Effect of Different Seed Sources and Planting Tube Sizes on Germination and Early Growth of Baobab Seedlings in Malawi
Abstract
Baobab is an important multipurpose fruit tree which provides food and economic values along the sub-Saharan African countries. Although there has been remarkable research on domestication of Adansonia digitata, understanding the variation on seed germination and early growth of baobab among different seed sources and planting tube sizes is essential for silvicultural practices at the nursery stages, massive domestication and conservation strategies. The study assessed the effect of different planting tube sizes on seed germination and early growth of baobab seedlings of 10 Malawi subpopulations. Two-way ANOVA revealed significant interaction (p < 0.001) between subpopulations and planting tube sizes on germination percentage and early growth in the number of leaves, root collar diameter, plant height, tuber length and tuber weight. Both seed source and different planting tube sizes significantly affected the germination and early growth of baobab. In conclusion, germination and growth of baobab is influenced by subpopulations and planting tube sizes. The results signify that different genotypes responded differently to the microclimate of tube sizes on germination and growth of baobab.
1. Introduction
In most of the African countries where food insecurity is present, rural communities rely on nontimber forest product (NTFP) to supplement for food, health and income deficit [1]. Adansonia digitata is one of the most important fruit trees in Africa. It belongs to the Malvaceae family and Bombacoideae subfamily. The genus Adansonia has eight species growing in the tropics [2]. The species A. digitata is distinguishable from the other endemic species found in Madagascar and Australia by its very big trunk which can reach up to 10 m diameter [3]. Adansonia digitata is a slow-growing tree endemic to the savannah biomes [4].
Baobab species occur in most of the sub-Saharan countries, spanning eastwards from Senegal to Sudan and Ethiopia and southwards to South Africa [3]. In Malawi, the baobab tree is mostly found in the southern region, but it also occurs in the northern and central regions, particularly along the lakeshore [5].
Baobab forms an integral part of the dietary condiments, traditional medicines and cultural commodities of rural communities in Africa [6]. Every part of a baobab tree (bark, leaves, fruits, roots, and seed) is usable as a source of food, traditional medicine or implements. For instance, fruits are highly edible and can further be converted into juices. Environmentally, baobab trees play a significant role in sequestration of huge quantities of carbon dioxide (CO2) from the atmosphere [7]. It is estimated that 1 ton/ha of CO2 is sequestered annually [8]. Thus, an increase in the number of stems of baobab trees in the African landscapes has potential to mitigate the effects of greenhouse gases (GHGs), particularly atmospheric CO2. Further, the species is highly resistant to forest fires [9, 10] Forest fires are a common occurrence particularly in savanna biomes where baobabs exist. Fire resistance is, therefore, an important property that makes A. digitata become well adapted to such fire-prone environments.
In spite of the high socioeconomic and environmental reverence and valorization of Adansonia digitata among users, the tree is pressured to extinction due to increased demand for its goods and services [4]. During the past decade, remarkable research efforts have been directed towards propagation and domestication of Adansonia digitata [11–13]. To avoid the extinction of baobab, the domestication of the tree can ensure its propagation.
Adansonia digitata can be propagated by seedling or vegetative multiplication. Seed germination and early growth depend on many factors that can be intrinsic or extrinsic and are specific for every species. Under optimal aeration and temperature conditions, nondormant seeds can easily germinate after sowing and watering. However, baobab seeds are known to stay so long in the soil before germination due to the dormancy. Baobab seeds have a very hard coat restrain the germination which usually less than 20% when planted without pretreatment [14]. The seed may be dormant for many reasons such as immature seeds, low permeability of seeds to water or oxygen and coat resistance to embryo growth [2]. Therefore, for a great germination rate, the dormancy has to be broken before sowing. Falemara et al. [12] suggested that baobab cultivation needs pretreated seeds to facilitate water and oxygen accessibility into the seeds before sowing in order to break the dormancy. Germination and early growth of seedling at the nursery stage may also depend on other factors such as seed source or the planting tube size.
