Effect of Environmental Variables on Plant Community Formation and Vegetation Dynamics in Northwest Ethiopia
Abstract
Identifying plant communities has been a central aspect of vegetation science for centuries, with emphasis on the distribution, composition, and classification of plant communities. This study aimed to assess how environmental variables influence plant community formation and vegetation dynamics in northwest Ethiopia. A systematic random sampling technique was employed to gather vegetation data from 50 plots, each measuring 20 × 20 m, arranged at 100-m intervals along seven transects. In each plot, the encountered species and their percentage cover-abundance were recorded, which were subsequently transformed into a modified Braun–Blanquet scale. Additionally, composite soil samples collected from 15 × 15 cm subplots were analyzed for 20 soil parameters. The Shannon–Wiener index was used to measure species diversity. Hierarchical clustering and ordination analyses (DCA and RDA) were conducted on the floristic and environmental data, respectively, using R software. A total of 69 woody plant species from 64 genera and 44 families were recorded at altitudes ranging from 2485 to 2747 m above sea level. The Shannon–Wiener diversity index (H) was 3.74, and the evenness index (J) was 0.90. These high species diversity indices showed ecosystem health, stability, reflecting effective species interactions, and resource utilization. Among 6 terrain variables and 14 edaphic factors, 12 of these environmental factors (altitude, slope, aspect, cutting, silt, pH, sand, organic carbon, organic matter, total nitrogen, phosphorus, and calcium) were found significantly to (p ≤ 0.05) explain the variation in species composition and community formation of four plant communities in the study area. The observed patterns of community formation underscore the need to design different conservation measures tailored to the specific environmental conditions at different elevations.
1. Introduction
Identifying plant communities has been a central aspect of vegetation science for centuries, with emphasis on the distribution, composition, and classification of plant communities [1]. According to Godoy et al. [2], a plant community is defined as “an assemblage of functionally similar species populations sharing a common environment that interact with each other at the same time, and integrated to a degree by competition, complementarities, and dependence.” Certain plant communities frequently coexist on the landscape because they share similar environmental requirements [3]. The concept of plant communities has been a topic of extensive debate throughout this century [4]. Two primary models have been proposed to explain plant community structure: the community-unit model by Clements [5] and the continuum model developed by Whittaker [6] and Curtis and McIntosh [7], the latter of which is based on Gleason’s concept of individualistic species distribution. The community-unit model states that communities are highly structured, repeatable, and identifiable associations of species shaped by climate. Clements’ model of vegetation is often illustrated as a series of nonoverlapping species response curves along an environmental gradient.
According to the continuum model, species are distributed individualistically along an environmental gradient, meaning that no two species respond identically to changes in space and time. This leads to the understanding that communities are simply loose collections of species coexisting in a particular area. Empirical evidence indicates that the continuum model, in its current form, does not fully capture the observed patterns of vegetation along the environmental gradient. Therefore, the concept of the hierarchical continuum has been proposed to better represent dynamic community structures along regional spatial gradients. This concept integrates the individualistic distribution of species, hierarchical assemblage structure, and the core-satellite species hypothesis [8]. The hierarchical continuum predicts that species distribution across sites in a region is polymodal, reflecting this hierarchical structure, and that the distribution and abundance of species exhibit spatial and temporal dynamics within and between sites.
In this research, both the community-unit model and the hierarchical continuum model were applied. The community-unit model was used to study distinct, classified plant communities, allowing for clear categorization and analysis. In contrast, the hierarchical continuum model explored the variability and dynamics of vegetation across environmental gradient, emphasizing how changes in factors influence species distribution and community composition. This model views plant communities as part of a gradient rather than as discrete units, highlighting the variability and overlap between different community types.
Vegetation dynamics is the scientific field that examines the concepts, theories, observations, and models related to changes in vegetation over time [9, 10]. The Earth’s vegetation is in a constant state of flux, as plant communities are assemblages of species that have evolved and reorganized throughout the history of the planet’s vegetation cover in response to changes in environmental factors [11]. A prominent example of drastic vegetation change can be seen in Ethiopia, which has suffered historical deforestation primarily due to agricultural expansion coupled with population growth [12, 13]. A plant community is typically characterized by its species composition, and changes in that composition indicate a change in the community itself [14].
Spatial and temporal patterns of vegetation change are influenced by a multitude of factors, including the physical environment, land use history, prior disturbances, and initial vegetation composition [15]. Plant species with comparable environmental affinities inhabit similar places across the landscape, connecting environmental gradient to variation in plant communities and species diversity [16, 17].
The plant species that will colonize a place can be predicted by physiographic and edaphic parameters since different plant species have different tolerances for different factors [18]. These variations can be used to explain broad-scale compositional differences among numerous resource gradients and have been identified as a driving force behind species coexistence in similar environments. There is a significant link between community structure, composition, and species diversity with elevation gradient and other environmental variables [15, 18]. Elevation is one of the determining elements affecting the spatial patterns of vegetation and species variety. This is due to the fact that elevation affects the spatiotemporal distribution of other environmental factors, such as temperature, precipitation, air pressure, soil, hydrology, and others [19]. These environmental factors, in turn, either directly or indirectly impact the growth and development of plants, as well as the dispersal configurations of the local flora [20].
Studying how vegetation reacts to environmental conditions is essential for understanding the structure of a forest community as well as for planning and implementing the community’s conservation strategy [21, 22]. Therefore, the purpose of this research was to examine the effects of environmental variables on plant community formation and vegetation dynamics in the Gibgodo Dry Evergreen Afromontane Forest in northwestern Ethiopia.
