Gold Nanoparticles Electrodeposition Onto Thick Diazonium Film for Sensitive and Long-Lasting Hg(II) Trace Sensing
Abstract
A new electrochemical sensor devoted to Hg(II) trace determination was developed by tailoring a hybrid organic/inorganic interface using diazonium salts and gold nanoparticles (AuNPs). The AuNPs were electrodeposited for the very first time onto glassy carbon (GC) electrodes functionalized by thick (ca. 4 nm) diazonium films. The thickness of the organic films was obtained using atomic force microscopy (AFM) in scratching mode, while the AuNPs were characterized by field emission gun scanning electron microscopy (FEG-SEM). Each step of the functionalization process was studied by cyclic voltammetry using ferricyanide and ruthenium hexaammine as the redox probes. The as-prepared electrode was used for Hg(II) trace detection through square wave anodic stripping voltammetry (SWASV). A linear response was observed in the 1.0–9.9 nmol·L−1 range using a preconcentration time of 300 s, yielding a normalized sensitivity of 0.03 μA·L·nmol−1·min−1. The limit of detection (LOD) was determined to be as low as 300 pmol·L−1. The effect of major interfering metal cations on the sensor’s response was also investigated. In addition, the hybrid organic/inorganic interface strongly enhanced the lifetime of the sensor, this latter being extended up to 4 weeks.
1. Introduction
The contamination of the environment, including air, water, and soils, by heavy metals or, more precisely, trace metal elements [1], is a worldwide distributed concern. Amongst these elements, mercury (Hg) is of particular interest and global importance, since it is probably the most toxic one. It is released in the natural media either by natural processes, mainly aerial and subaerial volcanism [2], or by anthropogenic discharges from mining, coal-burning plants, smelters, waste incinerators, and industrial and urban runoffs [3, 4]. Hg can be encountered in many different forms, namely, inorganic and organometallic species such as methylmercury (MeHg) [5, 6]. This latter is very toxic [7], because of its intrinsic hydrophobic properties and the strong affinity it exhibits towards biological compounds through sulfhydryl groups [8, 9] and DNA binding [10]. Acute or chronic MeHg exposure, even at low levels, may cause severe adverse effects for mammals, including humans, such as lung, heart, liver, kidney, or brain pathologies [11, 12]. Since it is now admitted that the production of MeHg is directly correlated to the (bio)-availability of inorganic Hg(II) [13], this latter represents a growing environmental [14, 15] and health threat [16, 17]. As a consequence, a guideline value of 6 μg·L−1 (ca. 30 nmol·L−1) for drinking water has been delivered by the World Health Organization [18], and the European Water Framework Directive set an even more strict standard of 0.07 μg·L−1 (ca. 0.3 nmol·L−1) in addition of classifying all forms of Hg as priority dangerous substances [19]. Thus, Hg(II) appears to be a priority target for sensing, and there is an urgent need for real-time, in situ and trace-sensitive Hg(II) tools in order to multiply monitoring points dedicated to early warning pollution alert [20].
Standard Hg(II) trace analysis is usually achieved by spectroscopic techniques such as cold-vapor atomic fluorescence spectrometry [21, 22] or cold-vapor atomic absorption spectrometry [23], the former allowing concentrations as low as 0.2 pmol·L−1 total Hg(II) to be measured [24]. However, these techniques suffer severe drawbacks as they require expensive materials and time-consuming, complex procedures. Most of the time, the materials are also heavy, which makes them unsuitable for on-field analysis. Consequently, rigorous storage and transportation conditions become mandatory, which are the critical steps in order to avoid speciation changes or external contamination in the collected samples [25, 26].