The seed provenances depict assemblages of seed populations growing at a particular and well-adapted geographical location [15]. Plant assemblages with wider geographical locations offer greater opportunities for the realization of genetically diverse of provenances. According to Broadhurst et al. [16], seed provenances with wider genetic base play an important role in the restoration or regeneration of superior plant species. Several studies have shown that variations exist among baobab provenances across sub-Saharan Africa [17, 18]. The variations in germination and early growth characteristics of baobab have been reported from tree phenotypic. In Benin, a large variation was observed on seed germination and early growth of the African baobab from three different ecological zones [15]. Wide differences have been reported in A. digitata seedling growth between seeds from Mali and Malawi [19]. Even at a local scale, Munthali et al. [20] observed seed germination and early seedling growth variation of A. digitata from different provenances within Malawi. For baobab restoration strategies, the use of local seed provenance is essential to ensure the efficient germination and growth because of less variation in local ecological conditions such as humidity and temperature [15].
Planting tubes are typically used for holding the growing media which, in turn, helps to anchor the seeds and seedlings. The size of containers like polybags plays a significant role in seed germination and health growth of seedlings [21]. The size of tube is important in seedling early growth as it determines the amount of growing medium available for the growth of the plant [22]. Researchers reported that small size of polybags contains small quantity of soil, hence have a negative effect on plant growth and biomass production [23, 24]. Soil moisture and nutrients, oxygen and carbon dioxide are some of the important elements that are found in the growing media. Thus, planting tubes or containers with different sizes may have an effect on seed germination and early growth of the seedling [25]. According to NeSmith & Duval [26], in a related study, a reduction of growth on shoot height and biomass of tomato had been observed in smaller tube sizes. In another study, Meyer & Cunliffe [27] reported that planting tube or container sizes had a significant and positive effect on root and shoot growth of the ornamental grasses. Similar observations are reported by Annapurna et al. [28] that the sizes of planting tubes influence the growth of many parts, such as plant height, collar diameter, tuber length and plant length, of the Indian sandalwood (Santalum album L). This is because the size of the container has the potential to change the soil structure, water availability and micro- and macronutrient availability [29]. Therefore, the study aimed to assess the effect of planting tube sizes and seed source on germination and early growth.
2. Material and Methods
2.1. Description of the Site
The study was carried out at Mzuzu University in the Department of Forestry and Environmental Management Nursery from 23 November 2020 to 23 April 2021 (Figure 1). The Department of Nursery lies within silvicultural zone M and sits at an altitude of 1270 m above sea level. Mzuzu University has a mean annual temperature range of 13.5°C to 24°C and mean annual rainfall range of 1150 to 1487 mm [30].

2.2. Seeds Provenance
Adansonia digitata seed was collected from Mangochi Dedza provenances (Figure 1). Six subpopulations were collected from Mangochi and four subpopulations from Dedza in 2019. Table 1 provides the characteristics of the two provenance sites.
| Site parameter | Mangochi | Dedza |
|---|---|---|
| Latitude | 14°25′.17″ S | 14°38′.17″ S |
| Longitude | 35°12′.43″ E | 34°32′.55″ E |
| Temperature range | 14.5°C to 33.5°C | 14°C to 21°C |
| Rainfall range | 658 to 1303 mm | 800 to 1200 mm |
| Annual temperature average | 26°C | 17.4°C |
| Annual rainfall average | 983 mm | 1109.8 mm |
2.3. Seed Pretreatment
The pretreatment of seed followed a methodology described by Hansohm et al. [33]. Seeds collected in 2019 were soaked in very hot water (boiling at 100°C) for 15 min then wrapped in a wet cloth for 3 days in order to soften the seed coat. After that, the coat of seeds was still hard so seed samples were again soaked in very hot water (boiling at 100°C) for 15 min, then wrapped again in the same wet cloth for 24 h. The seeds became soft, and the outer coat of each seed was nicked using a knife (a small hole to make the endosperm visible) and put in cold water to remove the floating seeds and wrapped again with a wet cloth for 24 h. This procedure was done to break the dormancy and facilitate the germination of the Baobab seeds.
2.4. Experimental Design
The study comprised 30 treatments (T1, T2, …, T30) emanating from a 10 × 3 factorial experiment. There were 10 subpopulations of baobab seeds sown in 3 planting tube sizes (S1: 7 × 12 cm, S2: 10 × 16 cm and S3: 15 × 24 cm). The experiment was set in a completely randomized block design (CRBD), and the treatments were replicated 4 times with 10 planting tubes per treatment/replicate giving a total of 1200 tubes. Treatment effects were blocked against the gradient. The seeds were sown on 23 November 2020 and water was applied when necessary to keep the planting medium moist.
