Multiple Anthropogenic and Climatic Factors Drive Tree Species Attributes in Ecologically Distinct Sacred Groves in Ghana
Abstract
Sacred groves are rich biodiversity hotspots serving as an important habitat for conserving species and providing ecosystem goods and services to meet societal needs. Despite the benefits these sacred groves offer, they are threatened by anthropogenic stressors coupled with climate change impacts, thereby limiting their maximum ability to offer essential ecological and cultural services. On a climatic gradient, we have explored differences in tree attributes (diversity and composition) and how they are shaped by multiple drivers of land-use change and climatic factors (rainfall and temperature) which drive underlying soil conditions in different sacred groves. These sacred grooves studied are the Mintimrim Kwaye, Antobia, Boako, Nsoatre Botene and Pimpimgyae sacred groves. Tree species diversity and composition differed between the sacred groves with those located in the forest zones more diverse than those located in the dry semideciduous or the savannah zones. These differences in tree attributes were mainly driven by the various degrees of anthropogenic stressors (mining, agricultural activities, logging and wildfire) coupled with variations in climatic factors driving underlying soil conditions. It is quite evident the role climate plays which highlights how tree species react in terms of distribution and composition in this era of climate uncertainty. This therefore calls for the need for conservation efforts to mitigate the consequences of climate change impacts on biodiversity and human societies.
1. Introduction
Protected ecosystems including sacred groves are essential components of conservation, serving as hotspot biodiversity areas and providing ecosystem goods and services to meet societal needs [1]. Notwithstanding the conservational benefits of protected ecosystems, they are underpinned by anthropogenic stressors coupled with climate change impacts [2–4]. Effects are seen in altering the distributional ranges of species and their community composition [5–7].
While the immensity of climate impacts is uncertain in some regions [8], numerous kinds of evidence of the negative impacts on biodiversity and human society have been observed in the tropical regions [9–11]. For instance, in the African subregion which supports numerous tropical ecosystems, the impact of climate change on biodiversity through changes in species life cycles, alteration in habitat and the distributional range of species has been established [12]. Similarly, the Intergovernmental Panel on Climate Change [13] reported a distributional shift of forest biomes, changes in species composition and increasing forest fires as a result of climate change in the tropics. Addressing these impacts requires sustainable approaches to safeguard tropical forests while minimising their effects on biodiversity and simultaneously maximising their benefits through livelihood support systems.
The tropical forests cover less than 10% of the total earth’s surface [14, 15] but harbour about two-thirds of all terrestrial biodiversity on Earth [16, 17]. Despite their small spatial coverage, they represent global hotspots of biodiversity and endemism [18] and directly and indirectly influence the well-being of billions of people globally [19, 20] through the provisioning of ecosystem goods and services including food, shelter and raw materials that support human livelihood [21]. Besides their role in sustaining biodiversity [22, 23], they serve as an essential net source or sink of atmospheric carbon dioxide [24] and account for about 40% of global terrestrial biomass [25, 26].
Species diversity in the tropics is typically driven by several factors, including their distinct hot and wet climates, as well as their nutrient recycling ability which provides ideal environmental conditions for species to thrive and colonise [27, 28] and by their large fragments which are minimally isolated from human pressures thereby allowing nature to thrive [25]. Within these tropical regions are established sacred groves which are defined by their form of governance (based on traditional directives and regulations), actors and powers which determine how they are used [1]. Approaches to the management of these sacred groves have seen the prohibition of harvesting certain tree species and the restrictions on entry on certain taboo days aiming at ensuring the rich biodiversity of such designated sacred places are protected from degradation.
The vegetation of Ghana is classified into various ecological zones based on climate which drives tree species distribution and the underlying soil conditions [29]. Nine main forest types or vegetation zones are delineated in Ghana [30] and further classified into three broad vegetation zones to include the high forest (HFZ), the transitional (TZ) and the savannah zones (SZ) [31]. Within the HFZ are different forest types together with the TZ and the SZ forming the focal ecosystems of this study. The assessment of vegetation attributes in this study focused on forest zones in the wet evergreen (WE), moist evergreen (ME), moist semideciduous (MSD), dry semideciduous (DSD) and the SZ each defined by their unique association of plant and animal species [32, 33]. Most of these sacred groves are persistently faced with the issue of unsustainable logging and encroachment for farming because of their rich biodiversity on fertile soils supporting the growth of high-economic value timber species. Besides logging, the rich mineral reserves located in some of these sacred groves have exposed them to indiscriminate mining activities causing numerous environmental problems, affecting biodiversity, and limiting the ecological benefits and services offered by these sacred groves [34]. Furthermore, the dry climate up north also subjects the sacred groves in the transitional and the DSD forests to extremely high temperatures with low or erratic rainfall patterns creating highly flammable fuel loads on the forest floors leading to frequent wildfire events [35] with the expected implications on biodiversity and tree species distribution.
Management approaches to biodiversity conservation in this changing climate era have frequently focused on understanding the underlying mechanisms driving species distribution. Unscrambling these mechanisms in sacred groves located in the various forest types in Ghana will inform adaptive strategies for sustainable forest management and enhance conservation efforts in the tropics. Understanding the dynamics of plant community assembly in sacred groves and their distribution across climate-delineated spatial scales under different land-use scenarios is fundamental in setting conservation goals and priorities as well as the design of adaptive frameworks aiming at counteracting climate change impacts. The goal of this study was to explore how climate, environmental and land-use factors affect tree species attributes within sacred groves located in different ecological zones. Specifically, we addressed (i) the differences in tree species diversity and community composition between different sacred groves located in different forest types on climatic gradients and (ii) explored how species attributes (diversity and composition) are shaped by multiple edaphic, land-use and climatic factors.