2. Methods
2.1. Description of the Study Area
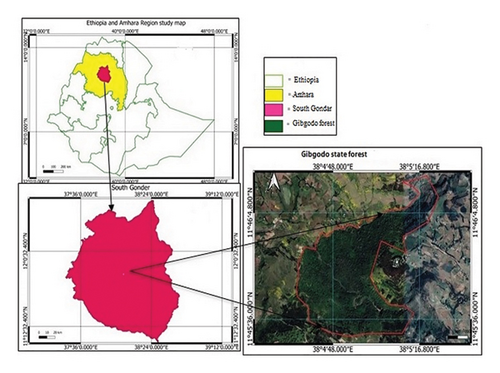
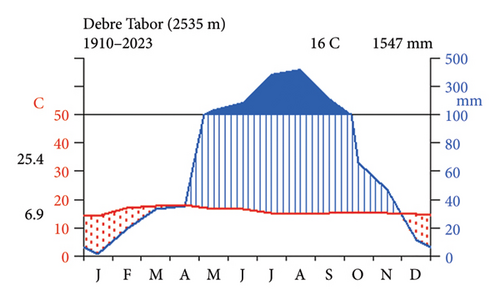
The study was conducted in the Farta district of the South Gondar Zone in the Amhara National Regional State, situated 666 km from Addis Ababa in the northwest highlands of Ethiopia (Figure 1). The district’s geography, which covers 82,982 ha and has an elevation range of 1920–4135 m above sea level, is composed of 29% flat ground, 45% mountains and hills, and 26% valley bottoms [23, 24]. The land use type in the Farta district is primarily composed of 57.43% cultivated land, 10.8% grazing land, 3.93% natural forest, 7.1% settlements, 4.34% wetlands, 10.07% plantations, and 6.33% other uses [25]. The district had seven soil types (Alisols, Nitisols, Luvisols, Vertisols, Cambisols, Regosols, and Leptosols) and was dominated by two major agroecological zones (Dega/highland = 42.5%) and Woina Dega/midland = 57.5% [26]. From the National Meteorological Center’s 14-year data (2010–2019), it was inferred that the area had a unimodal rainfall distribution pattern with 1547 mm of mean annual rainfall (Figure 2). The average annual temperature was around 16 degrees Celsius, with an average minimum of 6.9 degrees Celsius and an average maximum of 25.4 degrees Celsius, as depicted in Figure 2. This generally showed a favorable climate for plant growth and vegetation dynamics in the region. The central statistics agency projected that the population of Farta district would reach 234,143 in 2022, with the majority (80%) living in rural areas [27], which indicates significant anthropogenic pressure on the natural vegetation.
2.2. Data Collection and Analysis
2.2.1. Data Collection
The materials for plant data collection in the field included a plant press for preserving specimens, a data recording sheet for documenting observations, and secateurs for cutting voucher plant specimens. A Garmin GPS device was used to record coordinate points and elevation of plots, while a caliper was used to measure the diameter at breast height (DBH) of woody plants, with a tape measure for too big in diameter. Additionally, a calibrated bamboo stick was used to measure plant heights, and a hypsometer was utilized for plants too large in height. A digital camera was used to capture visual data, a hand lens allowed for close examination of microscopic plant parts, and a compass was used to determine orientation (aspect). A soil auger will be used for digging soil samples, and plastic bags will be used to store and transport the collected plant and soil samples.
Systematic random sampling was used to collect data on the vegetation and environmental characteristics of the study area [1, 28, 29]. Seven transect lines were established at 100-m intervals along an altitudinal gradient, and 50 plots measuring 20 × 20 m (400 m2) were distributed along these transects at 100-m intervals, covering an elevation range from 2485 to 2747 m.a.s.l. The procedures for sampling and gathering data on the vegetation were based on the methods described by Boehmer and Temam’s work [30]. All plant species, including trees, shrubs, and lianas, in each plot were recorded. Additional plant species occurring outside the plots but inside the forest within 10 m of the plots were also recorded as “present,” which were not used in the subsequent data analysis [31]. The percentage cover-abundance was estimated and later converted into a 1–9 Braun–Blanquet scale [1]. The cover-abundance scales were as follows: < 5% = 1, 0.5% < c ≤ 1.5% = 2, 1.5% < c ≤ 3% = 3, 3% < c ≤ 5% = 4, 5% < c ≤ 12.5% = 5, 12.5% < c ≤ 25% = 6, 25% < c ≤ 50% = 7, 50% < c ≤ 75% = 8, and > 75% = 9. During a guided field walk, each specimen will be numbered, pressed, and dried.
Identification was performed using the flora of Ethiopia and Eritrea, with support from experts at the University of Gondar. The recent scientific name change for a plant species will be verified using the World Flora Online [32]. Additionally, the endemic status and conservation assessments of the identified plant species were cross-referenced and validated against the comprehensive works of Vivero, et al. [33], Awas [34], Dagne [35], Ayalew, et al. [36], and the IUCN Red List of Threatened Species online database (https://www.iucnredlist.org/), which provide authoritative information on the endemic flora and conservation status of Ethiopia’s plants.
Terrain variables such as altitude, aspect, slope, and locations were measured and recorded for each sample plot. Altitude and location were measured using a Garmin GPS 60 device, slope (%) was measured using a Suunto Optical Reading Clinometer, and aspect of each plot was measured using a compass. The aspect values were codified based on Bekele [37] and Woldu [38], where N = 0, NE = 1, E = 2, SE = 3, S = 4, SW = 3.3, W = 2.5, and NW = 1.3 before analysis. To determine the environmental factors that govern the distribution of plant community types, elevation and geographical coordinates were measured at the center of each plot using a Garmin GPS device. The types of disturbances were recorded for each plot, and the intensity of anthropogenic disturbance was estimated as a cumulative effect of grazing/browsing intensity (on a scale of 0 to 4): 0 = no disturbance; 1 = slight; 2 = moderate; 3 = heavily; and 4 = destructive [39, 40], the number of trees and shrubs cut, the number of foot trails, and the number of seedlings trampled.