2.5. Data Collection
Data were collected on seed germination and early growth of seedlings in terms of number of leaves per plant, plant height, root collar diameter, tuber length, diameter and weight, leaf length and width. The following sections outline details of data collection variables that were collected.
2.6. Germination Counts
Seeds were considered germinated when two true leaves on surface of the growing medium had appeared [34]. Number of seeds germinated were counted and recorded from the first day of germination appearance (5 days after sowing) and then monitored every 2 days till 31 days after sowing (germination period) according to Assogbadjo et al. [15].
2.7. Early Seedling Growth Parameters for Planting Tube Size and Seed Provenance
Seedling growth parameters comprising plant height, root collar diameter and number of leaves, tuber diameter, weight and length were assessed in two phases. During the first phase (3 months after seed sowing), plant height was measured in centimetres using a metric transparent ruler and root collar diameter in millimetres using a digital Vernier Caliper. Counts were made on the number of leaves. During the second phase (5 months after sowing the seeds), plant height and root collar diameter were re-measured. The measurements were extended to tuber length in centimetres using a ruler, tuber diameter in millimetres using a digital Vernier Caliper and tuber weight in grams using a digital balance following the methodology used by Munthali et al. [20]. However, the number of leaves was not counted in the provenance, planting tube size and sowing espacement experiments because the plants had started shedding off some and budding new leaves.
2.8. Data Analysis
Germination speed was calculated as a germination percentage of each subpopulation on the 2-daily counts from the 5th to the 31st day of the experiment.
Two-way analysis of variance (ANOVA) was performed to compare main treatment means and interaction means at 5% level of significance of the different provenance x planting tube size experiments.
All data were analysed using R i386 Version 4.0.3 software. Graphs were produced using Microsoft Excel Version 2016. Data were subjected to normality and homogeneity of variances using Shapiro–Wilk’s and Levine’s tests, respectively. This was done to safeguard against violation of parametric test statistics.
3. Results
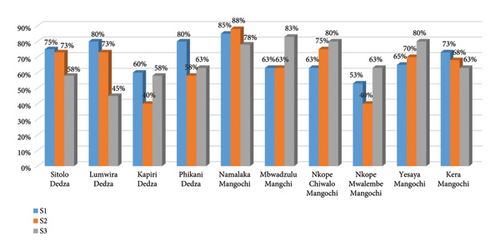
Germination rate between subpopulations and planting tube sizes interaction was statistically significant (p = 0.01). Figure 2 shows that Namalaka (Mangochi) had the highest germination percentage in S1 (88%) and in S2 (85%), while the lowest germination percentage was observed with Kapiri (Dedza) and Nkope Mwalembe (Mangochi) at (40%) in the planting tube size S2. In the planting tube S3, Mbwadzulu (Mangochi) had the highest germination rate (83%) whilst the lowest germination percentage (45%) was observed with Kapiri (Dedza).
3.1. Early Growth of Baobab Seedlings
3.1.1. Mean Number of Leaves/Plant
Three months after sowing seeds, the difference in mean number of leaves/plant was statistically significant (p = 0.01) among the subpopulations of Adansonia digitata seedlings. Planting tube sizes had a very high statistically significant effect on the number of leaves (p < 0.001). The interaction between planting tube size and subpopulations on number of leaves was highly statistically significant (p < 0.001).
Table 2 shows that, for all the subpopulations, the number of leaves increased as the planting tube size increased except for Kapiri (Dedza) subpopulation which decreased in planting tube size S2. In the planting tube size S1, Kapiri (Dedza) had a significantly higher number of leaves (7.16 ± 1.51) than Lumwira (Dedza) with the smallest number of leaves (5.36 ± 1.03). Yesaya (Mangochi) had a significantly higher number of leaves (8.2 ± 1.08) than Phikani (Dedza) (6.04 ± 1.87) in S2. Kera (Mangochi) planted in S3 had more number of leaves 9.13 ± 2.71 while Lumwira (Dedza) had less number of leaves 7.38 ± 1.19.