2. Materials and Methods
2.1. Study Area
Five protected areas (sacred groves) spreading across the high forests through the TZ to SZ of Ghana were selected for the assessment of tree community composition and species attributes (Figure 1).
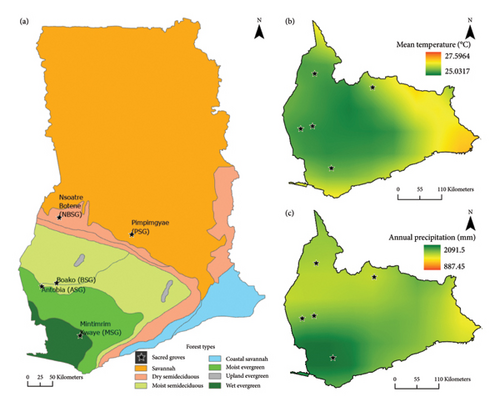
Mintimrim Kwaye Sacred Grove (MSG) is located in Prestea Nkwanta community on 2°11′35″W, 4°49′19″N in the Prestea-Huni Valley district of the Western Region of Ghana. MSG is found in the WE Forest type of Ghana and covers an area of about 0.52 km2. Antobia Sacred Grove (ASG) is found in the ME Forest of Ghana and located on 2°54′28″W, 6°16′13″N. ASG is closer to the Krokosua Hills Forest Reserve and located in the Western North region of Ghana, covering an area of about 0.21 km2. Boako Sacred Grove (BSG) is in the MSD forest, located on 2°35′24″W, 6°22′43″N, and covers an area of about 0.99 km2..BSG is found between the Asawinso and Sefwi Ayinabrim towns located in the Western North Region of Ghana. Nsoatre Botene Sacred Grove (NBSG) located within the Tain II Forest Reserve is a DSD forest covering about 6.29 km2 and located in the Bono Region of Ghana, on 2°27′41″W, 7°28′36″N. Frequent annual wildfire events, uncontrolled logging activities and unsustainable farming practices are the characteristic drivers of forest change in Tain II Forest Reserve [36], which houses the NBSG. Pimpimgyae Sacred Grove (PSG) falls in the SZ, between Asowaso and Sasenono communities near Ejura in the Ashanti Region of Ghana. It is located on 1°12′25″W, 7°10′32″N and covers about 0.48 km2 of land size. The dry climate of PSG exposes it to frequent annual wildfire events with implications for local biodiversity and human livelihoods [1].
2.2. Plot Establishment and Sampling
In each sacred grove, nine (9) plots (45 sampling units) with sizes 20 × 20 m were purposively stratified and demarcated for sampling tree and soil attributes. Prior to plots demarcation, stratification of the area of interest based on environmental strata (e.g., human impact and terrain) was carried out. Plots were kept at least 200 m from each other to limit spatial autocorrelation. All trees with diameter at breast height (dbh) ≥ 10 cm were identified for enumeration. Enumeration focused on species identification by an experienced botanist with the assistance of forest guards. Species identification was based on an identification manual from Hawthorne and Gyakari [37]. The diameter of trees was measured with a Vernier calliper, and tree heights estimated based on trigonometric calculations [38]. Five replicated samples of soil were taken with soil auger from each plot at a depth of 0–30 cm and thoroughly mixed to form a composite sample for further assessment of physical and chemical soil properties in the laboratory.
2.3. Climate Variables and Drivers of Forest Change
Climate data (average temperature and precipitation) at 1 km spatial resolution for each forest type were obtained from the ‘WorldClim’ database [39]. Drivers of forest change (logging, mining, wildfire and agriculture) were assessed based on visual observations of the presence or absence and the percentage coverage of such drivers (defined by their scope) in each plot [40].
2.4. Data Analysis
Plot level data were pulled together (average) to represent species abundance data for each sacred grove. Species diversity estimates were based on log-transformed (Log (x + 1)) pulled data and estimated using the ‘Diverse’ function in PRIMER v7 [41]. Statistically significant differences between the sacred groves for each species attribute (diversity and composition) were evaluated with a one-factorial permutational analysis of variance (PERMANOVA) using Bray–Curtis similarity [42], with sacred grove as fixed and plot types nested in sacred grove as random factors. A pairwise post hoc testing was carried out when differences existed between the sacred groves for any of the tree attributes tested. Species composition patterns were visually displayed using non-Metric Multidimensional Scaling Ordination (nMDS) [43] and species dominance was evaluated based on Similarity Percentage analysis (SIMPER) [44]. The influence of multiple environmental factors (climate, soil and anthropogenic stressors) on tree species composition was evaluated with the Distance-Based Linear Model (DistLM) and patterns were visually displayed with the distance-based redundancy analysis (dbRDA) [45].