Soil samples were collected at three soil layers of 10 cm thickness each (0–30 cm) from the five 15 × 15 cm2 subplots found at each corner and center of the main plot and mixed to produce a composite soil sample [41]. The samples were air-dried and sieved with a mesh size of 2 mm. Soil analyses were largely based on the standard procedures outlined in the International Soil Reference and Information Center [42] (Table 1).
| Soil physical/chemical parameters | Methods used | |
|---|---|---|
| Texture classes (sand, clay, and silt) (expressed as % weight) | Bouyoucos hydrometer method | |
| Exchangeable cation | Ca and Mg | Atomic absorption spectrophotometer (AAS) |
| Na and K | Flame emission spectrophotometer (FES) | |
| Electrical conductivity | Ammonium acetate (NH4OAc) | |
| Cation exchange capacity | ||
| Organic carbon/organic matter content | Walkley–Black method | |
| Soil pH | PH meter at 1:2.5 soil-distilled water suspension | |
| Total nitrogen | Kjeldahl method | |
| Available phosphorous | Olsen method | |
2.2.2. Data Analysis
2.2.2.1. Floristic Data Analyses
R Statistical Software Version 4.4.0 was used to perform a hierarchical cluster analysis on the abundance data set of 65 species in 50 sample quadrats in order to identify different plant community types. The similarity ratio was used in order to determine the resemblance function, and Ward’s method was employed to increase homogeneity within groups and increase heterogeneity among groups [1]. The communities generated during cluster analysis were refined further in a synoptic table, and occurrences of species were compiled as values of synoptic cover-abundance, where synoptic values are the product of the species frequency and average cover-abundance values [38]. The synoptic value of species was used to determine the dominant species in each community type, and the community types were then named using two dominant species. An indicator species analysis (ISA) was performed to quantify the strength of the association between individual species and the defined plant community types [43]. This analysis is used to understand the ecological significance of these species, assess the correlation between their presence and the environmental variables associated with different plant communities, and provide valuable insights for conservation efforts.
2.2.2.2. Species Diversity
Woody species diversity was calculated using the Shannon–Wiener diversity index formula , while woody species evenness was quantified using the Shannon–Wiener evenness index formula , where H′ is Shannon–Wiener index, s is species richness, Pi is the proportion of individuals, ln is the natural logarithm to base n, and J is evenness [44–47].
2.2.2.3. Environmental Data Analysis
Ordination is a multivariate method that articulates the relationships between species, plots, and environmental variables in a low-dimensional space using ordination diagrams [29]. Before selecting the ordination method, a preliminary detrended correspondence analysis (DCA) was performed to determine the appropriate model for gradient analysis [48]. The environmental variables that were relatively more important in explaining the species data were selected using the Monte Carlo technique and the Adonis function test [49]. A computation of the variance inflation factor (VIF) was also conducted to eliminate any collinear environmental variables [49, 50]. Finally, a one-way ANOVA followed by a post hoc Tukey HSD test was used to determine whether there were significant mean differences among the plant communities with respect to environmental variables [49, 51]. The choice between using a linear or unimodal model depends on the nature of the dataset. The gradient length calculated from the DCA indicates the heterogeneity in the community composition across the ordination axes. If the longest DCA gradient is greater than four, then the unimodal model is preferred. Conversely, if the longest gradient is shorter than three, the linear model is a better choice. When the longest gradient falls between three and four, both the linear and unimodal models can work well [52, 53]. In this study, the DCA revealed that the longest gradient was less than 3 (2.538), which supported the use of the linear redundancy analysis (RDA) ordination method.
3. Results and Discussion
3.1. Woody Plant Species Composition, Community Types, Richness, Diversity, and Evenness
From the forest, 69 different species of woody plants were discovered, representing 64 genera and 44 families (Table 2). Sixty-five plant species were recorded within the established quadrats, and additionally, four plant species were recorded outside of the quadrats using the extrapolation method for the complete collection and a holistic representation of the plant diversity. Among the total woody plant species recorded in the patches, 34 (49.3%) were trees, 27 (39.1%) were shrubs, and 8 (11.6%) were climbers. It showed that trees were the most dominant growth form, followed by shrubs and lianas. Nearly similar numbers of woody plant species were reported in Endiras Natural Forest (S = 73) [54], Kuandisha Afromontane Forest (S = 66) [55], Kafta Sheraro National Park Dry Forest (S = 70) [56], Wof Washa Natural Forest (S = 62) [57], and Woynwuha Natural Forest (S = 69) [58]. The study forest has a greater number of species than Gelawoldie Community Forest (S = 59) [59], Yemrehane Kirstos Church Forest (S = 39) [60], Amoro Forest (S = 57) [61], Kurib Forest (S = 39) [62], Gatira Georges (S = 27) [63], and Zengena (S = 50) [64] Dry Afromontane Forests of Amhara Region. However, Gibgodo Forest has considerably lower species richness than Tara Gedam and Abebaye Forests (S = 120) [65], Alemsaga Forest (S = 124) [66], Zerat Forest (S = 156) [67], Yegof Mountain Forest (S = 144) [68], and Sesa Mariam Monastery Forest (S = 113) [69]. The variations in species composition are probably due to differences in forest size, climate, geographical location, environmental heterogeneity, disturbance level ecological successional stage, extent of protection, and different levels of awareness in the communities.
