manufacturer’s instructions (ZymoBIOMICS DNA Miniprep Kit_D4300). Water samples were centrifuged, and pellets were pooled. Then, 250 μL of each homogenous pooled pellet from the water sample was transferred to a ZR BashingBead Lysis Tube (0.1 and 0.5 mm), and 750-μL ZymoBIOMICS lysis solution was added to mix in the tube. DNA extraction was conducted according to the manufacturer’s instructions and included a negative control of sterile water. The concentration of the resulting genomic deoxyribonucleic acid (gDNA) samples was measured using a Qubit fluorometer (Thermo Fisher Scientific, United States), following the manufacturer’s instruction. All DNA samples were stored at −20°C until shipping to EzBiome Inc. (Gaithersburg, MD, United States) for amplicon sequencing and bioinformatics analysis.
2.3. 16S rRNA Gene Amplicon Sequencing
Upon receipt of samples, gDNA concentration was further validated on a Qubit 4.0 Fluorometer using Qubit dsDNA High Sensitivity Assay Kit (ThermoFisher Scientific, Waltham, MA, United States). This procedure was followed by 16S rRNA gene amplicon sequencing using methods optimized by EzBiome Inc. (Gaithersburg, MD, United States). The 16S rRNA V3-V4 regions within the ribosomal transcript were amplified using the primer pair (Illumina-F: TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG and Illumina-R: GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC) which contains the gene-specific sequences and Illumina adapter overhang nucleotide sequences. Amplicon polymerase chain reaction (PCR) was performed to amplify the template out of input DNA samples as previously described [30]. Each 25 μL of PCR contained 12.5 ng of sample DNA as input, 12.5 μL 2x KAPA HiFi HotStart ReadyMix (Kapa Biosystems, Wilmington, MA), and 5 μL of each primer (1 μM). PCRs were carried out using the following protocol: an initial denaturation step at 95°C for 3 min followed by 25 cycles of denaturation (95°C, 30 s), annealing (55°C, 30 s), and extension (72°C, 30 s), and a final elongation for 5 min at 72°C. PCR products were cleaned up from the reaction mix with Mag-Bind RxnPure Plus magnetic beads (Omega Bio-tek, Norcross, GA). A second index PCR amplification, used to incorporate barcodes and sequencing adapters into the final PCR product, was performed in 25-μL reactions, using the same master mix conditions as described above. Cycling conditions were as follows: 95°C for 3 min, followed by 8 cycles of 95°C for 30 ″, 55°C for 30 ″, and 72°C for 30 ″. A final, 5-min elongation step was performed at 72°C. The libraries were then normalized and pooled. The pooled library was checked using an Agilent 2200 TapeStation and sequenced on the MiSeq (Illumina, San Diego, CA) on a 500-cycle (2 × 250 bp paired-end) run.
2.4. Bioinformatic Analysis
The bioinformatics analysis included amplicon taxonomic assignment and comparative statistical analyses [31]. The EzBioCloud microbiome taxonomy profiling platform (http://www.ezbiocloud.net) was used to perform taxonomic profiling of 16S sequencing paired-end reads as described by Yoon et al. [32]. EzBioCloud filtered out sequences of low quality by read length (< 438 bp or > 468 bp) and averaged Q values less than 25, while the DUDE-Seq software was used to denoise and extract non-redundant reads. To eliminate chimera sequencing, the UCHIME algorithm was applied against the EzBioCloud 16S chimera-free database. The USEARCH program was used to calculate sequence similarities of the query single-end reads for taxonomic assignment against the EzBioCloud 16S database. Using a cutoff set at 97% sequence similarity, the UPARSE algorithm was used to cluster sequencing reads into operational taxonomic units (OTUs) while the UCLUST tool was used to cluster each sample reads into many.