3. Results
3.1. Differences in Tree Species Richness and Diversity Between the Sacred Groves
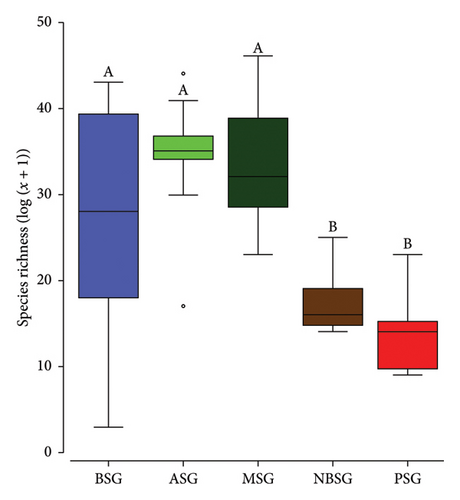
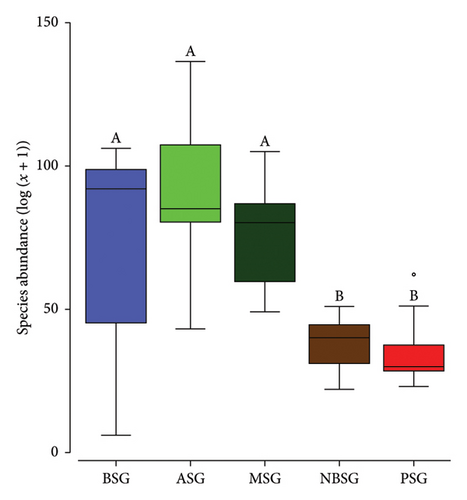
Tree stands in ASG were more diverse and abundant (s = 78, n = 825) than those in the MSG (s = 67, n = 679), BSG (s = 64, n = 652), NBSG (s = 47, n = 344) and the PSG (s = 31, n = 302) (Table 1). Species richness (F4,32 = 6.521; p < 0.001) as well as abundance (F4,32 = 5.042; p = 0.001) differed significantly between the sacred groves. A pairwise comparison revealed non-statistically significant differences between the species richness of the sacred groves in the forest zone (BSG, ASG and MSG). The sacred groves in the forest zones however differed from those in the DSD (NBSG) and the SZ (PSG) for both species richness and abundance (Figure 2). While the sacred groves did not differ significantly in terms of Pielou’s evenness (p > 0.05) and Simpson indices (p > 0.05), they differed in terms of Shannon indices (p = 0.002).
| Diversity | BSG | ASG | MSG | NBSG | PSG | p value |
|---|---|---|---|---|---|---|
| Tree richness (s) | 64 | 78 | 67 | 47 | 31 | < 0.001 |
| Tree abundance (N) | 652 | 825 | 679 | 344 | 302 | 0.001 |
| Pielou’s evenness (J′) | 0.899 (0.02) | 0.908 (0.02) | 0.929 (0.01) | 0.934 (0.01) | 0.912 (0.01) | 0.233 |
| Shannon (H′) | 2.781 (0.27) | 3.19 (0.10) | 3.242 (0.09) | 2.652 (0.06) | 2.339 (0.09) | 0.003 |
| Simpson (1-ƛ′) | 0.907 (0.04) | 0.948 (0.01) | 0.960 (0.01) | 0.941 (0.00) | 0.909 (0.01) | 0.162 |
- Note: Differences in indices between the sacred groves were tested with permutational analysis of variance (PERMANOVA). Mean values are provided for the diversity indices and standard deviations are in parentheses: n = 9. Significant p values are in bold (p < 0.05).
3.2. Differences in Tree Species Composition Between the Sacred Groves
The sacred groves differed significantly in terms of tree species composition which is confirmed by three main clusters (Figure 3; F4,32 = 7.612; p < 0.001). Dissimilarities were most pronounced between the sacred groves in the savannah (PSG) and the DSD forest (NBSG) as plots were separated far from the sacred groves in the three forest zones (BSG, ASG and MSG).
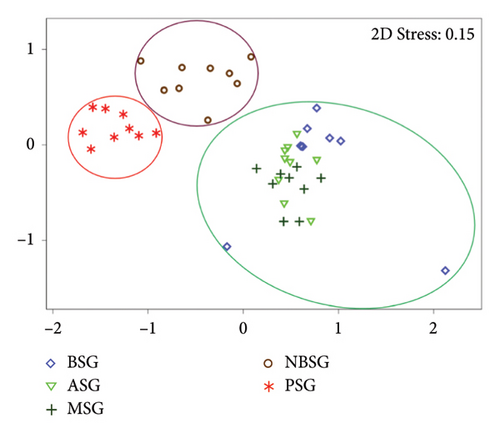
A pairwise comparison of species composition for the sacred groves revealed no statistical differences (p > 0.05; BSG, ASG and MSG) which was further confirmed by the NMDS ordination clusters (Figure 3). However, statically significant differences (p < 0.05) were observed between the BSG and NBSG, BSG and PSG, ASG and MSG, ASG and NBSG, ASG and PSG, MSG and NBSG, MSG and PSG and NBSG and PSG (Table 2).
| Groups | t | p (perm) | Unique perms | |
|---|---|---|---|---|
| BSG | ASG | 1.210 | 0.119 | 9921 |
| BSG | MSG | 1.464 | 0.052 | 9924 |
| BSG | NBSG | 2.415 | < 0.001 | 9908 |
| BSG | PSG | 3.503 | < 0.001 | 9944 |
| ASG | MSG | 1.325 | 0.039 | 9908 |
| ASG | NBSG | 2.676 | < 0.001 | 9942 |
| ASG | PSG | 4.123 | < 0.001 | 9924 |
| MSG | NBSG | 2.957 | < 0.001 | 9916 |
| MSG | PSG | 4.113 | < 0.001 | 9938 |
| NBSG | PSG | 2.754 | < 0.001 | 9929 |
- Note: Significant p values are in bold (p < 0.05).