| Scientific name | Family | Local name | H |
|---|---|---|---|
| Acanthus sennii Chiov.LC | Acanthaceae | Kosheshile | S |
| Albizia gummifera (J.F.Gmel.) C.A.Sm.LC | Fabaceae | Sessa | T |
| Apodytes dimidiata E.Mey. ex Arn. | Metteniusaceae | Donga | T |
| Astropanax abyssinicum (Hochst. ex A.Rich.) Seem. | Araliaceae | Getem | T |
| Baccharoides calvoana subsp. leucocalyx (O.Hoffm.) Isawumi, El-Ghazaly & B.Nord. | Asteraceae | Kotikoto | S |
| Bersama abyssinica Fresen | Francoaceae | Azamir | T |
| Bridelia micrantha (Hochst.) Baill. | Phyllanthaceae | Yenbirtifr | T |
| Brucea antidysenterica J. F. Mill. | Simaroubaceae | Abalo | S |
| Buddleja polystachya Fresen. | Scrophulariaceae | Anfar | S |
| Calpurnia aurea (Aiton) Benth. | Fabaceae | Zigta | S |
| Carissa spinarum L. | Apocynaceae | Agam | S |
| Cassipourea malosana (Baker) Alston | Rhizophoraceae | Tikur enchet | S |
| Clausena anisata (Willd.) Hook.f. | Rutaceae | Lmbich | T |
| Clematis hirsuta Guill. & Perr. | Ranunculaceae | Azo hareg | L |
| Clutia abyssinica Jaub. & Spach | Peraceae | Fyel fej | S |
| Croton macrostachyus Hochst. ex Delile | Euphorbiaceae | Bisana | T |
| Discopodium penninervium Hochst. | Solanaceae | Almiti | S |
| Dodonaea viscosa subsp. angustifolia (L.f.) J.G.West | Sapindaceae | Kitikta | S |
| Dombeya torrida (J.F.Gmel.) Bamps | Malvaceae | Wulkifa | T |
| Dovyalis abyssinica (A.Rich.) Warb. | Salicaceae | Koshim | T |
| Ekebergia capensis Sparrm. | Meliaceae | Lol | T |
| Embelia schimperi Vatke | Primulaceae | Enkoko | T |
| Erica arborea L. | Ericaceae | Asta | T |
| Erythrina brucei Schweinf.LC | Fabaceae | Korch | T |
| Eucalyptus globulus Labill. | Myrtaceae | Nech baharzaf | T |
| Euphorbia abyssinica J.F.Gmel. | Euphorbiaceae | Kulkual | T |
| Ficus sur Forssk. | Moraceae | Emibela shola | T |
| Galiniera saxifraga (Hochst.) Bridson | Rubiaceae | Yewof shola | T |
| Gymnanthemum amygdalinum (Del.) Sch.Bip.ex Walp. | Asteraceae | Girawa | T |
| Gymnosporia addat Loes. VU | Celastraceae | Kermo ayderk | T |
| Gymnosporia arbutifolia (Hochst. ex A.Rich.) Loes. | Celastraceae | Atat | S |
| Gymnosporia obscura (A.Rich.) Loes. | Celastraceae | Kumbel | T |
| Hibiscus macranthus Hochst. ex A.Rich. | Malvaceae | Nacha | S |
| Hypericum revolutum Vahl | Hypericaceae | Amja | S |
| Jasminum abyssinicum Hochst. ex DC. | Oleaceae | Abita hareg | L |
| Jasminum fluminense Vell. | Oleaceae | Tibitiba hareg | L |
| Juniperus procera Hochst. ex. Endl. | Cupressaceae | Habesha tisde | T |
| Laggera tomentosa (A.Rich.) Sch.Bip. ex Oliv. & HiernNT | Asteraceae | Sheteto | S |
| Lobelia giberroa Hemsl. | Campanulaceae | Jibra | S |
| Maesa lanceolata Forssk. | Primulaceae | Kuraba | T |
| Marsdenia abyssinica (Hochst.) Schltr. | Apocynaceae | Tikur moyder | L |
| Maytenus undata (Thunb.) Blakelock | Celastraceae | Seged | T |
| Myrica salicifolia Hochst. ex A.Rich. | Myricaceae | Shinet | T |
| Myrsine africana L. | Primulaceae | Kechem | S |
| Nuxia congesta R.Br. ex Fresen | Stilbaceae | Atikuar | T |
| Ocimum lamiifolium Hochst. | Lamiaceae | Dama kesse | S |
| Olea europaea subsp. cuspidata (Wall. & G.Don) Cif. | Oleaceae | Weyra | T |
| Olea welwitschii (Knobl.) Gilg & G.Schellenb | Oleaceae | Wegeda | T |
| Olinia rochetiana A. Juss. | Penaeaceae | Tifie | T |
| Osyris lanceolata Hochst. & Steud. | Santalaceae | Keret | S |
| Pavetta abyssinica Fresen | Rubiaceae | Debenie keleb | S |
| Periploca linearifolia Quart.—Dill. & A.Rich | Apocynaceae | Key moyder | L |
| Phytolacca dodecandra L’Hér | Phytolaccaceae | Endod | L |
| Pittosporum abyssinicum Delile | Pittosporaceae | Ahot | T |
| Prunus africana (Hook.f.) Kalkman | Rosaceae | Homa | T |
| Pterolobium stellatum (Forssk.) Brenan | Fabaceae | Kentefa | S |
| Rhamnus prinoides L’Hér. | Rhamnaceae | Gesho | S |
| Rhamnus staddo A. Rich | Rhamnaceae | Tedo | S |
| Ritchiea albersii Gilg | Capparaceae | Tota kolet | T |
| Rosa abyssinica R.Br. ex Lindl. | Rosaceae | Kega | T |
| Rubus apetalus Poir. | Rosaceae | Enjorerit | S |
| Rumex nervosus Vahl | Polygonaceae | Embacho | S |
| Salix mucronata Thunb. | Salicaceae | Shunshuna | S |
| Scepocarpus hypselodendron (Hochst. ex A.Rich.) T.Wells & A.K. Monro | Urticaceae | Lankuso | L |
| Searsia glutinosa subsp. abyssinica (Hochst. ex Oliv.) Moffett LC | Anacardiaceae | Embs | T |
| Senegalia polyacantha (Willd.) Seigler & Ebinger | Fabaceae | Neche girar | T |
| Solanecio gigas (Vatke) C. Jeffrey | Asteraceae | Lib agba | S |
| Vepris nobilis (Delile) Mziray | Rutaceae | Sihil | S |
| Zehneria scabra Sond. | Cucurbitaceae | Nech hareg | L |
- Note: Superscript bold indicates endemic species.
- Abbreviations: H = habit, L = lianas, LC = least concern, NT = near threatened, S = shrub, T = tree, VU = vulnerable.
The dominant family was Fabaceae, represented by 5 species. It is the second-largest family in the flora of Ethiopia and Eritrea [70]; this is due to its efficient and successful dispersal strategies as well as its better adaptation to a wide range of ecological conditions. Fabaceae is not only dominant in the study area but also in other Afromontane forests in Ethiopia [71]. According to Kitessa Hundera and Tsegaye Gadissa [72], Fabaceae was found to be dominant in the Belete Forest with 8 species, accounting for 10% of the identified species in that area. Similarly, in the Komto Forest, [73] reported 12 species of Fabaceae, representing 12% of the total species. Additionally, in Jibat Forest, [74] found 14 species of Fabaceae, which accounted for 8% of the identified species. Overall, Fabaceae’s ecological and economic significance, along with its ability to adapt to various ecological conditions, contributes to its dominance and prevalence in Afromontane forests and other ecosystems in Ethiopia and around the world [56, 75].