To estimate the functional profiles of the microbiome identified using 16S rRNA gene amplicon sequencing, the EzBioCloud 16S-based microbiome taxonomic profile (MTP) pipeline used the PICRUST algorithm. The KEGG (Kyoto Encyclopedia of Genes and Genomes) orthology and pathway database were used to bioinformatically annotate the functional abundance profiles of the microbiome wherein the vector of gene counts for each OTU was multiplied by the abundance of that OTU in each sample. The subsampling, generation of taxonomy plots/tables and rarefaction curves, and calculation of species richness, coverage, and alpha and beta diversity indices were performed in the EzBioCloud App [32]. Abundance-based coverage estimator (ACE), Chao1, Jakknife, and the number of OTUs found in the MTP index were used to measure microbial richness. Using the Wilcoxon rank-sum test, the diversity for each group was estimated using the Shannon, Simpson, and Phylogenetic α-diversity indices. The Bray–Curtis dissimilarity distances based on the taxonomic abundance profiles were used to calculate the beta diversity. The statistical significance of β-diversity was measured using permutational multivariate analysis of variance (PERMANOVA). Principal component analysis (PCoA) was used to cluster different groups based on the abundance Jaccard distance metric.
3. Results
3.1. Bacteriome Profiles
Nine out of the 10 DNA samples underwent successful PCR amplification of the targeted V3–V4 regions of the 16S rRNA gene, followed by adequate sequencing data generation for microbiome analysis. From the nine samples, representing three sample types, that is, gills, intestines, and pond water, a total of 162,687 valid reads were obtained, ranging from 10,134 to 26,433 reads per sample. All valid reads underwent taxonomic assignment through clustering, using a sequence similarity threshold of 97%. The mean number of valid reads that were identified at the species level in gills, intestines, and pond water were 11,152, 16,091, and 16,616, respectively. The bacteriome profile of all sample types’ analysis revealed an incredibly rich and taxonomically diverse bacterial community, comprising 30 bacterial phyla, 72 classes, 144 orders, 308 families, 678 genera, and 1056 species across samples.
3.1.1. Bacteriome Profile at Phylum Level
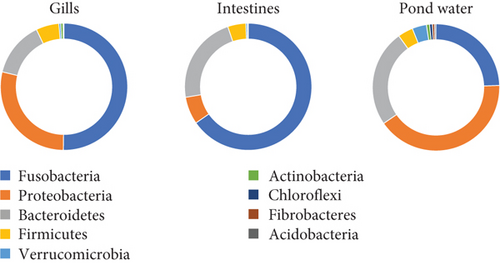
In the taxonomy assignment at the phylum level across the three sample types, a total of 30 phyla were identified (Figure 2). Among these, 12 phyla (Fusobacteria, Bacteroidetes, Proteobacteria, Firmicutes, Verrucomicrobia, Actinobacteria, Chloroflexi, Saccharibacteria_TM7, Spirochaetes, Cyanobacteria, Planctomycetes, and Acidobacteria) were found to be shared among all three sample types, while 10 phyla were shared specifically between the gills and pond water bacteriome (Figure 3). The gill and intestinal samples each exhibited one distinct phylum, while pond water samples revealed six unique phyla.
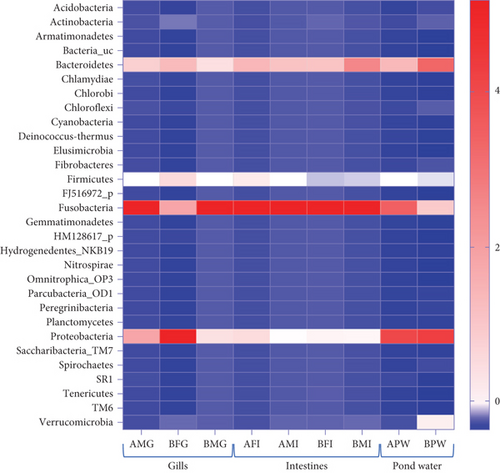

Among the 12 shared phyla, Fusobacteria, Bacteroidetes, Proteobacteria, Firmicutes, and Verrucomicrobia were predominant, with each representing over a 3% mean percent abundance in at least one sample type and comprising over 93% of all identified phyla (Figure 4). The percentage abundance of Proteobacteria was higher in pond water (40.24%) compared with catfish gills and intestines (28.51% and 6.98%, respectively). Bacteroidetes had a mean percent abundance of 22.44% and 24.28% in intestines and pond water samples, respectively, with the lowest abundance observed in gill samples (14.03%). The mean percent abundance of Fusobacteria, Firmicutes, and Verrucomicrobia were not significantly different (p < 0.05) across sample types. Fusobacteria exhibited slightly higher mean percent abundance in intestines (65.3%) and gills (49.98%) and lowest in pond water (24.12%). Firmicutes showed slightly higher abundance in the gills and intestines, while Verrucomicrobia was slightly more abundant in pond water samples. The contribution of all other reported phyla summed up to approximately 6.3% of all identified phyla.