Species composition differences between the BSG and ASG were mainly driven by the abundance of five species. A higher abundance of Celtis mildbraedii, Alstonia boonei and Ceiba pentandra was observed in ASG than in BSG. Entandrophragma angolense and Ficus exasperata were more abundant in BSG than ASG (Table 3). While a higher abundance of Azadirachta indica, Tectona grandis and Parkia bicolor was observed in PSG and the NBSG, no records were found in BSG, ASG and MSG. Species including Funtumia elastic, Funtumia africana, Microdesmis puberula and Entandrophragma angolense were completely absent in the sacred grove in the SZ (PSG). Species composition details for each forest zone are provided in Appendix 1.
| Species | Av. abund BSG | Av. abund ASG | Contrib % | Species | Av. abund BSG | Av. abund MSG | Contrib % |
|---|---|---|---|---|---|---|---|
| Celtis mildbraedii | 5.44 | 8.78 | 6.47 | Entandrophragma angolense | 7.78 | 0.89 | 5.83 |
| Entandrophragma angolense | 7.78 | 1.56 | 5.71 | Celtis mildbraedii | 5.44 | 1.33 | 4.07 |
| Alstonia boonei | 1.33 | 5.44 | 4.41 | Funtumia africana | 1.11 | 4.33 | 3.91 |
| Ficus exasperata | 2.89 | 2.33 | 3.86 | Funtumia elastic | 0.56 | 4.56 | 3.88 |
| Ceiba pentandra | 1.11 | 4.44 | 3.45 | Baphia nitida | 2.89 | 4.33 | 3.85 |
| ASG | MSG | BSG | NBSG | ||||
| Celtis mildbraedii | 8.78 | 1.33 | 6.76 | Entandrophragma angolense | 7.78 | 1.00 | 6.68 |
| Alstonia boonei | 5.44 | 2.00 | 4.32 | Celtis mildbraedii | 5.44 | 0.78 | 4.81 |
| Funtumia africana | 0.11 | 4.33 | 3.64 | Funtumia africana | 1.11 | 4.11 | 4.14 |
| Funtumia elastic | 1.67 | 4.56 | 3.55 | Tectona grandis | 0.00 | 3.33 | 3.97 |
| Baphia nitida | 4.11 | 4.33 | 3.28 | Microdesmis puberula | 3.11 | 0.00 | 3.13 |
| ASG | NBSG | MSG | NBSG | ||||
| Celtis mildbraedii | 8.78 | 0.78 | 6.93 | Funtumia africana | 4.33 | 4.11 | 5.10 |
| Alstonia boonei | 5.44 | 1.67 | 4.14 | Baphia nitida | 4.33 | 0.00 | 4.52 |
| Ceiba pentandra | 4.44 | 0.22 | 3.98 | Funtumia elastic | 4.56 | 1.33 | 4.19 |
| Microdesmis puberula | 4.44 | 0.00 | 3.80 | Tectona grandis | 0.00 | 3.33 | 3.47 |
| Baphia nitida | 4.11 | 0.00 | 3.72 | Ceiba pentandra | 3.00 | 0.22 | 3.05 |
| BSG | PSG | ASG | PSG | ||||
| Entandrophragma angolense | 7.78 | 0.00 | 6.34 | Celtis mildbraedii | 8.78 | 0.11 | 6.69 |
| Azadirachta indica | 0.00 | 5.00 | 5.81 | Azadirachta indica | 0.00 | 5.00 | 4.41 |
| Tectona grandis | 0.00 | 4.33 | 4.86 | Ceiba pentandra | 4.44 | 0.22 | 3.79 |
| Celtis mildbraedii | 5.44 | 0.11 | 4.72 | Tectona grandis | 0.00 | 4.33 | 3.72 |
| Parkia bicolor | 0.00 | 3.00 | 3.51 | Microdesmis puberula | 4.44 | 0.00 | 3.59 |
| MSG | PSG | NBSG | PSG | ||||
| Azadirachta indica | 0.00 | 5.00 | 4.96 | Azadirachta indica | 0.44 | 5.00 | 8.01 |
| Tectona grandis | 0.00 | 4.33 | 4.18 | Funtumia africana | 4.11 | 0.00 | 6.82 |
| Baphia nitida | 4.33 | 0.67 | 4.10 | Parkia bicolor | 1.00 | 3.00 | 4.67 |
| Funtumia elastic | 4.56 | 0.00 | 4.03 | Tectona grandis | 3.33 | 4.33 | 4.30 |
| Funtumia africana | 4.33 | 0.00 | 3.87 | Acacia albida | 0.33 | 2.33 | 3.86 |
3.3. Factors Influencing Species Composition Differences Across the Sacred Groves
dbRDA revealed environmental variables (mainly climate and soils) to be explaining about 23% of the variation in species composition among the sacred groves (Figure 4(a)). Higher temperature values characterised PSG in the SZ, with higher rainfalls characterising the sacred groves in the forest zones (BSG, ASG and MSG). Similarly, about 30% of the variation in species composition was also explained by multiple environmental stressors. While wildfire, agriculture and mining activities were high in PSG and NBSG, logging was however high in the sacred groves in the forest zone (BSG, ASG and MSG; Figure 4(b)).