Four woody plant species, such as Albizia gummifera, Ekebergia capensis, Croton macrostachyus, Prunus africana, Juniperus procera, and Olea welwitschii, were found in the forest and have been identified as national priorities for conservation efforts in Ethiopia. These species have been designated as priorities based on their ecological, economic, and cultural significance within the country, as well as the threats they currently face, such as overexploitation, deforestation, and habitat loss [76].
Out of the 69 recorded plant species, 6 (8.7%) were found to be endemic to the forest (Table 2). Among these, 1, 1, and 4 were vulnerable (VU), near threatened (NT), and least concern (LC), respectively. Endemism, which refers to the exclusive occurrence of species within a specific geographic region or habitat, can arise through different mechanisms. One important factor contributing to endemism is the geographical and ecological isolation of species from other regions or habitats [77]. A nearly similar number of endemic species was recorded, like Alemsaga Forests [66], represented by 7 species. The highest number of endemic plants was recorded compared with Yemrehane Kirstos Church Forest [60], and Woynwuha [58] Forests, each represented by only one endemic species, followed by Kurib [62], and Zegie Peninsula [78] Forests with two endemic species each; the lowest endemism record was compared with Zerat Forest [67] with a total of 17 species. Various topographic barriers that inhibit pollination and dispersal would cause species to become isolated and undergo independent evolution, giving rise to a large number of endemic species [79].
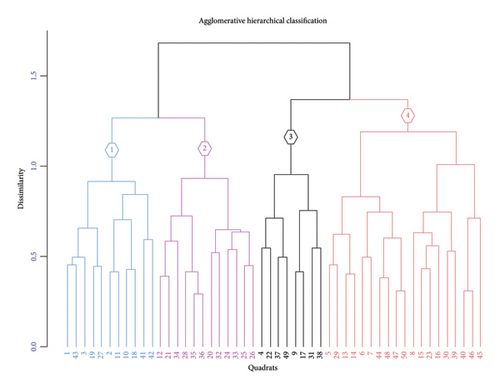
The hierarchical cluster combination with indicator value species analysis was used to derive four distinct community types, as shown in Figure 3 and Table 3.
| Species | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 |
|---|---|---|---|---|
| Acanthus sennii | 6.09 | 0.17 | 2.62 | 4.63 |
| Albizia gummifera | 5.82 | 1.83 | 0 | 0.26 |
| Olinia rochetiana | 1.36 | 6.5 | 0 | 2.42 |
| Apodytes dimidiata | 5.64 | 5.5 | 3 | 4.63 |
| Discopodium penninervium | 2.73 | 1 | 6.38 | 1.79 |
| Dovyalis abyssinica | 2.64 | 3.25 | 4.62 | 2.84 |
| Vernonia hymenolepis | 0 | 1.75 | 4.61 | 5.32 |
| Bersama abyssinica | 5.27 | 1.67 | 4 | 5.16 |
| Bridelia micrantha | 4.18 | 2.33 | 1 | 1.16 |
| Brucea antidysenterica | 3.91 | 0.42 | 1 | 0.63 |
| Buddleja polystachya | 3.27 | 0 | 1.88 | 2.21 |
| Calpurnia aurea | 3.27 | 2 | 3 | 1.47 |
| Carissa spinarum | 3.18 | 5.48 | 3.25 | 1.47 |
| Clausena anisata | 3.09 | 1.83 | 1.75 | 1.47 |
| Clematis hirsuta | 2.91 | 0.75 | 1.75 | 2.05 |
| Clutia abyssinica | 2.82 | 0.58 | 1.12 | 1.63 |
| Dombeya torrida | 2.73 | 0.92 | 0.5 | 4.26 |
| Ekebergia capensis | 2.45 | 2.08 | 1 | 2.47 |
| Embelia schimperi | 2.45 | 2.08 | 1.88 | 0.16 |
| Erythrina brucei | 2.36 | 0 | 1.88 | 0 |
| Euphorbia abyssinica | 2.27 | 0.83 | 0 | 0.11 |
| Galiniera saxifraga | 2.27 | 3.17 | 2.38 | 3.21 |
| Jasminum abyssinicum | 2.18 | 0.83 | 0.5 | 0.26 |
| Maesa lanceolata | 2.09 | 3.17 | 4 | 3.26 |
| Maytenus addat | 2 | 1.67 | 0.75 | 1.42 |
| Maytenus arbutifolia | 2 | 3.17 | 3.5 | 1.05 |
| Maytenus obscura | 1.82 | 1.17 | 0 | 0 |
| Maytenus undata | 1.73 | 1.83 | 0 | 0.63 |
| Myrica salicifolia | 1.55 | 0.5 | 0 | 1.47 |
| Nuxia congests | 1.45 | 3.08 | 0.62 | 4.11 |
| Osyris quadripartita | 1.09 | 0.25 | 0 | 1.47 |
| Pavetta abyssinica | 0.82 | 2.08 | 1.62 | 1.53 |
| Periploca linearifolia | 0.73 | 1.25 | 0 | 0.84 |
| Phytolacca dodecandra | 0.64 | 0 | 2.75 | 0 |
| Pittosporum abyssinicum | 0.64 | 3.92 | 2.38 | 1.58 |
| Prunus africana | 0.55 | 2.08 | 1.62 | 1.63 |
| Rhus glutinosa | 0.45 | 3.17 | 0 | 3.42 |
| Rosa abyssincia | 0.36 | 1.92 | 0.62 | 1.63 |
| Schefflera abyssinica | 0.27 | 1.42 | 1.5 | 0.79 |
| Solanecio gigas | 0.27 | 2.75 | 2.5 | 0.79 |
| Teclea nobilis | 0.18 | 1.5 | 2 | 1.58 |
| Urera hypselodendron | 0 | 1 | 2.5 | 0.95 |
- Note: Bold numbers indicate dominant species by which communities named.