In this context, electrochemistry appears as a good alternative to face the challenge of building cheap and portable devices dedicated to Hg(II) trace determination [27]. Indeed, electrochemical sensors are mostly reagentless. They also can be easily miniaturized, which makes them particularly well-suited for automatic in situ measurements with minimal sample changes. Experimental data can be obtained in real time or with a few minutes time-lag, thus allowing for quite fast analyses. Finally, limits of detection (LOD) in the pmol·L−1 range can be reached by combining anodic stripping voltammetry (ASV) as a preconcentration step and pulsed techniques such as differential pulse (DPV) or square wave voltammetry (SWV) [28, 29]. A wisely chosen strategy for electrode surface functionalization also helps in improving both selectivity and sensitivity. The wide range of possible functionalizations has been recently reviewed [29, 30]. Amongst all these strategies, gold nanoparticles (AuNPs) have attracted tremendous attention in the last 2 decades, due to their specific physicochemical properties which confer them manifold advantages with respect to electrochemical sensing [31]: these include higher active surface area, enhanced diffusion of electroactive species, (electro)catalytic activity, and higher signal-to-noise ratio [32]. Our group has recently reported on an electrochemical sensor devoted to Hg(II) trace determination based on glassy carbon (GC) functionalized by chemically prepared [33] or electrodeposited AuNPs (GC/AuNPs) [34]. After the first results obtained by depositing AuNPs using cyclic voltammetry (CV), the influence of the electrodeposition technique on the deposit structuration has been studied, and it was shown that the analytical performances of the sensor are directly correlated with AuNPs’ average diameter and density on GC [35]. Further improvement was brought by studying the influence of chloride anions on Hg(II) trace detection [36] and by including a chloride desorption step in the electroanalytical procedure [37]. Using this protocol and the optimal AuNPs deposit, a normalized sensitivity of 0.60 μA·L·nmol−1·min−1 and a LOD as low as 80 pmol·L−1 were achieved for a preconcentration time of 300 s. Tests in natural water were also performed, allowing the measurement of a Hg(II) concentration of 19 ± 3 pmol·L−1, thanks to a preconcentration time that increased up to 50 min. However, the GC/AuNPs electrode failed stability tests, since the detection of a given amount of Hg(II) was possible only over a few consecutive days. To address this critical issue of sensor lifetime, a two-step hybrid organic/inorganic functionalization of the interface was considered [38]. In the first step, diazonium compounds were first grafted onto the GC surface in order to favor AuNPs stabilization on the interface. In a second step, chemically prepared AuNPs were drop-casted on the diazonium-functionalized GC surface. This strategy led to a significant enhancement of the sensitivity toward Hg(II), with a normalized value of up to 3.94 μA·L·nmol−1·min−1 depending on the diazonium used. However, no improvement in the interface stability was observed. Several alternative strategies have been reported in the literature for the development of hybrid organic/inorganic interfaces. Guo and Li have immobilized metal cations on a surface functionalized by an aryl monolayer and then reduced the cations [39, 40]. However, the interface stability vs. time has not been studied. Liu et al. have reported on the diazotization of aminated nanoparticles (NPs) followed by diazonium reduction and grafting [41]. In this case, a pretty good stability of 10 weeks was obtained, but this strategy appears unsuitable for trace metal elements determination due to the presence of the capping ligands on the NPs surface, which may hinder metal accumulation during the preconcentration process. On the contrary, electrodeposition actually provides ligand-free NPs. However, very few reports in the literature deal with the electrodeposition of NPs on aryldiazonium films. Mirkhalaf and coworkers have electrodeposited AuNPs on a inhomogeneous, submonolayer 4-nitrosophenyl film [42], while González et al. have reduced HAuCl4 by CV on carbon substrates functionalized by a 4-nitrobenzenediazonium monolayer [43, 44]. It is noteworthy that none of these studies were focused on sensor development. Furthermore, in all three cases, the diazonium film thickness was limited to a monolayer or submonolayer. Such a precaution was mandatory keeping in mind that multilayer, thick aryldiazonium films always induce a strong barrier effect [45, 46], which can severely hinder further electrochemical processes such as electrodeposition. However, thick aryldiazonium films should be considered as they could favor interactions with AuNPs and thereby improve their stability on the functionalized electrode surface. In the present work, we report on the development of a GC/diazonium/AuNPs interface and its application for Hg(II) trace determination, focusing on the lifetime of the interface. The functionalized interface was achieved by electrodepositing for the very first time AuNPs onto a thick diazonium film, which was expected to be insulating. The successful reduction of Au(III) onto the thick organic film was confirmed through electrochemical analysis. The resulting hybrid organic/inorganic interface was characterized by CV, atomic force microscopy (AFM) and field emission gun scanning electron microscopy (FEG-SEM). The as-prepared electrode was used for Hg(II) trace detection and its analytical performance was evaluated. A study of potential interferences from metal cations was also conducted along with an examination of the critical issue of the interface stability and sensor lifetime in several media.
2. Experimental Section
2.1. Chemicals and Apparatus
All the chemicals were purchased from commercial suppliers and used without further purification. Detailed information is provided in the Supporting Information. All the solutions were prepared using ultrapure water (Milli-Q, Millipore, 18.2 MΩ·cm).