| Mean number of leaves/plant | |||
|---|---|---|---|
| Provenance | S1 (7 × 12 cm) | S2 (10 × 16 cm) | S3 (15 × 24 cm) |
| Kapiri | 7.16 ± 1.51 | 6.54 ± 1.66 | 8.48 ± 1.75 |
| Kera | 6.81 ± 1.21 | 7.66 ± 2.29 | 9.13 ± 2.71 |
| Lumwira | 5.36 ± 1.03 | 7.05 ± 2.11 | 7.38 ± 1.19 |
| Mbwadzulu | 6.83 ± 1.46 | 7.57 ± 1.57 | 8.11 ± 1.52 |
| Namalaka | 5.89 ± 1.39 | 7.80 ± 2.11 | 8.22 ± 1.89 |
| Nkope Chiwalo | 5.53 ± 2.14 | 7.31 ± 1.62 | 7.83 ± 2.01 |
| Nkope Mwalembe | 6.12 ± 1.53 | 6.80 ± 2.36 | 8.76 ± 1.52 |
| Phikani | 6.15 ± 1.87 | 6.04 ± 1.87 | 8.58 ± 1.93 |
| Sitolo | 6.12 ± 1.09 | 6.87 ± 1.55 | 8.46 ± 1.35 |
| Yesaya | 5.50 ± 1.19 | 8.20 ± 1.08 | 7.87 ± 1.76 |
- Note: Bold numbers represent the highest and lowest value of each planting tube size.
3.1.2. Plant Height
Three months after sowing, results on subpopulations and planting tube sizes showed that there was a very highly significant difference (p < 0.001) in plant height. There was significant interaction between subpopulation and tube size (p = 0.01) (Figure 3).
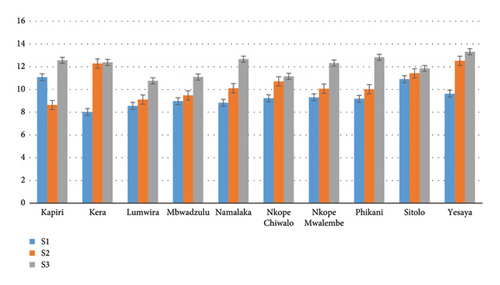
Figure 3 shows that, except for Kapiri (Dedza), all the subpopulations had higher plant height when the planting tube size was increased.
At 5 months after sowing, there was highly significant interaction between subpopulations and planting tube sizes (p < 0.001) in plant height.
Figure 4 shows that, except Kapiri and Phikani (Dedza), all the subpopulations had higher plant height when the planting tube was increased. In S1, Kapiri (Dedza) had the tallest seedling (12.73 ± 5.68 cm) and the shortest seedling (9.5 ± 3.18 cm) was found with Kera (Mangochi). In S2, Kera had the tallest seedling (14.48 ± 3.24 cm) while Kapiri had the shortest (9.83 ± 4.21 cm). Yesaya had the tallest seedlings in S3 (16.96 ± 3.51 cm) while Lumwira (Dedza) had the shortest seedlings in S3 (12.9 ± 3.22 cm).
3.1.3. Root Collar Diameter
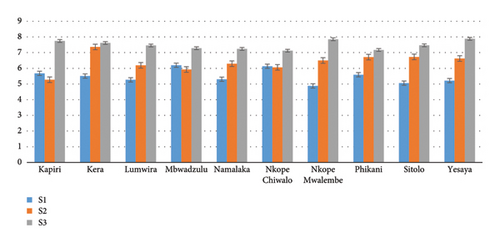
Root collar diameter was found to be statistically significant among the subpopulations (p = 0.035) 3 months after sowing. There was a highly significant difference in collar diameter between subpopulations and planting tube sizes (p < 0.001).
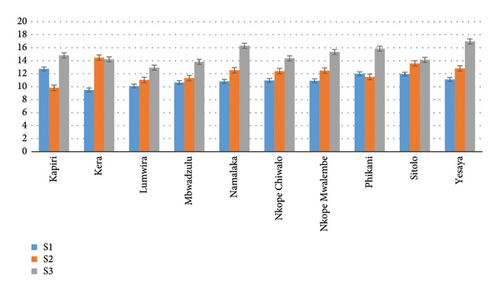
Figure 5 shows that the collar diameter increases with the increase of the planting tube size except Phikani (Dedza) which decreased in the planting tube size S2. In planting tube size S1, Mbwadzulu (Mangochi) had the biggest collar diameter (5.95 ± 1.13 mm) and the smallest collar diameter (4.52 ± 1.13 mm) was found at Namalaka (Mangochi). Kera had the biggest collar diameter (6.71 ± 0.99) and Kapiri the smallest (5.16 ± 1.01 mm) among other subpopulations. Although in S3, Yesaya dominated the collar diameter size (7.49 ± 1.58 mm), the smallest collar diameter (6.68 ± 1.17 mm) was found in Nkope Chiwalo.