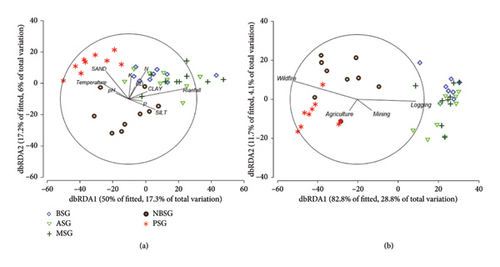
Only rainfall and temperature were significant environmental factors which could cumulatively explain about 18% of the variation in tree species composition across the sacred groves (Table 4). In terms of environmental stressors, wildfire and logging were the most significant stressors explaining the differences in tree species composition across the sacred groves (Table 5).
| Variable | R2 | SS (trace) | Pseudo-F | p | Prop | Cumul | res. df |
|---|---|---|---|---|---|---|---|
| +Rainfall | 0.103 | 14,038 | 4.949 | 0.001 | 0.103 | 0.103 | 43 |
| +Soil moisture | 0.179 | 10,252 | 3.854 | 0.002 | 0.075 | 0.179 | 42 |
| +SAND | 0.211 | 4413.7 | 1.686 | 0.081 | 0.032 | 0.211 | 41 |
| +P | 0.234 | 3129.9 | 1.202 | 0.243 | 0.023 | 0.234 | 40 |
| +BD | 0.258 | 3255.8 | 1.258 | 0.215 | 0.024 | 0.258 | 39 |
| +pH | 0.278 | 2740.2 | 1.061 | 0.382 | 0.020 | 0.278 | 38 |
| +N | 0.297 | 2524.8 | 0.977 | 0.434 | 0.018 | 0.297 | 37 |
| +CLAY | 0.315 | 2432.2 | 0.939 | 0.487 | 0.018 | 0.315 | 36 |
| +K | 0.333 | 2454.6 | 0.946 | 0.479 | 0.018 | 0.332 | 35 |
| +Temperature | 0.346 | 1794.4 | 0.686 | 0.795 | 0.013 | 0.346 | 34 |
- Note: Significant p values are in bold (p < 0.05).
| Variable | R2 | SS (trace) | Pseudo-F | p | Prop | Cumul | res. df |
|---|---|---|---|---|---|---|---|
| +Wildfire | 0.284 | 38,572 | 17.021 | 0.001 | 0.283 | 0.284 | 43 |
| +Logging | 0.323 | 5408.6 | 2.468 | 0.008 | 0.039 | 0.323 | 42 |
| +Agriculture | 0.338 | 1957.4 | 0.891 | 0.620 | 0.014 | 0.338 | 41 |
| +Mining | 0.348 | 1380.8 | 0.623 | 0.915 | 0.010 | 0.348 | 40 |
- Note: Significant p values are in bold (p < 0.05).
4. Discussion
We explored the role of protected areas in biodiversity conservation by characterising and comparing species diversity and community composition of tree species in sacred groves across various ecological zones and forest types in Ghana. These sacred groves are characterised by distinct climatic conditions and exposed to various degrees of anthropogenic stressors. Our findings revealed differences in tree species attributes (mainly diversity and composition) between the sacred groves which were predominantly driven by the various degrees of anthropogenic stressors (e.g., mining activities, agriculture, logging and wildfire) characterising the sacred groves as well as the differences in climatic conditions (rainfall and temperature) driving underlying soil properties. Our results conform with the findings of other studies in the tropics where rainfall and temperature differences as well as anthropogenic stressors were the underlying mechanisms driving species distribution and community composition [46–49]. The influence of multiple drivers in shaping tree species communities has been extensively discussed in contemporary literature. For instance, Thammanu et al. [50] assessed tree species communities in a mixed deciduous forest and attributed topographic, edaphic and anthropogenic factors as the underlying drivers of species distribution. Furthermore, it was evident in the works of Rahman, Rahman and Chowdhury [51] that soil, topography and climatic factors cumulatively influenced species attributes in a humid forest of the Western Himalayas. In Ghana, Appiah-Badu et al. [52] highlighted land use and soil conditions as the major drivers of tree species diversity in the ME Forest of Ghana, with Mensah et al. [46] attributing climate and soil factors as the major drivers of tree species and aboveground carbon patterns in the semiarid SZs of Ghana. It is quite evident in our study that increasing resource availability driven by soil nutrient concentration and climatic factors influenced tree species diversity and community composition.
Scared groves in the forest zone (Mintimrim Kwaye: MSG, Boako: BSG and Antobia: ASG) were diverse than those in the DSD (Nsoatre Botene: NBSG) and the savannah (Pimpimgyae: PSG) ecological zones, reflecting the merits of appropriate climatic conditions on species recruitment, survival and growth [48]. While overlapping patterns (very little to no variation) in terms of species composition were observed for the three sacred groves in the forest zones (MSG, ASG and BSG), the sacred groves in the savannah (PSG) and the DSD forests (NBSG) showed clear separation of clusters affirming the climate gradient effects on tree species composition [53]. Precipitation and temperature are usually strong drivers of species composition at both spatial and temporal scales [54, 55] and are known to promote mineralisation and improve soil nutrients as well as the overall plant net primary productivity [56] which commensurate well with higher species diversity. Therefore, the amount of optimum climate can increase the length of the growing season through climate water availability which allows plants to grow under favourable climatic conditions [54].