Electrochemical measurements were conducted using a Metrohm Autolab PGSTAT128N potentiostat controlled by NOVA 2.1.6. The experimental setup has been described in detail in previous works from our group [37] (see Supporting Information for details).
2.2. Electrode Preparation and Functionalization
All working electrodes were carefully polished prior to use. The exact polishing procedure is described elsewhere (see Supporting Information for details).
Diazonium grafting was performed either by CV or constant potential electrolysis at a given potential value for 300 s. 4-Nitrobenzene diazonium (NBD) was used from a commercial batch without further treatment. 4-Thiophenol diazonium (TPD) was prepared from 4-aminothiophenol using a previously published procedure [38] (see Supporting Information for detailed synthesis).
AuNPs electrodeposition was carried out at 22°C either by CV or constant potential electrolysis in a 0.1 mol·L−1 NaNO3 solution containing 0.25 mmol·L−1 HAuCl4. For CV, AuNPs were obtained by scanning the working electrode from 0.90 to −0.5 V at a scan rate of 50 mV·s−1 for a given number of scans. Constant potential electrolysis was performed by applying a potential of 70 mV lower than that corresponding to the reduction peak of Au(III) in Au(0) for various durations from 30 to 600 s. After electrodeposition, AuNPs were activated by recording 10 consecutive CVs from 0.2 to 1.4 V in a 0.5 mol·L−1 H2SO4 solution using a scan rate of 100 mV·s−1.
2.3. Interface Characterization
AFM from the Institut des Technologies Avancées en Sciences du Vivant (ITAV) and FEG-SEM from the Atelier Interuniversitaire de Micro-nano Électronique (AIME) were used to fully characterize the functionalized interface. Details of both experimental setups are provided in the Supporting Information.
2.4. Stripping Voltammetric Detection of Hg(II)
To enable direct comparison, the analytical procedure for Hg(II) trace determination was the same as that reported in a previous study from our group [37] (see Supporting Information for detailed synthesis [available here]).
2.5. Stability of the Interfaces Under Different Storage Conditions