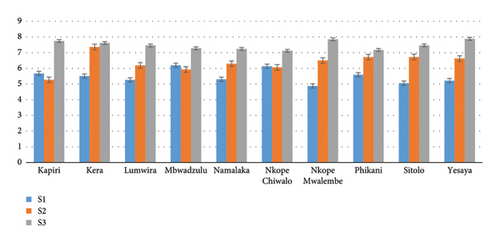
The root collar diameter growth between subpopulations in planting tube sizes was found to be highly statistically significant (p < 001) at 5 months after sowing the seeds.
The root collar diameter increases with an increase in the planting tube size except for Kapiri (Dedza) and Mbwadzulu (Mangochi), which decreases in the planting tube size S2. In the planting tube S1, Mbwadzulu and Nkope Chiwalo (Mangochi) had the biggest root collar diameter 6.19 ± 11.25 mm and 6.13 ± 2.19 mm, respectively (Figure 6). Further, in S2, Kera (Mangochi) had the biggest root collar diameter 7.36 ± 0.99 mm, while the smallest (5.27 ± 0.96 mm) was found in Kapiri (Dedza). Yesaya (Mangochi) planted in S3 had the biggest root collar diameter (7.88 ± 1.26 mm) while Nkope Chiwalo had the smallest root collar diameter (7.12 ± 1.03 mm).
3.1.4. Tuber Length
Five months after sowing, there was a high significant difference (p < 0.001) among tuber length of subpopulations with respect to planting tube size.
Table 3 shows that tuber length increases with an increase in planting tube size except for Kapiri (Dedza) and Phikani (Dedza) which decreases in the planting tube size S2 and S3, respectively. In S1, Kera (Mangochi) had the longest tuber of 10.75 ± 3.14 cm and Sitolo (Dedza) the shortest tuber of 8.27 ± 2.35 cm. In S2, Yesaya (Mangochi) had the longest tuber (12.84 ± 3.32 cm) while the shortest was observed in Kapiri (Dedza) of 8.41 ± 2.82 cm. Namalaka (Mangochi) dominated in tube size S3 with 15.07 ± 4.67 cm and Lumwira (Dedza) had the smallest tuber length of 11.88 ± 2.27 cm (Table 3).
| Tuber height (cm) | |||
|---|---|---|---|
| Provenance | S1 (7 × 12 cm) | S2 (10 cm) | S3 (15 cm) |
| Kapiri | 9.57 ± 2.29 | 8.41 ± 2.82 | 14.48 ± 2.67 |
| Kera | 10.75 ± 3.14 | 10.98 ± 1.38 | 13.07 ± 3.05 |
| Lumwira | 8.78 ± 2.49 | 12.73 ± 4.78 | 11.88 ± 2.27 |
| Mbwadzulu | 9.35 ± 1.65 | 11.22 ± 2.02 | 12.00 ± 2.95 |
| Namalaka | 8.72 ± 1.85 | 10.54 ± 2.39 | 15.07 ± 4.67 |
| Nkope Chiwalo | 9.08 ± 1.52 | 10.83 ± 2.35 | 13.03 ± 2.71 |
| Nkope Mwalembe | 8.32 ± 2.64 | 10.83 ± 2.75 | 14.13 ± 3.93 |
| Phikani | 9.12 ± 1.84 | 10.18 ± 3.32 | 13.39 ± 2.97 |
| Sitolo | 8.27 ± 2.35 | 9.62 ± 1.96 | 12.75 ± 2.87 |
| Yesaya | 9.58 ± 2.31 | 12.84 ± 3.21 | 14.55 ± 3.12 |
- Note: Bold numbers represent the longest and shortest value from each planting tube size.
3.1.5. Tuber Diameter
Tuber diameter was found to be statistically different (p = 0.01) among subpopulations planted in different tube sizes.