Soil parameters together with climatic factors cumulatively explained about 23% of the variation in species composition between the sacred groves. Soil conditions in the forest zones were better and nutrients were available in appropriate concentrations than those in the DSD and SZs, which presumably accounted for the differences in tree community composition. According to the ‘soil fertility hypothesis’, an increase in diversity, recruitment and growth of species is driven by high soil nutrient availability which promotes niche differentiation and facilitation [46, 57, 58]. Mensah et al. [46] affirmed that soil conditions (mainly nutrients and moisture) can filter plant community assembly and determine productivity due to gradients in soil resources. When appropriate soil conditions are met, plants respond positively. In a seminal study, Damptey et al. [59] affirmed the positive role of improved soil conditions in ensuring the emergence of diverse plant community. Appropriate soil conditions make available essential resources, thereby increasing the overall species diversity of an ecosystem [60]. Accordingly, soil with higher concentrations of nutrients tends to eliminate competition between plants (because there are enough nutrients for all other competitors), thereby increasing the chances of other tree species’ survival and diversity [61].
Anthropogenic stressors including logging, mining, agricultural activities and wildfire events accounted for about 33% of the variation in tree species composition across the sacred groves. The degree to which these stressors influenced tree species compositions was different for all sacred groves driven by the extent and intensity of these stressors. For instance, while wildfire, agricultural and mining activities were high in the sacred grove located in the savannah and the DSD forests, those located in the forest zones rather experienced a higher degree of logging activities. As much as anthropogenic stressors facilitate environmental changes [62], they also lead to depletion of essential soil nutrients, thereby limiting species’ emergence, growth and survival [49]. These stressors are known to influence species succession as well as forest dynamics [63]. Similar to the results of this study, Bentsi-Enchill et al. [49] observed a significant influence of anthropogenic stressors (mainly logging, farming and mining activities) on species attributes of an upland evergreen forest in Ghana with these stressors causing excessive depletion of soil nutrients and or direct destruction of plant species.
The sacred grove in the SZ was mainly characterised by dry savannah species including Azadirachta indica, Tectona grandis and Parkia bicolor. These three species contributed more than 50% to the species composition of the savannah sacred grove which has historically and frequently been affected by wildfire events. Apart from Tectona grandis which was also recorded in the forest zones, none of the three savannah species were found in the sacred groves located in any of the forest zones. According to Attua and Pabi [64], these savannah and drought-tolerant species are able to withstand wildfire impacts or could germinate easily after wildfire events making them characteristic species in fire-prone areas.
5. Conclusion
Sacred groves despite their smaller size areas proved efficient in harbouring diverse tree species across climatically distant forests exposed to various degrees of anthropogenic stressors. While sacred groves in the forest zone (Mintimrim Kwaye, Boako and Antobia) could harbour diverse tree species compared to the sacred grove in the SZ (Pimpimgyae), the SZ had some few tree species which were not found in the other sacred groves. The variation in tree species diversity and community composition in this study was mainly driven by differences in climatic factors across the various sacred groves which influenced underlying soil conditions. Subsequently, anthropogenic stressors including mining, logging, agricultural activities and wildfire also played an important role in shaping tree communities of each sacred grove. Sacred groves offer an important conservation approach to biodiversity conservation, and hence sustainable strategies need to be implemented to ensure they are not degraded, thereby losing their conservational and ecological significance.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this study.
Acknowledgements
We are grateful to the staff of Forest Services Division—Ashanti Region, Kumasi and ForstAid Ghana, Accra, for their support during the data collection. Messrs Adoma Owusu and Peter Kwasi Akomatey of RMSC (Conservation Unit) provided assistance with the data entry.