3.1.2. Bacteriome Profile at Genus Level
At the genus level, taxonomic assignment revealed a total of 678 genera across the three sample types. Figure 5 provides an overview of how these genera are shared among the samples. Specifically, 71 genera were shared among gills, intestines, and pond water, while 184 genera were shared among pond water and gill samples. Pond water contained the highest proportion of distinct genera identified, with a total of 275 distinct genera. Overall, the most abundant genera identified were Cetobacterium, found in all three sample types with a mean abundance of 49.93%, 65.19%, and 23.85% in the gills, intestines, and pond water samples (Figure 6), respectively.
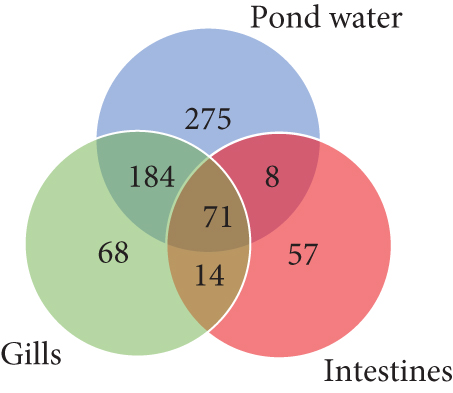
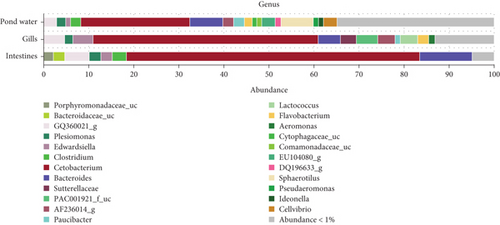
Figure 6 illustrates the mean abundance of the most abundant genera identified. Within intestinal samples, OTUs of the genera Porphyromonadaceae_uc (2.19%) and Bacteroidaceae_uc (2.54%) were slightly more abundant, whereas Lactococcus (3.83%), Aeromonas (1.46%), PAC001921_f_uc (4.7%; class: Betaproteobacteria) and Sutterellaceae_uc (3.56%) were more abundant in the gill samples. Pond water samples exhibited eight genera with a mean abundance greater than 1%, compared to less than 1 in the other samples. These included Sphaerotilus (7.06%), Cellvibrio (3.05%), EU104080_g (2.91%), DQ196633_g (1.3%), Pseudaeromonas (1.12%), Comamonadaceae_uc (1.07%), Cytophagaceae_uc (1.06%) and Ideonella (1.05%). Paucibacter (gills: 1.18%; pond water 2.36%), AF236014_g (gills: 3.83%; pond water 2.33%) and Flavobacterium (gills: 2.48%; pond water 1.64%) genera were relatively more abundant in gills and pond water samples, while Clostridium was relatively more abundant in intestines (3.08%) and pond water (2.21%) samples.
3.2. Shared and Unique Bacteriome Taxa of Catfish and Pond Water Samples
Table 1 shows bacterial genera accounting for ≥ 1% of sequences within each sample type. Shared taxa are present in all three sample types. Bacterial genera shared across fish and pond water included Cetobacterium, the most prevalent bacterial genera, and the fish pathogenic genera Edwardsiella, Aeromonas, Plesiomonas, and Flavobacterium. Shared bacteria also included human gut bacteria of the genus Clostridium and Bacteroides, as well as bacteria of the probiotic genera of Lactococcus. Bacteria of the genus Sphaerotilus, Cellvibrio, and Paucibacter were shared among the gills and pond water, though more abundant in pond water. Sutterellaceae_uc and PAC001921_f_uc OTUs, which belong to bacteria of the phylum proteobacteria, were found exclusively in the gills.