Community 1 (Acanthus sennii–Albizia gummifera) was found within an altitudinal range of 2494 to 2570 m.a.s.l. and on slopes ranging from flat to 40%. This community type was associated with 11 plots and had 16 indicator species, 3 of which had significant indicator values (p < 0.05) (Table 4).
| Name of indicator species | Community type | Indicator value | p value |
|---|---|---|---|
| Cassipourea malosana | 1 | 0.273 | 0.011 ∗ |
| Nuxia congests | 1 | 0.381 | 0.011 ∗ |
| Rhus glutinosa | 1 | 0.469 | 0.001 ∗∗ |
| Apodytes dimidiata | 2 | 0.348 | 0.028 ∗ |
| Carissa spinarum | 2 | 0.407 | 0.003 ∗∗ |
| Embelia schimperi | 2 | 0.316 | 0.025 ∗ |
| Maytenus undata | 2 | 0.31 | 0.025 ∗ |
| Olinia rochetiana | 2 | 0.576 | 0.001 ∗∗ |
| Periploca linearifolia | 2 | 0.349 | 0.007 ∗∗ |
| Discopodium penninervium | 3 | 0.669 | 0.001 ∗∗ |
| Phytolacca dodecandra | 3 | 0.508 | 0.002 ∗∗ |
| Ritchiea albersii | 3 | 0.25 | 0.019 ∗ |
| Acanthus sennii | 4 | 0.344 | 0.03 ∗ |
| Dombeya torrida | 4 | 0.395 | 0.009 ∗∗ |
| Hypericum revolutum | 4 | 0.211 | 0.037 ∗ |
| Vernonia hymenolepis | 4 | 0.388 | 0.002 ∗∗ |
- ∗p < 0.5.
- ∗∗p < 0.01.
Community 2 (Olinia rochetiana–Apodytes dimidiata) was distributed from 2485–2596 m.a.s.l., slope from flat to 65%, and composed of 18 indicator species and 6 indicator species with significant indicator values (p < 0.05) (Table 4).
Community 3 (Discopodium penninervium–Dovyalis abyssinica) was found within an altitudinal range of 2541 to 2747 m.a.s.l. and on slopes from flat to 60%. This community type was associated with 19 plots and consisted of 11 indicator species, 3 of which had significant indicator values (p < 0.05) (Table 4).
Community 4 (Bersama abyssinica–Vernonia hymenolepis) was distributed within an altitudinal range of 2497 to 2571 m.a.s.l. with slope gradient varying from flat to 63%. This community type was comprised of 12 plots and had 20 associated indicator species, 4 of which exhibited significant indicator values (p < 0.05) (Table 4).
The Shannon–Weiner diversity and evenness of the forest were 3.74 and 0.90, respectively (Table 5). Community 3 was the most diverse and evenly distributed, followed by Community 1 and Community 2. The observed, Shannon–Weiner diversity was higher than Kurib Forest (H′ = 1.8) [62], Tara Gedam (H′ = 2.98) [65], and Gosh Wona Forest (H′ = 2.78) [71], Ylat Forest (H′ = 2.94) [80], Yemrehane Kirstos Church Forest (H′ = 2.88) [60], Zegie Peninsula (H′ = 2.49) [78], but lower than Bore–Anferara–Wadera Forest (H′ = 3.84) [81], Gebra-Dima Forest (H′ = 4.18) [49], and Agama Forest (H′ = 4.04) [82]. The Shannon–Weiner diversity index varies between 1.5 and 3.5 and rarely exceeds 4.5 [83]. According to Kent and Coker [83], Shannon–Weiner diversity is high when it is above 3.0, medium when it is between 2.0 and 3.0, and low when it is smaller than 2.0. Thus, the H′ value of the Gibgodo Forest was within the normal range that indicated high plant species diversity in the studied forest.
| Community | Quadrats | Quadrats (number) | Altitudinal range | (S) | H′ | J |
|---|---|---|---|---|---|---|
| 1 | 1, 2, 3, 10, 11, 18, 19, 27, 41, 42, 43 | 11 | 2494–2570 | 50 | 3.58 | 0.91 |
| 2 | 4, 9, 17, 22, 31, 37, 38, 49 | 8 | 2485–2596 | 37 | 3.41 | 0.94 |
| 3 | 5, 6, 7, 8, 13, 14, 15, 16, 23, 29, 30, 39, 40, 44, 45, 46, 47, 48, 50 | 19 | 2541–2747 | 55 | 3.61 | 0.90 |
| 4 | 12, 20, 21, 24, 25, 26, 28, 32, 33, 34, 35, 36 | 12 | 2497–2571 | 50 | 3.53 | 0.90 |
| Forest | 65 | 3.74 | 0.90 | |||
The Shannon–Weiner evenness (J = 0.90) in the Gibgodo Forest was also higher than Zegie Peninsula (J = 0.58) [78], Wanzaye Natural Forest (J = 0.81) [84], Tara Gedam (J = 0.65) [65], and Sesa Mariam Monastery (J = 0.85). This indicated a relatively much more equitable distribution of individuals among various species in the study area. According to Kent and Coker [83], the species evenness value was between 0 and 1. An evenness value of 0 indicated that the area was dominated by a single species, and when it was 1, the species were evenly distributed in the area [85]. The differences in species diversity between community types may be attributed to variations in topographic parameters that result in the formation of microhabitats and disturbance level [86]. High species diversity indicated ecosystem health, stability, and appropriate species interaction [1].