Appendix 1: Species Presence or Absence for Each Forest Zone
| Species | Moist semideciduous | Moist evergreen | Wet evergreen | Dry semideciduous | Savannah zone |
|---|---|---|---|---|---|
| Acacia albida | X | X | |||
| Acacia pentagona | X | X | X | ||
| Adansonia digitata | X | ||||
| Adiantum incisum | x | ||||
| Aframomum strobilaceum | X | ||||
| Afzelia africana | X | X | |||
| Albizia adianthifolia | X | X | X | ||
| Albizia ferruginea | X | ||||
| Albizia zygia | X | x | X | X | X |
| Alchornea cordifolia | X | X | |||
| Allanblackia parviflora | X | X | X | X | |
| Allophylus africanus | X | X | |||
| Alstonia boonei | X | X | X | X | X |
| Amphimas pterocarpoides | X | X | X | X | |
| Annona senegalensis | X | X | |||
| Aningeria altissima | X | X | |||
| Aningeria robusta | X | X | |||
| Annickia polycarpa | X | X | X | X | |
| Anogeissus leiocarpus | X | X | |||
| Anthocleista nobilis | X | X | X | X | |
| Anthonotha fragrans | X | X | |||
| Anthonotha macrophylla | X | X | X | ||
| Antianis toxicaria | X | X | X | X | |
| Aubrevillea kerstingii | x | X | |||
| Aulacocalyx jasminiflora | x | X | 2 | ||
| Avicennia marina | x | ||||
| Azadirachta indica | X | ||||
| Baissea breviloba | X | ||||
| Baphia nitida | X | X | X | X | |
| Baphia pubescens | X | X | X | X | |
| Beilschmiedia mannii | X | ||||
| Berlinia confusa | X | X | |||
| Berlinia tomentella | X | ||||
| Berlinia tomentella | X | X | |||
| Blighia sapida | X | X | X | X | X |
| Blighia welwitschii | X | X | X | ||
| Bombax buonopozense | X | X | X | X | x |
| Borassus aethiopum | x | ||||
| Brachiaria deflexa | X | ||||
| Bridelia feruginea | X | X | |||
| Buchholzia coriacea | X | X | |||
| Bussea occidentalis | X | X | X | ||
| Caesalpinia bonduc | x | ||||
| Calpocalyx brevibracteatus | X | x | x | x | |
| Calycobolus africanus | x | X | |||
| Calycobolus goodii | X | X | |||
| Canarium schweinfurthii | X | X | X | X | |
| Carapa procera | X | ||||
| Cecropia peltata | X | X | X | ||
| Ceiba pentandra | X | X | X | X | X |
| Celtis adolfi-friderici | X | X | X | ||
| Celtis mildbraedii | X | X | X | X | |
| Celtis philippensis | X | ||||
| Celtis wightii | |||||
| Celtis zenkeri | X | X | X | X | |
| Cercestis afzelii | X | ||||
| Chidlowia sanguinea | X | ||||
| Chrysophyllum pruniforme | X | X | |||
| Chrysophyllum albidum | X | X | X | ||
| Chrysophyllum giganteum | X | X | |||
| Chrysophyllum subnudum | X | ||||
| Cleidion gabonicum | X | X | |||
| Cleistopholis patens | X | X | |||
| Cnestis ferruginea | X | ||||
| Cola caricifolia | X | X | X | X | |
| Cola chlamydantha | X | X | X | ||
| Cola gigantea | X | X | X | X | X |
| Cola lateritia | X | ||||
| Cola nitida | X | X | X | X | |
| Cola umbratilis | X | X | |||
| Cola verticillata | X | ||||
| Combretum zenkeri | X | X | X | ||
| Commelina benghalensis | X | X | |||
| Cordia millenii | X | ||||
| Corynanthe pachyceras | X | X | X | X | |
| Crudia gabonensis | X | ||||
| Culcasia angolensis | X | X | X | X | |
| Cylicodiscus gabunensis | X | X | X | ||
| Cynometra ananta | X | ||||
| Dacryodes klaineana | X | X | x | X | |
| Daniellia ogea | X | x | |||
| Daniellia thurifera | X | X | |||
| Deinbollia grandifolia | X | ||||
| Desplatsia dewevrei | X | ||||
| Dialium aubrevillei | X | X | X | ||
| Dialium dinklagei | X | ||||
| Dichapetalum madagascariense | X | X | |||
| Dicranopteris linearis | X | X | X | ||
| Dioclea reflexa | X | ||||
| Dioscorea minutiflora | X | X | |||
| Diospyros canaliculata | X | X | |||
| Diospyros gabunensis | X | x | |||
| Diospyros kamerunensis | X | X | X | ||
| Diospyros monbuttensis | X | X | |||
| Discoglypremna caloneura | X | X | X | X | |
| Distemonanthus benthamianus | X | x | X | X | |
| Dracaena mannii | X | ||||
| Dracaena scabra | X | X | |||
| Dracaena surculosa | X | ||||
| Drypetes aubrevillei | X | X | |||
| Drypetes aylmeri | X | X | X | ||
| Drypetes gilgiana | X | X | X | ||
| Drypetes principum | X | ||||
| Duguetia staudtii | X | X | |||
| Entandrophragma angolense | X | X | X | ||
| Entandrophragma candollei | X | ||||
| Entandrophragma cylindricum | X | X | |||
| Eremospatha macrocarpa | X | X | |||
| Ficus exasperata | X | X | X | ||
| Ficus sur | X | X | X | X | |
| Funtumia africana | X | X | X | X | |
| Funtumia elastic | X | X | X | X | |
| Gilbertiodendron limba | X | ||||
| Gilbertiodendron preussii | X | ||||
| Gongronema latifolium | X | X | |||
| Griffonia simplicifolia | X | x | |||
| Guarea cedrata | X | X | X | ||
| Guarea thompsonii | X | X | |||
| Guibourtia ehie | X | x | |||
| Hallea ledermannii | X | X | |||
| Hallea stipulosa | X | ||||