The functionalized electrodes were stored under three different conditions over a period of 4 weeks. The first batch of electrodes was left at ambient air without particular precautions; the second was immersed in 0.01 mol·L−1 HCl at 22°C; finally, the third batch was stored in a 0.05 mol·L−1 phosphate buffer solution (PBS) (pH 7) at 22°C. For each measurement, the electrodes underwent an activation process in 0.5 mol·L−1 H2SO4 followed by detection of 4 nmol·L−1 Hg(II) to assess the stability of the interface and the performance of the sensors over time.
3. Results and Discussion
3.1. Functionalization and Characterization of the Electrode
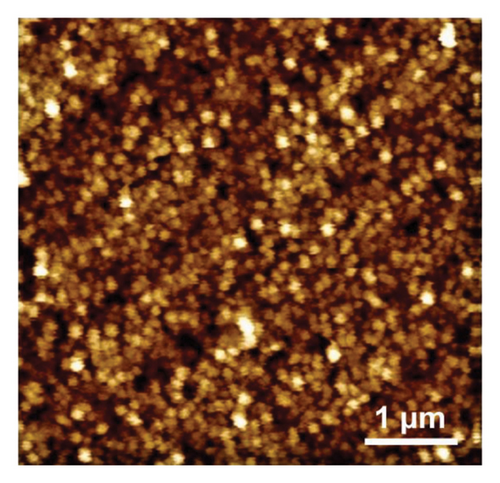
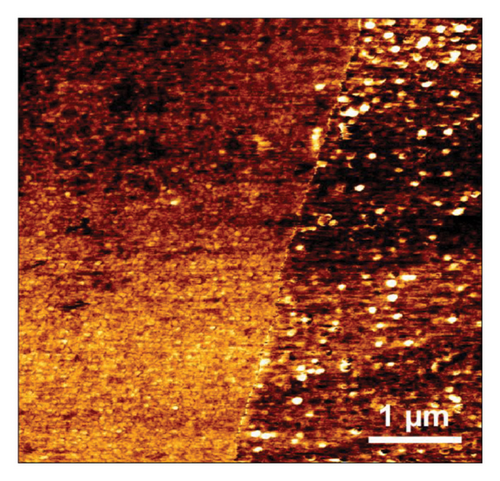
NBD was chosen because of its commercial availability and due to the fact that its reactivity has been extensively studied by many authors, including ourselves [47, 48]. By its side, TPD is expected to favor AuNPs stabilization via the strong covalent bonding between Au and S atoms [49–51], the corresponding binding energy being 154.4 kJ·mol−1 [52]. However, it is worth noting that the AuNPs used in combination with TPD were always obtained via chemical route and never by electrodeposition techniques. The electrochemical behavior of NBD and TPD was first examined by CV in acid media (Figures 1(a) and 1(b)). Both voltammograms exhibited the typical shape for diazonium compounds, with a large reduction peak at 0.02 and −0.3 V, respectively, during the first scan. This peak corresponds to the reduction and subsequent grafting of the diazonium onto the GC surface. The difference in peak potential between the two diazoniums was consistent with the stronger electron-withdrawing effect of the NO2 group compared to that of the SH one, as previously reported [38]. On the second scan, this reduction peak disappeared in accordance with the self-inhibiting nature of diazonium compounds. To form thick, self-inhibiting organic films, constant potential electrolysis was used at a potential approximatively 200 mV lower than the reduction peak potential, around −0.18 and −0.5 V for NBD and TPD, respectively, to carry out the diazonium reduction for 300 s. The resulting films were examined using AFM (Figures 1(c) and 1(d)). The micrographs revealed homogeneous films across the GC surfaces, with some “mushroom-like” or globular features. These latter structures, which have been previously