3.2. Relationship Between Community Types and Environmental Factors
An Adonis test was conducted to assess the influence of 6 terrain variables and 14 edaphic factors on community formation and species distribution patterns. The Monte Carlo test result showed that 12 of these parameters were statistically significant (p ≤ 0.05) in determining community formation and species distribution. The significant variables included altitude, slope, aspect, and cutting regime, as well as soil properties such as pH, sand, silt, organic carbon (OC), organic matter (OM), total nitrogen, phosphorus, and calcium (Table 6). However, due to issues of collinearity, the VIF for four of the variables was found to be greater than 4, leading to their elimination from the final output of the Monte Carlo test as a function of Adonis. The box plot analysis (Figure 4) visually highlighted the variations among the different community types, despite some overlaps, indicating clear differentiation in their responses to the significant environmental factors. The box-and-whisker plots provide a useful tool to make visual comparisons of the differences in the measured variables across the various community types.
| Variable | Df | Sum of squares | Mean of squares | F. model | R2 | Pr (> F) |
|---|---|---|---|---|---|---|
| Altitude | 1 | 0.8773 | 0.87725 | 7.4216 | 0.09653 | 0.01 ∗∗ |
| Slope | 1 | 0.3560 | 0.35605 | 3.0122 | 0.03918 | 0.02 ∗ |
| Aspect | 1 | 0.4432 | 0.44321 | 3.7496 | 0.04877 | 0.01 ∗∗ |
| Browsing | 1 | 0.0948 | 0.09482 | 0.8022 | 0.01043 | 0.69 |
| Cutting | 1 | 0.3687 | 0.36873 | 3.1195 | 0.04057 | 0.01 ∗∗ |
| Grazing | 1 | 0.1918 | 0.19175 | 1.6222 | 0.02110 | 0.08. |
| pH | 1 | 0.3119 | 0.31192 | 2.6389 | 0.03432 | 0.01 ∗∗ |
| EC | 1 | 0.1842 | 0.18415 | 1.5579 | 0.02026 | 0.11 |
| Sand | 1 | 0.2341 | 0.23410 | 1.9805 | 0.02576 | 0.02 ∗ |
| Silt | 1 | 0.3093 | 0.30927 | 2.6165 | 0.03403 | 0.02 ∗ |
| OC | 1 | 0.3225 | 0.32252 | 2.7285 | 0.03549 | 0.01 ∗∗ |
| OM | 1 | 0.2272 | 0.22720 | 1.9221 | 0.02500 | 0.03 ∗ |
| TN | 1 | 0.3406 | 0.34059 | 2.8814 | 0.03748 | 0.01 ∗∗ |
| P | 1 | 0.4736 | 0.47361 | 4.0067 | 0.05211 | 0.01 ∗∗ |
| Ca | 1 | 0.2747 | 0.27471 | 2.3240 | 0.03023 | 0.02 ∗ |
| Na | 1 | 0.1772 | 0.17718 | 1.4989 | 0.01950 | 0.13 |
| Residuals | 33 | 3.9007 | 0.11820 | 0.42923 | ||
| Total | 49 | 9.0878 | 1.00000 | |||
- Note: F. = F test model, R2 = coefficient of determination. Significant factors were indicated by the asterisk at their p value. Significance codes: 0.001 “ ∗∗,” 0.01 “ ∗,” and 0.05 “.”
- Abbreviations: DF = degree of freedom, Pr = probability.
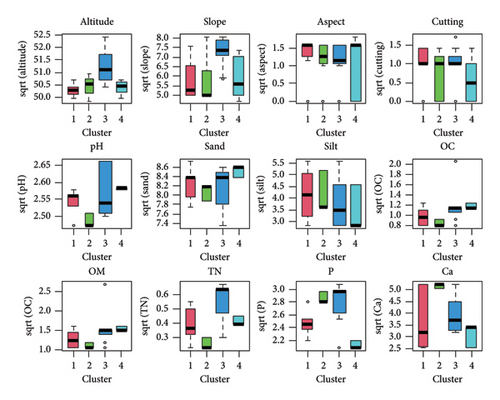
Tukey’s pairwise comparison test identified both significant and nonsignificant environmental factors responsible for the significant demarcation between community types (Figure 5). Specifically, comparisons where the mean difference confidence interval did not cross zero indicated statistically significant differences between the groups. In contrast, comparisons where the mean difference confidence interval did cross zero suggested statistically nonsignificant differences. As shown in Figure 5, factors such as altitude, slope, pH, sand, OC, OM, nitrogen (N), phosphorus (P), and calcium (Ca) were responsible for the significant demarcation between the community types. In contrast, aspect, cutting, and silt content did not reveal clear distinctions among the plant community type.
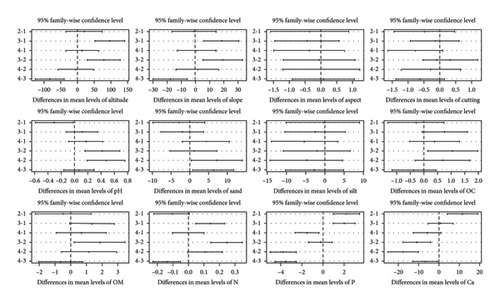
Terrain factors responsible for the demarcation between community types were as follows: altitude between 3-1, 3-2, and 4-3; slope between 3-1, 3-2, and 4-3; and sand between 4-2 and 4-3 community types. There is a significant link between community structure, composition, and species diversity with elevation gradient and other environmental variables [87]. Elevation is one of the determining elements affecting the spatial patterns of vegetation and species variety. This is due to the fact that elevation affects the spatiotemporal distribution of other environmental factors, such as temperature, precipitation, air pressure, soil, hydrology, and others [19]. These environmental factors, in turn, either directly or indirectly impact the growth and development of plants, as well as the dispersal configurations of the local flora [20]. The angle and orientation of slopes can influence soil erosion, drainage, and exposure to sunlight, all of which affect vegetation patterns and community structure [88]. Sandy soils typically have different drainage and nutrient retention characteristics compared to other soil types, impacting the types of plants that can establish and persist in those areas [89].
Likewise, edaphic factors responsible for the demarcation between community types were as follows: pH between 2-1, 3-2, and 4-2; OC was between 3-2; OM between 3-2; N between 3-1, 3-2, and 4-3; P between 2-1, 3-1, 4-1, 4-2, and 4-3; Ca between 2-1, 3-2, 4-2, and 4-3 community types. Soil pH defines the relative acidity or alkalinity of the soil. Soil reaction, usually expressed as pH value, is a crucial factor in forest ecosystems, as it affects nutrient availability, microbial activity, and root growth of trees and other plants [90]. Soil pH is determined by the particular chemical, mineralogical, and biological environment in the soil, and it is one of the most important chemical characteristics of the soil solution, as higher plants respond markedly to their chemical environment [91]. Most plants grow better in a pH range of 5.5 to 7.5 [92]. Higher OC content generally indicates better soil fertility and structure, influencing plant growth and community composition. OM contributes to soil health by enhancing nutrient availability, moisture retention, and overall soil structure, which can affect plant community dynamics. Nitrogen is an essential element of all amino acids, a component of nucleic acids, which form the DNA of all living things and hold the genetic code, a component of chlorophyll, which is the site photosynthesis [93]. Nitrogen stimulates cell development and division during the vegetative phase, thereby promoting stem, leaf, and root growth [94]. It is vital to plant growth found in every living plant cell. It is involved in several key plant functions, including energy transfer, photosynthesis, transformation of sugars and starches, nutrient movement within the plant, and transfer of genetic characteristics from one generation to the next [95]. It is a constituent of plant cells, essential for cell division and development of the growing tip of the plant [96]. For this reason, it is vital for seedlings and young plants. Ca is essential for cell wall structure, root growth, and nutrient uptake [97].