Table 4 indicates that tuber diameter increases with an increase in the planting tube size except Mbwadzulu and Kera (Mangochi) which decreases in the planting tube size S2 and S3, respectively. In the planting tube size S1, Mbwadzulu (Mangochi) had the biggest tuber diameter (19.88 ± 4.74 mm) while Lumwira (Dedza) had the smallest tuber diameter (16.25 ± 4.26 mm). On the other hand, Kera (Mangochi) had the biggest diameter of 23.38 ± 3.82 mm and Mbwadzulu (Mangochi) the smallest diameter of 18.64 ± 4.13 mm as observed in the planting tube S2. In S3, Yesaya (Mangochi) had the biggest diameter tuber of 25.78 ± 4.28 mm whilst Phikani (Dedza) had the smallest tuber diameter of 21.56 ± 4.03 mm (Table 4).
| Tuber diameter (mm) | |||
|---|---|---|---|
| Provenance | S1 (7 × 12 cm) | S2 (10 × 16 cm) | S3 (15 × 25 cm) |
| Kapiri | 16.41 ± 475 | 18.74 ± 4.29 | 23.29 ± 4.9 |
| Kera | 16.95 ± 5.49 | 23.38 ± 3.82 | 22.86 ± 4.69 |
| Lumwira | 16.25 ± 4.26 | 20.32 ± 4.43 | 24.44 ± 5.06 |
| Mbwadzulu | 19.88 ± 4.74 | 18.64 ± 4.13 | 22.17 ± 3.86 |
| Namalaka | 17.52 ± 5.51 | 20.87 ± 4.29 | 24.23 ± 5.49 |
| Nkope Chiwalo | 18.63 ± 3.22 | 21.36 ± 5.92 | 22.51 ± 4.52 |
| Nkope Mwalembe | 18.92 ± 4.18 | 19.47 ± 4.63 | 22.05 ± 4.39 |
| Phikani | 19.27 ± 2.93 | 21.02 ± 4.34 | 21.56 ± 4.03 |
| Sitolo | 16.56 ± 3.74 | 21.99 ± 5.04 | 24.41 ± 5.6 |
| Yesaya | 17.25 ± 2.71 | 22.25 ± 4.17 | 25.78 ± 4.28 |
- Note: Bold numbers represent the highest and lowest value of each planting tube size.
3.1.6. Tuber Weight
There was a high statistically significant difference (p < 0.001) in tuber weight among the different subpopulations based planting tube sizes.
Table 5 shows that, tuber weight increases with an increase in planting tube size in all the subpopulations. In the planting tube S2 Mbwadzulu (Mangochi) had the biggest tuber of 21.94 ± 9.04 g and 37.55 ± 12.67 g in planting tuber sizes S1 and S2, respectively. Furthermore, Lumwira and Kapiri (Dedza) had the smallest tuber weight of 14.8 ± 4.95 g and 20.85 ± 9.11 g in S1 and S2, respectively. Yesaya (Mangochi) yielded the biggest tuber weight (58.5 ± 17.81 g) while Phikani (Dedza) had the smallest tuber weight (39.89 ± 15.83 g) from planting tube S3 (Table 5).
| Tuber weight (g) | |||
|---|---|---|---|
| Provenance | S1 (7 × 12 cm) | S2 (10 × 16 cm) | S3 (15 × 24 cm) |
| Kapiri | 20.38 ± 5.64 | 20.85 ± 9.11 | 43.9 ± 13.99 |
| Kera | 21 ± 10.83 | 35.35 ± 12.18 | 50.75 ± 14.55 |
| Lumwira | 14.8 ± 4.95 | 42.05 ± 11.87 | 47 ± 14.64 |
| Mbwadzulu | 21.94 ± 9.04 | 37.55 ± 12.67 | 48.2 ± 16.14 |
| Namalaka | 17.85 ± 2.72 | 23.9 ± 9.19 | 52.5 ± 17.26 |
| Nkope Chiwalo | 18.63 ± 5.79 | 28.75 ± 9.92 | 55.45 ± 12.86 |
| Nkope Mwalembe | 18.8 ± 5.65 | 26.93 ± 13.83 | 51.55 ± 17.59 |
| Phikani | 21.7 ± 5.22 | 27.42 ± 10.77 | 39.89 ± 15.83 |
| Sitolo | 16.7 ± 3.88 | 29.8 ± 11.36 | 51.75 ± 12.83 |
| Yesaya | 17.25 ± 5.18 | 37.16 ± 11.92 | 58.5 ± 17.81 |
- Note: Bold numbers represent the highest and lowest value of each planting tube size.