reported for diazonium films, are a result of the nucleation and growth process of the layer [43, 53]. Notably, the NBD film (Figure 1(c)) displayed a much more granular structure compared to the TPD film (Figure 1(d)). Therefore, the roughness of the NBD film can be assumed to be greater than that of the TPD film. Scratching experiments (see Figure 1(d) with the scratch line on the left and the organic film on the right side of the micrograph) allowed a thickness of approximatively 4 nm to be measured for both films, consistent with prior results from our group [38, 48], and in agreement with the formation of multilayered films [54].
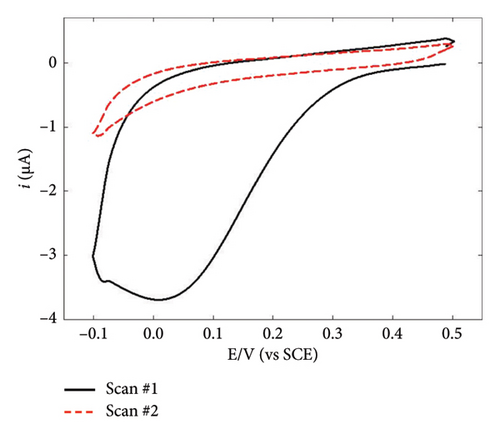
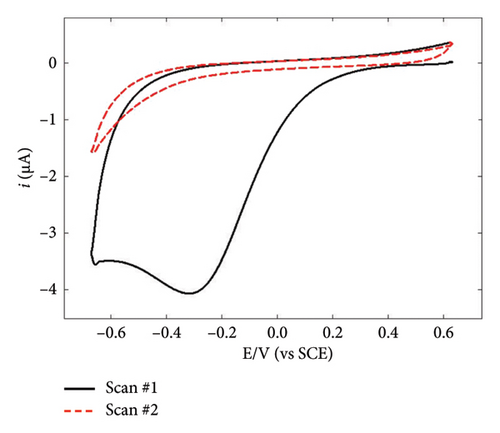
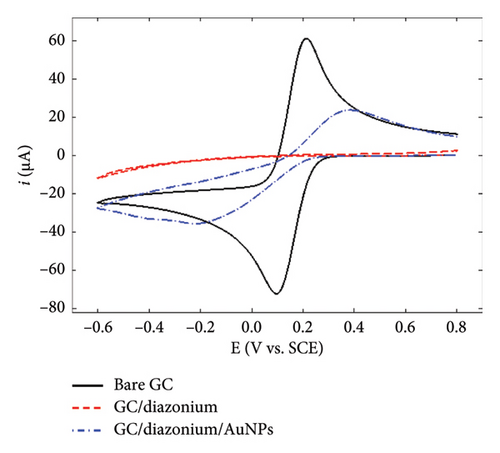
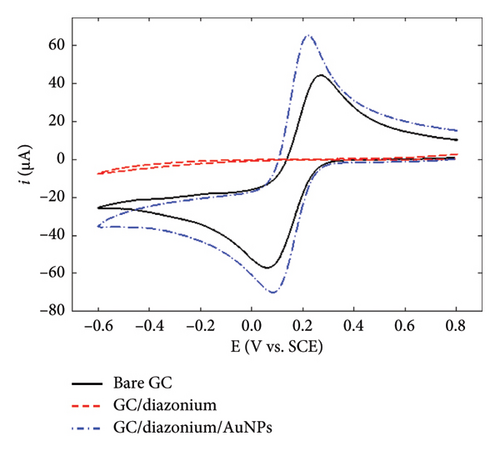
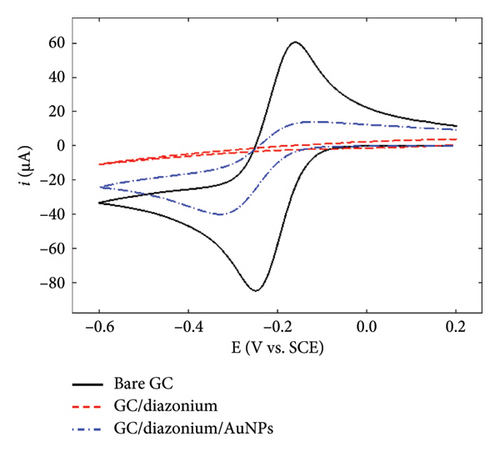
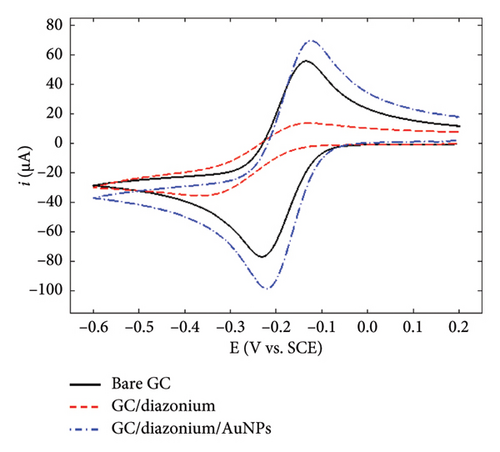
To gain further insights into the GC/NBD and GC/TBD electrodes’ features, CVs were recorded in a 0.1 mol·L−1 KNO3 solution containing 5 mmol·L−1 of either ferricyanide Fe(CN)63− or ruthenium hexaammine Ru(NH3)63+ (Figure 2). Upon grafting the diazonium compounds onto GC, the redox signal corresponding to the quasireversible system of ferricyanide was nearly completely suppressed (Figures 2(a) and 2(b)), in accordance with the barrier effect induced by the organic films and the well-known inner-sphere mechanism of this probe. Ru(NH3)63+ exhibited a different behavior related to the nature of the substituent attached to the organic film. On the GC/NBD electrode (Figure 2(c)), the redox signal was almost completely suppressed, similar to what was observed for Fe(CN)63−. This behavior is unusual for a redox probe, the electron transfer of which is known to proceed via an outer-sphere mechanism. One possible explanation for this result could be the exceptionally high compactness of the organic film. In contrast, on the GC/TPD electrode (Figure 2(d)), the redox signal of Ru(NH3)63+ was still present, albeit significantly slowed (ΔEp = 139 mV) and with much smaller peak currents compared to those recorded on unmodified GC. These findings further confirmed the presence of grafted organic films that effectively covered the entire electrode surface and exhibited very few defects or pinholes.