| Classification | Abundance (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Phylum | Class | Order | Family | Genus | Gills | Intestines | Pond water | |
| Shared taxa | Fusobacteria | Fusobacteria_c | Fusobacteriales | Fusobacteriaceae | Cetobacterium | 54.35 | 64.39 | 26.29 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | 4.98 | 11.33 | 6.63 | |
| Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | GQ360021_g | 4.56 | 5.01 | 2.87 | |
| Proteobacteria | Gammaproteobacteria | Enterobacterales | Hafniaceae | Edwardsiella | 4.37 | 2.82 | 1.17 | |
| Firmicutes | Clostridia | Clostridiales | Clostridiaceae | Clostridium | 0.83 | 3.5 | 2.5 | |
| Proteobacteria | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | Plesiomonas | 1.69 | 3.07 | 2.38 | |
| Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroidaceae_uc | 0.12 | 3.1 | 0.62 | |
| Proteobacteria | Betaproteobacteria | Rhodocyclales | Azovibrio_f | AF236014_g | 3.17 | 0.52 | 1.84 | |
| Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | Porphyromonadaceae_uc | 0.3 | 2.38 | 0.95 | |
| Firmicutes | Bacilli | Lactobacillales | Streptococcaceae | Lactococcus | 3.42 | 0.05 | 0.45 | |
| Bacteroidetes | Flavobacteria | Flavobacteriales | Flavobacteriaceae | Flavobacterium | 2.3 | 0.01 | 1.48 | |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Sutterellaceae | DQ196633_g | 0.45 | 0.36 | 1.08 | |
| Proteobacteria | Gammaproteobacteria | Aeromonadales | Aeromonadaceae | Aeromonas | 1.26 | 0.02 | 0.31 | |
| Proteobacteria | Gammaproteobacteria | Aeromonadales | Aeromonadaceae | Pseudaeromonas | 0.39 | 0.01 | 1.14 | |
| Unique taxa | Proteobacteria | Betaproteobacteria | Burkholderiales | Comamonadaceae | Sphaerotilus | 0.07 | — | 8.27 |
| Proteobacteria | Gammaproteobacteria | Cellvibrionales | Cellvibrionaceae | Cellvibrio | 0.38 | — | 3.7 | |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Sutterellaceae | Sutterellaceae_uc | 3.75 | — | — | |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Comamonadaceae | Paucibacter | 1.02 | — | 2.46 | |
| Proteobacteria | Betaproteobacteria | PAC001921_o | PAC001921_f | PAC001921_f_uc | 3.58 | — | — | |
| Bacteroidetes | Sphingobacteriia | Saprospirales | Saprospiraceae | EU104080_g | 0.03 | — | 2.47 | |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Comamonadaceae | Comamonadaceae_uc | 0.29 | — | 1.1 | |
| Bacteroidetes | Cytophagia | Cytophagales | Cytophagaceae | Cytophagaceae_uc | 0.02 | — | 1.01 | |
3.3. Diversity Analysis
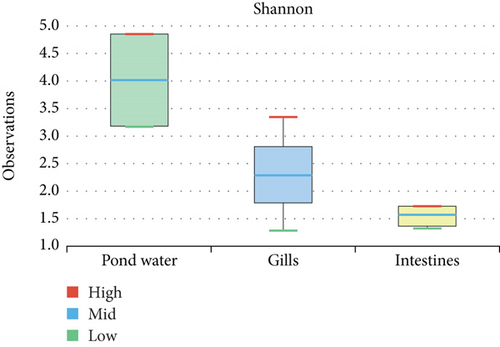
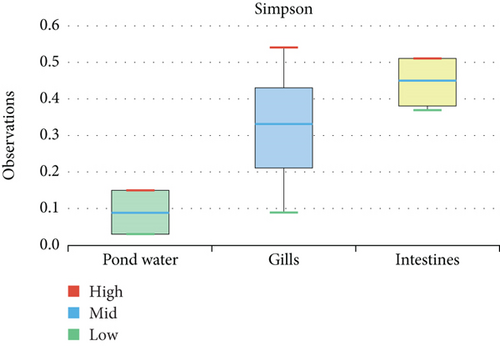
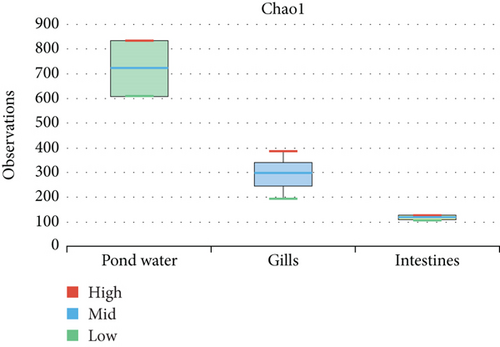
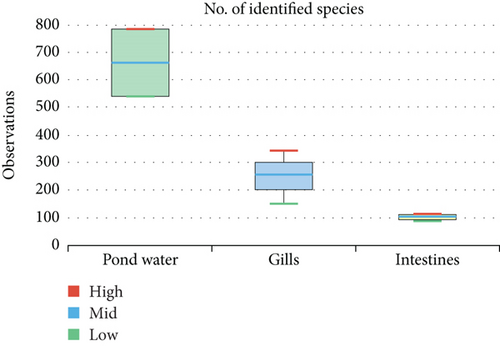