These findings from Tukey’s pairwise comparison, presented with a 95% family-wise confidence level, provide a robust statistical validation of the underlying environmental factors shaping the observed plant community typology in the study area.
Preliminary analysis using DCA on the vegetation data revealed a short length (gradient) of the first DCA axis (2.538), which was less than 3 (Table 7 and Figure 6). This indicates the presence of lower species turnover or more homogeneous vegetation data, likely due to a linear relationship between the species and the environmental variables measured. RDA is a constrained ordination method that is better suited for identifying and visualizing the linear relationships between community composition and environmental predictors. The short gradient length from the DCA suggests that the species–environment relationships are more linear in nature, making RDA a more powerful and informative technique compared to the unimodal-based DCA. The variable with the highest score of 0.553 (55.3%) associated with the first axis in the analysis was altitude, which was the most important variable in weighting and interpreting this axis. This finding is consistent with similar studies conducted in other Afromontane forests of Ethiopia, which have also confirmed the importance of altitude as a major determinant of vegetation distribution along altitudinal gradient [98–100]. Altitudinal change leads to changes in various environmental factors, such as humidity, temperature, and soil type, which in turn influence the growth and development of plants and ultimately determine the patterns of vegetation distribution [101]. Additional research carried out in Ethiopia has also reported that height has a significant impact on vegetation distribution [102] and that altitude was the most significant environmental variable for determining vegetation variance [99, 103].
| DCA axes | DCA1 | DCA2 | DCA3 | DCA4 |
|---|---|---|---|---|
| Eigen values | 0.201 | 0.173 | 0.1220 | 0.098 |
| Additive eigenvalues | 0.201 | 0.173 | 0.1219 | 0.099 |
| Decorana values | 0.218 | 0.164 | 0.110 | 0.077 |
| Axis lengths | 2.538 | 1.964 | 2.187 | 1.340 |
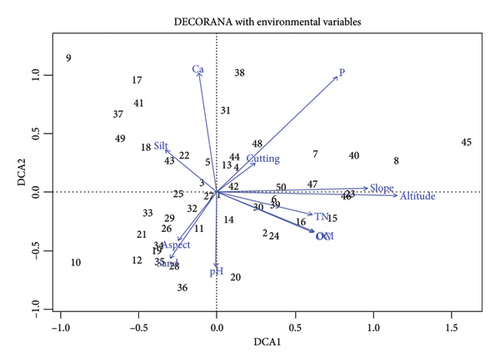
The findings of this study indicate that slope 0.499 (49.95) and aspect 0.215 (21.5%), followed by altitude, were also the most important constraining variables in weighting the first axis of the ordination analysis (Table 8 and Figure 6). The importance of slope and aspect in determining plant community distributions has been well documented in previous studies conducted in similar Afromontane ecosystems [104]. Variations in slope and aspect can lead to differences in soil moisture, exposure to sunlight, and other microclimate conditions, which in turn influence the establishment and growth of plant species [105, 106]. Through control of solar insolation, which directly affects soil moisture and temperature regimes, slope and aspect can limit the evolution of local plant and ecosystem types [107, 108].
| Environmental variables | RDA1 | RDA2 |
|---|---|---|
| Altitude | −0.553 | 0.543 |
| Slope | −0.499 | 0.403 |
| Aspect | 0.215 | −0.058 |
| Cutting | −0.175 | 0.089 |
| pH | 0.221 | 0.172 |
| Sand | 0.413 | 0.139 |
| Silt | −0.048 | −0.532 |
| OC | −0.139 | 0.478 |
| OM | 0.141 | 0.476 |
| TN | −0.251 | 0.249 |
| P | −0.251 | −0.022 |
| Ca | −0.459 | −0.535 |
| Eigenvalue | 27.737 | 18.003 |
| Proportion explained | 0.259 | 0.168 |
| Cumulative proportion | 0.259 | 0.427 |
4. Conclusions and Recommendations
The findings indicate that the Gibgodo State Forest is one of the most ecologically significant and biodiverse ecosystems in the country. These forests serve as crucial in situ conservation sites and a repository for plant genetic resources, including endemic and threatened plant species recognized in IUCN categories. The diversity in the forest is high, evenly distributed, and it also harbors a reasonable number of endemic plants. The diversity of the woody species demonstrates that the forest is still healthy and in a stable condition, wherein the natural ecological processes are taking place normally. The results also revealed that among all of the investigated environmental factors, pH, sand, OC, OM, total nitrogen, phosphorus, and calcium were found to significantly explain variation in species composition and community formation of four plant communities in the study area.
The variations in the patterns of community formation and species distribution highlight the importance of designing different conservation strategies for the Gibgodo State Forest. It is recommended that the forest be placed under strict conservation measures to preserve its ecological significance and biodiversity. Additionally, the management of the forest should focus on maintaining the optimal levels of the identified significant environmental variables, such as soil properties and topographic features, to ensure the continued thriving of the diverse plant communities within the ecosystem. The conservation strategies should also consider the differences in species distribution and community formation across the various elevations within the forest.
Ethics Statement
This study is primarily ecological in nature and did not involve any plant species experiments. Therefore, the sections pertaining to ethics approval and participant consent do not apply to this investigation.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies.
Open Research
Data Availability Statement
The data supporting the findings of this study are presented in the tables and figures within the article.