The functionalization of the GC/diazonium electrodes was completed through electrodeposition of AuNPs. In order to get information on this functionalization step, this latter was first performed using CV (Figures 3(a) and 3(b)). Unexpectedly, Au(III) reduction actually occurred on both GC/NBD and GC/TPD electrodes despite the presence of the thick organic films. This can be rationalized considering that Au(III) reduction primarily proceeds via an outer-sphere mechanism. As a result, the reduction process was only slowed on GC/diazonium electrodes compared to bare GC. Indeed, the CVs showed a reduction peak corresponding to the reduction of Au(III) to Au(0) at 0 and 0.12 V for GC/NBD and GC/TPD, respectively, whereas Au(III) reduction on bare GC is typically reported to occur around 0.48 V [34, 35]. The slight difference in potential shift between GC/NBD and GC/TPD can be attributed to the subtle influence of the substituent on the organic films. Indeed, Au(III) reduction was slightly easier on the GC/TPD film than on GC/NBD (with the corresponding peak potential being 120 mV higher), likely due to the stronger affinity of sulfur for Au which facilitates the approach of Au(III). In contrast, nitro groups tend to exert a repulsive electrostatic effect [38]. More generally, Au is known to interact more readily with sulfur rather than with nitrogen [55]. This difference can be also noticed in the shape of the CV scans. On the first scan recorded on GC/NBD (Figure 3(a)), two distinct reduction peaks were observed, at 0 and −0.16 V, suggesting that Au(III) experiences multiple electronic environments during the reduction process. This behavior may be linked to the GC/NBD film granular structure, which likely resulted in a variable thickness and inhomogeneous reactivity with respect to Au(III) reduction. In contrast, the first scan recorded on GC/TPD (Figure 3(b)) showed a single, well-defined peak for Au(III) reduction at 0.12 V. The overall shape of this latter CV scan closely resembled that of Au(III) reduction onto bare GC, the only difference being the shift to lower potentials. In both films, a current crossover was observed at 0.35 V during the first backwards scan. The presence of this crossover, and its location at the same potential value regardless of the substituent borne by the organic film, indicated that Au(III) reduction was successful and led to the formation of AuNPs. This was further confirmed by the second scan recorded on both GC/NBD and GC/TPD, where a reduction peak centered at 0.6 V was observed. This latter peak actually corresponded to the reduction of Au(III) on the AuNPs formed during the first scan. The fact that the corresponding peaks occurred at the same potential for both films indicated that the reduction of Au on AuNPs was independent of the organic film substituent, confirming that the influence of the substituent had diminished once the AuNPs were formed. However, the films slowed the kinetics of the reduction process, as the reduction of Au(III) onto previously formed AuNPs has been reported to occur around 0.78 V when NPs electrodeposition was performed on bare GC [34, 35]. On the GC/NBD electrode, a second reduction peak was observed at around −0.18 V, indicating that Au(III) reduction took place in a different electronic environment. However, no further investigation was carried out to explore this latter peak, and its origin remains unclear.
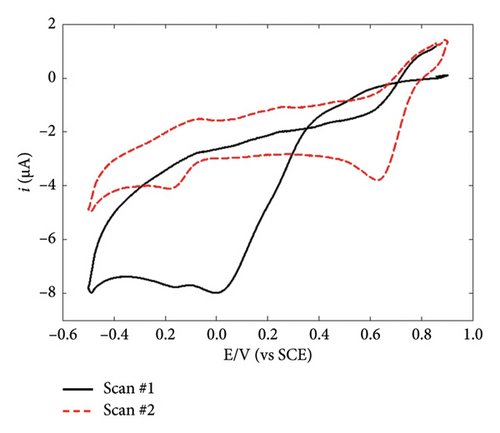
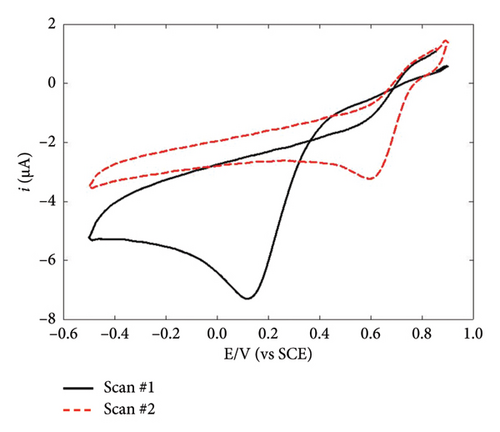
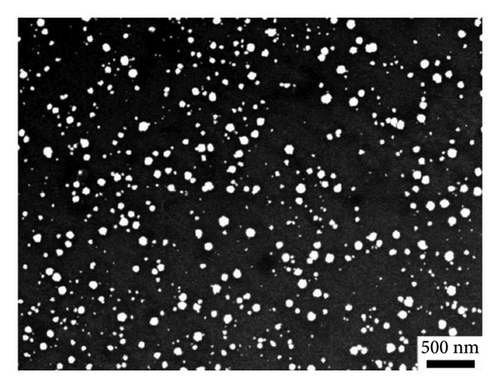
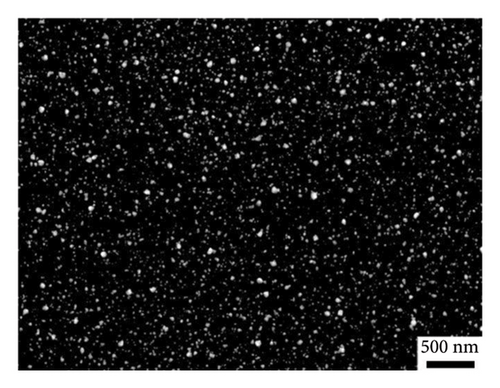