The alpha diversity assessment of the three sample types was performed using Shannon, Simpson, Chao1, and observed species diversity indices. These indices provide insights into species richness, evenness, and community composition. These revealed that microbial diversity was highest in pond water as compared to gills and intestines, with the lowest diversity observed in intestines. Chao1 (species richness) and number of OTUs (Figure 7) demonstrated the significant differences of alpha diversity between gills and intestinal samples following Wilcoxon rank-sum test (p value = 0.034). Thus, the alpha diversity of the gills and intestines is different. The beta diversity indices as presented by the Bray–Curtis dissimilarity index showed that the intestinal samples clustered together separately from other samples of the two types (Figure 8). PERMANOVA analysis showed a significant difference between gills and intestinal samples (p value = 0.033 and q value = 0.084).
3.4. Functional Abundance of Kyoto Encyclopedia of Genes and Genomes Orthologies (KOs)
The most abundant KOs annotated from taxonomic data were significantly different among the sample types (Table 2). In fact, analysis revealed that the most abundant KOs were from the microbiome of the intestines with K21572 (0.495) that encodes for the starch-binding outer membrane protein, SusD/RagB family, and K02014 that encodes for the iron complex outer membrane receptor protein (0.427) significantly highly expressed in intestines as compared to pond water and gill microbiome. KOs, which were significantly highest in pond water, included K00059 (0.306) that encodes for the 3-oxoacyl-[acyl-carrier protein] reductase and K03392 (0.394) that encodes for the aminocarboxymuconate-semialdehyde decarboxylase.
| Orthology | Definition | p value | Intestines | Gills | Pond water |
|---|---|---|---|---|---|
| K02014 | Iron complex outermembrane recepter protein | 0.040 | 0.427 | 0.188 | 0.241 |
| K21572 | Starch-binding outer membrane protein, SusD/RagB family | 0.040 | 0.495 | 0.130 | 0.177 |
| K03088 | RNA polymerase sigma-70 factor, ECF subfamily | 0.043 | 0.312 | 0.127 | 0.203 |
| K03585 | Membrane fusion protein, multidrug efflux system | 0.047 | 0.166 | 0.114 | 0.080 |
| K04763 | Integrase/recombinase XerD | 0.047 | 0.193 | 0.104 | 0.069 |
| K03654 | ATP-dependent DNA helicase RecQ | 0.047 | 0.136 | 0.093 | 0.065 |
| K21573 | TonB-dependent starch-binding outer membrane protein SusC | 0.040 | 0.319 | 0.090 | 0.115 |
| K03832 | Periplasmic protein TonB | 0.047 | 0.117 | 0.081 | 0.071 |
| K07114 | Ca-activated chloride channel homolog | 0.047 | 0.154 | 0.077 | 0.069 |
| K01190 | Beta-galactosidase | 0.050 | 0.197 | 0.076 | 0.073 |
| K07720 | Two-component system, response regulator YesN | 0.047 | 0.124 | 0.070 | 0.063 |
| K07407 | Alpha-galactosidase | 0.047 | 0.119 | 0.067 | 0.040 |
| K01187 | Alpha-glucosidase | 0.047 | 0.126 | 0.060 | 0.041 |
| K12340 | Outer membrane protein | 0.047 | 0.117 | 0.059 | 0.056 |
| K07636 | Two-component system, OmpR family, phosphate regulon sensor histidine kinase PhoR | 0.050 | 0.112 | 0.058 | 0.064 |
| K07165 | Transmembrane sensor | 0.047 | 0.111 | 0.038 | 0.055 |
| K05349 | Beta-glucosidase | 0.040 | 0.107 | 0.031 | 0.045 |
| K07760 | Cyclin-dependent kinase | 0.047 | 0.017 | 0.099 | 0.092 |
| K03392 | Aminocarboxymuconate-semialdehyde decarboxylase | 0.047 | 0.038 | 0.286 | 0.394 |