4. Discussion
The germination which started 5 days after sowing for all the subpopulations and most of the viable seeds had fully germinated by the 19th day signifies that the pretreatment procedures which were used indeed broke the dormancy. Seed source seems to have had no effect on the germination speed because most of the subpopulations started to germinate within 5–19 days of sowing and this could be attributed to the fact that under optimal environmental conditions, baobab pretreated seeds (nondormant) need just access to water to start germinating after sowing [2]. These results are in agreement with the findings of Munthali et al. [20], who reported that seed sources do not have significant influence on germination time necessitating the need for deploying seed pretreatment as more important than the provenance subpopulation of its origin.
The interaction between subpopulations and planting tube sizes on germination percentage may be due to many factors. For instance, the Namalaka (Mangochi) subpopulation had the highest germination rate in S1 and S2 while in S3 the highest germination percentage was observed on the Mbwadzulu (Mangochi) subpopulation signifying the fact that different genotypes may respond differently to microclimatic factors. Generally, the Mangochi provenances had the highest germination percentage compared to the Dedza provenances. For instance, seeds of most of the subpopulations from Mangochi had higher germination percentage values with Namalaka (Mangochi) topping the list at 88% than seeds from Dedza and Kapiri in particular with the lowest germination rate (40%) (Figure 2). However, germination percentage variations within subpopulations of baobab seeds were present which increase or decrease depending on their ecological area of origin and the tube size they are planted in. Similar observations were made by Bischoff et al. [35], who found a high variation on germination rate among different provenances of four plant species. Further, the variation in germination percentage among the provenances can be caused by the local conditions under which the seeds matured [36]. According to Assogbadjo et al. [15], this scenario could be linked to the fact that morphological characteristics of the seed vary with the climate of an area and ecological gradient. Furthermore, the variation in germination percentage in this study could likewise be attributed to genetic factors (family variation), fruit maturity at collection as well as the environmental or original ecological factors [20]. In addition, the germination capacity (%) of seeds can be markedly influenced by maternal factors, such as position of the seed in the fruit/tree and the age of the mother plant during seed maturation [37, 38] though not assessed in this study. In most of the plant species, seeds vary in their level of germination among and within individuals as well as between and within populations [39]. The significant variation in germination percentage among the subpopulations shows that there is a need for adequate selection of seed provenance for these types of germination studies in order to gain insights for domestication programmes.
The results have further revealed a generally high germination percentage among the subpopulations planted in the smallest tube size, S1, where it is speculated that the smallest planting tube gave higher germination rates due to higher temperature absorption rates compared to the big ones [40]. However, germination rate for some subpopulations planted in the medium S2 and biggest tube size S3 was less than 50% (Figure 2). The germination percentages had slight variations among the different planting tube sizes though it was not statistically significant. The slight variations that existed may probably be due to the effect of temperature, which slightly increases the germination rate [40]. However, contrary findings by James et al. [41] revealed that the biggest planting tube size (10 × 16 cm) influenced the germination percentage of Eucalyptus torelliana more than the smallest tube sizes (12 × 8 cm). This may be attributed to a number of local environmental factors such as moisture content potential and exposure to light energy.
The significant interaction between subpopulations and planting tube sizes which revealed that seed from Namalaka (Mangochi) germinated better than from Lumwira (Dedza) in the planting tube sizes S1 and S2 could be explained by the fact that Mangochi has higher temperatures than Dedza. It is also believed that the small planting tubes provide more heat which is ideal for increasing seed germination unlike the big planting tubes. The germination rate in S3 was dominated by Mbwadzulu (Mangochi). This could probably be attributed to the generally higher germination capacity of seeds from Mangochi.