| Hannoa klaineana | x | X | X | X | 1 |
| Heritiera utilis | X | ||||
| Hexalobus crispiflorus | X | X | |||
| Hildegardia barteri | X | ||||
| Holoptelea grandis | X | X | |||
| Homalium letestui | X | ||||
| Hunteria eburnea | X | ||||
| Hunteria umbellata | X | X | X | ||
| Hymenostegia afzelii | X | X | X | X | |
| Hypselodelphys poggeana | X | ||||
| Isolona campanulata | X | ||||
| Khaya ivorensis | X | X | |||
| Klainedoxa gabonensis | X | X | |||
| Landolphia dulcis | X | ||||
| Lannea acida | X | X | |||
| Lannea welwitschii | X | X | X | X | X |
| Lecaniodiscus cupanoides | X | x | x | ||
| Leptaulus daphnoides | X | ||||
| Lonchocarpus sericeus | X | ||||
| Lovoa trichilioides | X | X | X | ||
| Macaranga barteri | X | X | X | ||
| Macaranga heterophylla | X | X | |||
| Macaranga hurifolia | X | X | |||
| Maesobotrya barteri | X | X | X | X | |
| Malacantha alnifolia | x | ||||
| Mallotus oppositifolius | x | ||||
| Mammea africana | X | X | |||
| Manniophyton fulvum | X | X | X | ||
| Mansonia altissima | X | ||||
| Maranthes chrysophylla | X | X | |||
| Maranthes robusta | X | ||||
| Marantochloa leucantha | X | X | X | ||
| Mareya micrantha | X | X | |||
| Margaritaria discoidea | X | X | X | x | |
| Massularia acuminata | X | ||||
| Memecylon afzelii | X | X | |||
| Microdesmis puberula | X | X | X | ||
| Milicia excelsa | X | X | X | X | |
| Millettia chrysophylla | X | X | |||
| Millettia zechiana | X | X | |||
| Monodora myristica | X | x | x | ||
| Morinda lucida | X | X | |||
| Morinda morindoides | x | X | |||
| Morus mesozygia | x | ||||
| Motandra guineensis | X | X | |||
| Musanga cecropioides | X | X | |||
| Myrianthus arboreus | X | ||||
| Myrianthus libericus | X | x | X | X | |
| Napoleonaea vogelii | x | X | X | X | |
| Nauclea diderrichii | X | ||||
| Nesogordonia papaverifera | X | X | X | ||
| Newbouldia laevis | X | X | |||
| Omphalocarpum ahia | X | ||||
| Parinari excelsa | X | X | X | ||
| Parkia bicolor | x | x | |||
| Parkia biglobosa | X | X | |||
| Parquetina nigrescens | X | ||||
| Paullinia pinnata | X | ||||
| Pentaclethra macrophylla | X | X | X | ||
| Pentadesma butyracea | X | X | X | ||
| Petersianthus macrocarpus | X | X | |||
| Phyllocosmus africana | x | X | |||
| Piper guineense | X | ||||
| Piptadeniastrum africanum | X | x | x | x | |
| Psydrax arnoldiana | X | ||||
| Pterocarpus erinaceus | X | X | |||
| Pterygota macrocarpa | X | X | X | ||
| Pycnanthus angolensis | X | X | X | X | X |
| Raphia hookeri | X | ||||
| Rauvolfia vomitoria | X | X | X | ||
| Rhizophora racemosa | X | ||||
| Rhodognaphalon brevicuspe | X | X | |||
| Ricinodendron heudelotii | X | X | X | X | X |
| Rinorea ilicifolia | X | ||||
| Rothmannia hispida | X | X | |||
| Rothmannia longiflora | X | X | |||
| Salacia leptoclada | X | X | X | ||
| Scaphopetalum amoenum | X | ||||
| Scleria boivinii | X | ||||
| Scottellia klaineana | X | X | X | ||
| Scytopetalum tieghemii | X | ||||
| Senna siamea | X | X | |||
| Smeathmannia pubescens | X | X | X | ||
| Smilax kraussiana | X | X | X | ||
| Sphenocentrum jollyanum | X | X | X | ||
| Spondias mombin | X | ||||
| Sterculia oblonga | X | X | X | ||
| Sterculia rhinopetala | X | X | X | X | |
| Sterculia tragacantha | x | X | X | X | X |
| Strephonema pseudocola | x | x | X | ||
| Strombosia glaucescens | X | X | X | X | |
| Strombosia pustulata | X | X | X | ||
| Synsepalum afzelii | X | x | X | ||
| Tabernaemontana africana | X | X | |||
| Tectona grandis | X | X | |||
| Terminalia catappa | X | ||||
| Terminalia ivorensis | x | X | X | ||
| Terminalia superba | X | X | X | X | |
| Tetrapleura tetraptera | X | X | |||
| Tetrorchidium didymostemon | X | X | X | X | |
| Tieghemella heckelli | X | X | X | X | |
| Tiliacora dielsiana | X | X | X | ||
| Trema orientalis | X | ||||
| Tricalysia discolor | X | ||||
| Trichilia monadelpha | X | X | X | X | X |
| Trichilia prieureana | X | X | X | X | |
| Trichilia tessmannii | X | X | X | ||
| Trichoscypha arborea | X | ||||
| Trilepisium madagascariense | X | X | X | ||
| Triplochiton scleroxylon | X | X | X | X | |
| Turraeanthus africanus | x | x | |||
| Uapaca guineensis | X | ||||
| Uapaca heudelotii | X | X | |||
| Vepris suaveolens | X | X | |||
| Vernonia amygdalina | X | X | X | ||
| Vitex paradoxum | X | X | |||
| Vitex grandifolia | X | ||||
| Xylia evansii | X | ||||
| Xylopia aethiopica | X | ||||
| Xylopia quintasii | X | X | X | ||
| Xylopia staudtii | X | ||||
| Zanthoxylum gilletii | X | X | |||
| Zanthoxylum leprieurii | x | x | x | x |
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