| K00059 | 3-Oxoacyl-[acyl-carrier protein] reductase | 0.046 | 0.144 | 0.211 | 0.306 |
| K02020 | Molybdate transport system substrate-binding protein | 0.040 | 0.047 | 0.098 | 0.132 |
| K00798 | Cob(I)alamin adenosyltransferase | 0.047 | 0.041 | 0.083 | 0.112 |
| K09678 | [Heparan sulfate]-glucosamine 3-sulfotransferase 4 | 0.030 | 0.003 | 0.062 | 0.204 |
| K12132 | Eukaryotic-like serine/threonine-protein kinase | 0.043 | 0.076 | 0.053 | 0.117 |
| K00010 | Myo-inositol 2-dehydrogenase/D-chiro-inositol 1-dehydrogenase | 0.030 | 0.047 | 0.033 | 0.114 |
- Note: Highest values are in bold.
4. Discussion
The importance of fish microbiota on fish health has been widely recognized in various studies. Dominant bacterial species within the gut microbiota are particularly considered strong candidates for fish probiotics, especially in fish aquaculture settings. Despite the significance of catfish aquaculture in Cameroon and the broader sub-Saharan region, microbiome studies characterizing microbial communities associated with catfish and related environments are limited, with none reported in Cameroon or within the sub-Saharan region. Given the scale of catfish aquaculture in the country and on the continent, there is a notable gap in understanding the microbiome associated with these fish species, their differences, and potential role in fish health. This microbiome study represents a preliminary attempt to investigate the bacteriome associated with the gills and intestines of the African catfish Clarias gariepinus, as well as its pond water aquaculture in Cameroon. By shedding light on the microbial communities inhabiting these crucial environments, the study aims to provide useful insights to drive understanding of the microbial factors influencing catfish health as well as potentially contribute to the development of catfish aquaculture-oriented probiotics in the region.
Analyzing the bacterial taxa present in fish gills and intestines, the prevalence and abundance of Fusobacteria across all sample types, with Cetobacterium emerging as the most dominant genus, aligns with findings from previous studies. Indeed, research by [33–36] has consistently reported Cetobacterium as a major component of freshwater fish gut microbiota. This observation holds for other freshwater fish species, including tilapia [37, 38], as well as more recently for the African catfish, as indicated by Skvortsova et al. [28]. These findings suggest that Cetobacterium may indeed be a common resident of the bacterial microbiota in catfish and their environment, playing a significant role in catfish biology. The widespread presence of Cetobacterium across different freshwater fish species [34, 35] implies its importance as a key component of the fish gut microbiota in these aquatic environments. This supports the notion that globally, Cetobacterium could be considered a characteristic species within the guts of freshwater fishes, highlighting its potential significance in understanding fish health and ecology.