To achieve deposits with controlled morphology, AuNPs were electrodeposited via constant potential electrolysis at −0.07 and 0.05 V for GC/NBD and GC/TPD, respectively (ca. 70 mV lower than the peak potential), for various durations ranging from 30 to 600 s. The characterization of the resulting deposits was performed by FEG-SEM (Figures 3(c) and 3(d)). In both cases, the micrographs clearly showed the presence of AuNPs on the electrode surface, thus definitely proving that Au(III) reduction as well as subsequent formation and precipitation of AuNPs occur on the thick diazonium films. As far as we know, this is the first time that the effective electrodeposition of AuNPs is reported on thick, insulating organic films. However, the morphology of the deposits differed significantly in terms of particle density and average diameter depending on the diazonium used. On the GC/NBD electrode (Figure 3(c)), AuNPs with an average diameter of 55 nm were observed, with a density of 21 NPs μm−2. Two distinct populations of NPs could be identified, based on their average diameter. The first population consisted of “larger” AuNPs, with an average diameter of around 90 nm, and represented the predominant population. The second population comprised much smaller NPs. On the GC/TPD electrode (Figure 3(d)), a much more homogeneous and denser deposit (ca. 158 NPs μm−2) was observed, with AuNPs having an average diameter of 27 ± 3 nm. Some larger particles with an average diameter of around 60 nm were also present but were far less numerous. In addition to the influence of the substituent borne by the film, which could either facilitate or hinder the approach of Au(III) and the formation of AuNPs, the difference in roughness of the films may also explain the variations in the morphology of the deposits. This is consistent with the prior observation of the granular structure of the GC/NBD film, as well as the fact that two different peaks were observed on the CV for Au(III) reduction.
Finally, the GC/NBD/AuNPs and GC/TPD/AuNPs electrodes were characterized using Fe(CN)63− and Ru(NH3)63+ as redox probes (Figure 2). The results varied significantly depending on the type of film. On the GC/NBD/AuNPs electrode (Figures 2(a) and 2(c)), the initial signals observed on bare GC were only partly restored. The CVs exhibited slower redox processes, with larger peak separations (ΔEp = 590 and 180 mV for Fe(CN)63− and Ru(NH3)63+, respectively) and much smaller peak currents. For Fe(CN)63−, two reduction peaks were observed at −0.21 and −0.38 V on the forward scan, suggesting that the redox probe experienced different electronic environments within its reduction process. In contrast, on the GC/TPD/AuNPs electrode (Figures 2(b) and 2(d)), the initial redox signal for both redox probes was restored and even enhanced, likely due to the increase of the active surface area provided by the AuNPs. In addition, for both redox probes, the ΔEp value decreased, indicating faster electron transfer kinetics. This suggests that, in this case, the diazonium film no longer had a significant effect on the redox probe approach.
3.2. Electrochemical Response Toward Hg(II) Trace Detection
All the electrodes prepared by AuNPs electrodeposition by constant potential electrolysis for durations ranging from 30 to 600 s on both NBD- and TPD-functionalized GC were tested for the detection of a given concentration of Hg(II) (ca. 4 nmol·L−1, results not shown). Regardless of the electrodeposition duration, the GC/NBD/AuNPs electrodes exhibited poor repeatability and reproducibility and were therefore not used for Hg(II) trace detection. In contrast, all the GC/TPD/AuNPs electrodes successfully detected 4 nmol·L−1 Hg(II), with the best results in terms of reoxidation peak current obtained for AuNPs electrodeposition of 300 s. Thus, this latter electrode was chosen for calibration and further testing. This difference in responses to Hg(II) detection highlights the influence of the organic film, and more specifically the substituents it contains, on the detection performance. A similar trend was reported with NBD and TPD when AuNPs were chemically prepared and then drop-casted onto diazonium-functionalized GC electrodes [38]. In addition to the difference in deposit morphology induced by the substituent on the diazonium films, it can be inferred that SH groups either promote or at least do not hinder Hg(II) approach during the preconcentration step of the analysis procedure, whereas NO2 groups clearly inhibit this approach.