The knowledge of variation in growth of plant species is very important and prerequisite before starting a tree breeding programme [20]. The variation in seedling growth at the nursery stage is usually considered of genetic or phenotypic origin or both if seeds are sown under the same environmental conditions [42]. In Table 2, the results on seedling growth showed that seed provenance had a significant influence on early growth of baobab plants under study, where findings displayed variations in a number of seedling growth parameters such as number of leaves, plant height, root collar diameter, tuber length, diameter and weight. The number of leaves/plant and plant height varied significantly among the subpopulations of baobab seedlings 3 and 5 months after sowing in the nursery (Table 2). Generally, the subpopulations from Mangochi had dominated in all the growth parameters (number of leaves, plant height, root collar diameter, tuber length, diameter and weight). Subpopulations Yesaya, Kera, Namalaka and Mbwadzulu always displayed the higher growth values than the other subpopulations. The lowest growth values were found mostly among the Dedza provenances particularly, Kapiri, Lumwira and Phikani. Generally, subpopulations of Mangochi, where there are higher temperature averages than Dedza (Table 1), had the most outstanding growth in most traits. These findings could be linked to the fact that there is adequate genetic variability for seedling early growth in height and number of leaves in the present study. Similar results have been reported by Loha et al. [39], studying provenance variation in germination and seedling growth of Cordia africana. These present results mean that seed source has a great effect on early growth of baobab seedlings. The findings are in conformity with the observations of Fredrick et al. [43], who reported considerable significant differences in number of leaves and plant height among provenances at all ages of Faidherbia albida. From this study, it can be revealed that farmers who would like to establish nurseries for production of baobab leaves (as vegetable) have to choose the Mangochi provenance especially the subpopulations: Namalaka, Kera and Yesaya as their first choice and avoid the Lumwira and Phikani subpopulations from Dedza due to their inferiority in mean number of leaves produced per plant.
Seed provenance was not found to have a significant effect on root collar diameter and tuber diameter during the early growth stage at 5 months which could be attributed to the fact that seeds were sown in an area different from their original ecological zones. The findings agree with the results of Aigbe et al. [36], which displayed that there is no significant difference in collar diameter among different provenances of Heinsia crinita (Afzel.). These results are also in agreement with the findings where the plant in question is planted. Therefore, there is a risk to consider root collar and tuber diameter criteria as sole indicators for seed selection in baobab.
Traditional nurseries use polythene bags (polybags or tubes or container) in raising seedlings as a potting media. The planting tube size is critical for the growth of tree seedlings as it increases shoot and root biomass and leaves of the plant [26]. In this study, early growth of baobab seedlings had been affected by planting tube sizes. All the growth parameters used in the study were highly significant among the three different tubes sizes. The tube size, S3 (15 × 24 cm) had the superior early growth for all the parameters followed by S2 (10 × 16 cm) except for Kapiri and Phikani subpopulations which had less number of leaves and smaller tuber length in S2 than S1. The lowest early growth for all the parameters was observed in the smallest tube size S (7 × 12 cm) at 3 and 5 months after sowing except for Kapiri and Phikani subpopulations. The present findings are in agreement with the results of James et al. [41], which showed that the different planting tube sizes used in the nursery under the same geographical and weather conditions had significant influence on the growth performance of Eucalyptus torelliana. The results are also in conformity with the studies done by Ouma [44] and Abugre & Oti-Boateng [45], who reported that the growth of plant growth parameters increase with the size of the planting tube. The higher early seedling growth values of different parameters in the big tube sizes could be attributed to efficient water holding capacity and soil nutrients. The large planting tube size enables free tuber growth without restrictions, hence increasing dry matter production which facilitates the growth of the plant. Ouma [44] further reports that large-size planting tubes allow the tubers to increase the volume of soil exploited and hence nutrient uptake. In addition, the temperatures in large planting tubes could be cooler than those in smaller ones because of the soil volume effect. Large soil volumes tend to delay heat absorption from the outer surfaces to the inner core. Thus, this could help the water to stay longer in the big planting tube sizes without evaporation, hence the higher moisture content available for the seedling in these tube sizes reference.
5. Conclusion
This study has revealed substantial variation between and within the provenances in germination and early growth of Adansonia digitata. These variations have shown that source of the seeds has a significant effect on germination and early growth of baobab at the nursery stage.
Results on planting tube sizes have shown that the seed germination is not greatly affected by the use of different planting tube sizes in the nursery. Planting tube S3 (15 × 24 cm) displayed better growth performance than the smaller sizes. The subpopulations Yesaya, Namalaka, Kera from Mangochi displayed superior seed germination and seedling growth trends to the other subpopulations, including those from Dedza. However, there is significant interaction between subpopulation and planting tube size on germination percentage of baobab seeds. This means the tube sizes have a great impact on the germination and early growth of baobab seedlings, and this depends on provenance subpopulation seed source. It was further revealed that the big planting tube sizes give better overall growth performance as compared to the small-sized ones except for mean number of leaves/plant which was not statistically different among the planting tube sizes.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was funded by European Union (EU) in collaboration with African Union (UA), under the Intra-Africa Academic Mobility Scheme REFORM (Regional Academic Exchange for Enhanced Skills in Fragile Ecosystems Management in Africa) project.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author on request.