The capability of Cetobacterium species to produce cobalamin (Vitamin B12), along with antimicrobial metabolites, underscores their potential significance as functional symbionts with freshwater fish guts, including those of the African catfish C. gariepinus. Previous studies have demonstrated that Vitamin B12 produced by Cetobacterium somerae can enhance host resistance against pathogen infection by promoting beneficial interactions within the gut microbiota [39, 40]. Given these properties, Cetobacterium could indeed serve as an important functional symbiont [36] in freshwater fish guts, including the African catfish C. gariepinus. Harnessing the presence of Cetobacterium as a core member of the catfish bacteriome is a promising prospect for the development and testing of tailored feeds suitable for local species or specific aquaculture practices within Cameroon. Incorporating feeds enriched with Cetobacterium or its beneficial metabolites could improve fish health, increase yields, and enhance economic returns for aquaculture operations. This highlights the importance of understanding the functional roles of key bacterial symbionts within fish microbiomes and leveraging this knowledge to optimize aquaculture practices for the benefit of both fish health and production efficiency.
The exploration of the bacteriome across fish gills, intestines, and pond water environments revealed varying abundances of Cetobacterium across these environments. Overall, Cetobacterium emerged as the most abundant bacterial genus in this study. In the gills, bacteria belonging to the phylum Fusobacteria constituted 49.95% of the phyla, with Cetobacterium accounting for 49.87% of all genera. Similarly, in the intestines, Fusobacteria were the most abundant phylum (65.3%), with Cetobacterium species accounting for 65.18% of all bacterial genera. However, such dominance of the Fusobacteria phyla was not reflected in pond water. Instead, Proteobacteria emerged as the most dominant phylum, accounting for 40.24% of all phyla identified. Bacteroidetes and Fusobacteria made up 24.28% and 24.12% of the phyla in pond water, respectively. Although Cetobacterium species remained the most abundant genus in pond water, they constituted a much lower proportion of the overall phyla and genera compared to gills and intestines. This suggests a distinct microbial composition and distribution across different aquatic environments, with Cetobacterium being particularly prevalent within the internal organs of fish than in the surrounding water. The differential abundance of Cetobacterium highlights its potential role as an important member of the fish microbiome, particularly in the gastrointestinal tract, and underscores the need for further investigation into its functional significance in different ecological contexts.
The current study identified prevalent bacteria genera, recognized as potential fish pathogens such as Edwardsiella, Aeromonas, Plesiomonas, and Flavobacterium across fish and pond water samples. This finding aligns with reports of gut-associated pathogenic microbiota in other freshwater aquaculture fish like tilapia [41]. Of these, Edwardsiella and Aeromonas species are recognized as emerging infectious bacterial pathogens [42, 43] in freshwater fish, while Plesiomonas are increasingly being recognized as an important pathogen to various fish [44]. Flavobacterium is also noted as a significant pathogen in catfish aquaculture [45, 46]. Investigations of bacterial communities in recirculating catfish aquaculture systems have similarly identified Edwardsiella and Aeromonas species, along with other key fish pathogenic species such as Mycobacterium, Pseudomonas, Flavobacterium, Yersinia, and Rickettsia in the aquaculture system [47]. Therefore, these pathogens pose significant concerns for fish health and aquaculture management, underscoring the importance of continued monitoring and mitigation efforts in aquaculture environments.
Shared bacteria in aquaculture systems included human gut bacteria like Clostridium and Bacteroides. Clostridium species include Clostridium botulinum, an important food-borne pathogen [48] among the most recurrent fish-related foodborne illnesses affecting humans because of their wide environmental distribution and spore-forming ability. Detailed investigations of the aquaculture ecosystems within this under-researched region are crucial for identifying major circulating fish pathogens, aiding effective disease surveillance and prevention.
In addition to Cetobacterium, which is the most prevalent bacteria genera with potential probiotic benefits for fish physiology [36], Lactococcus species have also been identified. Mainly classified as lactic acid bacteria (LAB), Lactococcus includes species such as Lactococcus lactis and Lactococcus garvieae. While Lactococcus lactis is a well-known probiotic bacterium, Lactococcus garvieae is recognized as a fish pathogen in marine and aquaculture environments [49, 50]. Lactococcus lactis has been studied as a probiotic for aquaculture, demonstrating its antimicrobial properties against Lactococcus garvieae, the causative agent of lactococcosis in fish [49, 50]. This therefore suggests the potential use of Lactococcus lactis as a probiotic agent in aquaculture for disease management.
The annotated functional profile of the bacteriome associated with catfish aquaculture revealed highly abundant KOs (K21572, K01190, K00059, and K03392) with potential involvement in the metabolism of carbohydrates, lipids, and amino acids (Figure 2). In addition to the potential probiotic species earlier highlighted, these functional profiles can offer insights that can be used to increase nutrient availability for cultured catfish with more locally available feed material [51]. Analysis also revealed the functional profile K00798, a cob(I)alamin adenosyltransferase that converts cobalamin (Vitamin B12), highly produced by Cetobacterium, to adenosylcobalamin, which is required as a cofactor for the activity of other enzymes [52]. Also important to highlight is the high expression of functional profiles (K02014 and K2157) with high affinity for binding iron under iron-limiting conditions. This can also serve to balance iron in the pond environment given that excess iron can cause stress in fish [53].
Analysis also revealed the presence of KO K09678, coding for a sulfotransferase expressed by sulfur-oxidizing bacteria that can be very useful in the metabolism and removal of hydrogen sulfide in an aquaculture environment [54]. The KO K03585, highly expressed in intestinal samples, demonstrates the presence of antimicrobial efflux pumps in the gut bacteriome of the African catfish. This presence can have implications for the use of antibiotics in aquaculture and the spread of AMR. Knowledge of the high expression KOs of the microbiome of catfish intestines and pond water sheds light on the composition of the gut microbiome in healthy catfish individuals. This insight can be used to design and develop strategies to promote catfish gut health.
The bacteriome of catfish gills, intestines, and pond water was predominantly composed of bacteria of the order Fusobacteriales and genus Cetobacterium. Additionally, pathogenic genera like Edwardsiella, Aeromonas, Plesiomonas, and Flavobacterium, along with human gut bacteria like Clostridium and Bacteroides, were identified. Furthermore, genera like Lactococcus, known for their probiotic properties, were also identified. These findings provide preliminary insight into the natural bacteriome of healthy catfish and pond water within the aquaculture environment in Cameroon. It underscores the potential role of freshwater fish aquaculture in harboring both human pathogenic bacteria and important probiotic species. Understanding the composition of these microbial communities and their delicate balance is crucial for managing fish health and ensuring the safety of aquaculture practices in Cameroon. Continued research is vital for optimizing aquaculture practices and ensuring food safety.
Ethics Statement
Before sample collection, all fish manipulation procedures were submitted for ethical approval. Ethical clearance was obtained from the University of Buea Institutional Animal Care and Use Committee (UB-IACUC) with reference number UB-IACUC No. 24/2023. Sample collection and processing were performed following methods approved by the UB-IACUC ethical committee.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare that they have no competing interest. In the interest of transparency, Dr. Nur A. Hasan’s institute, EzBiome Inc., received partial support for 16S rRNA gene amplicon sequencing. However, all bioinformatics and statistically analysis, data interpretation, and visualization were provided free of charge and in the spirit of this collaboration.
Author Contributions
Conceived study and coordinated: G.T.N., B.T.F., and S.W. Sample collection and laboratory analysis: G.T.N., R.A.S., and A.A.A. Data analysis: G.T.N., A.L.K.T., B.T.F., and N.A.H. Developed the first draft of the manuscript: G.T.N. and B.T.F. Critical review of the article: S.W., B.T.F., N.A.H., J.F.-C., A.L.K.T., A.A.A., and R.A.S. All authors approved the manuscript.
Funding
The study received no external funding. S.W. is a senior Fellow Plus of EDCTP2 (TMA2019SFP-2814).
Acknowledgments
We acknowledge the owner of the commercial fish farm for access to his farm for sample collection. We also acknowledge “EzBiome” for facilitating sample transportation to EzBiome Inc., Gaithersburg, United States, sequencing, and bioinformatics. S.W. is a senior Fellow Plus of EDCTP2 (TMA2019SFP-2814).
Open Research
Data Availability Statement
The datasets generated and used during the current study are presented in the article and the sequences deposited in the NCBI database under the Accession Number PRJNA1171439.