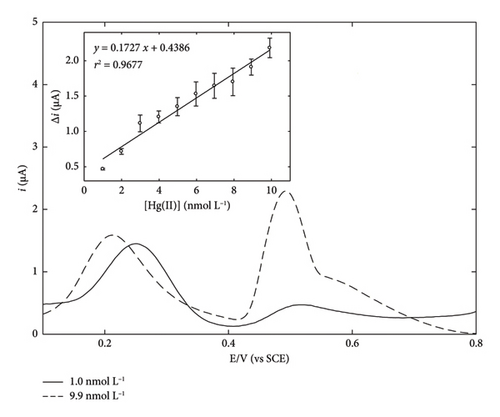
Figure 4 shows the typical square wave ASVs (SWASVs) recorded for increasing concentrations of Hg(II) using GC/TPD/AuNPs, with AuNPs prepared by constant potential electrolysis for 300 s (for the sake of clarity, only two Hg(II) amounts are depicted), along with the corresponding calibration plot obtained (inset). The reoxidation of preconcentrated Hg(0) was observed at 0.50 V. The peak current progressively increased while increasing Hg(II) amount. Another peak located around 0.2–0.25 V was attributed to the chloride adsorption/desorption process on the Au surface, as previously reported [36]. The electrode exhibited a linear response in the 1.0–9.9 nmol·L−1 concentration range, with a correlation coefficient of 0.9677. An F-test conducted on the whole dataset afforded a F-value of 240, confirming a good linearity of the response. A normalized sensitivity of 0.03 μA·L·nmol−1·min−1 was determined from the slope of the linear regression curve. All the relevant analytical features are summarized and compared to literature data in Table 1. While this value is not the highest sensitivity in the literature (see Table 1 for comparative features), it is noteworthy that electrogenerated AuNPs were much less reactive than chemically prepared ones, as highlighted in a previous work from our group [38]. Nevertheless, the sensor was sensitive enough to provide reliable analytical responses in the nmol·L−1 range with a short preconcentration time (ca. 5 min). The error bars observed in this experiment were significantly larger than those usually reported in our previous works [34, 35, 37], likely due to the influence of the organic film during the various steps of the analytical procedure. Finally, the LOD was experimentally determined by adding successive aliquots of Hg(II), each corresponding to a 100 pmol·L−1 concentration, and measuring the corresponding SWASV (data not shown). Using this approach, a LOD of 300 pmol·L−1 was obtained.
| Electrode functionalization | Cl− desorption step | Linear range (nmol⋅L−1) | Normalized sensitivity (μA⋅L·nmol−1·min−1) | LOD (pmol⋅L−1) | Reference |
|---|---|---|---|---|---|
| Elect.a AuNPs | No | 0.64–4.0 | 0.274 | 420 | [33] |
| Elect. AuNPs | No | 0.8–9.9 | 0.23 | 400 | [34] |
| MESb/elect. AuNPs | No | 5–500 | 0.009 | 660 | [56] |
| Elect. AuNPs | No | 50–250 | 0.65 | 7.5 | [57] |
| Elect. AuNPs | Yes | 0.4–6.0 | 0.60 | 80 | [36] |
| NBD/Chem.c AuNPs | No | 1–10 | 0.37 | — | [37] |
| TPD/Chem. AuNPs | No | 1–10 | 3.94 | — | [37] |
| TPD/Elect. AuNPs | Yes | 1.0–9.9 | 0.03 | 300 | This work |
- aElect., electrodeposited.
- bMES, mercaptoethanesulfonate monolayer–modified AuNPs.
- cChem., chemically prepared AuNPs.
3.3. Interfering Species
A study was conducted to evaluate interfering metallic species commonly found in natural water samples. For this purpose, a 4 nmol·L−1 Hg(II) concentration was measured in the presence of various excess ratios of different metal cations. The results are summarized in Table 2. Amongst all the tested metal cations, Zn2+ was the least disruptive one to the sensor response. Even at a 50-fold excess, no significant change in the Hg(0) reoxidation peak current was seen, and at a 100-fold excess, 72% of the initial signal was still measured. The other metal cations can be divided into two groups, based on their influence on the Hg(0) reoxidation peak current. In the first group, Co2+, Pb2+, V5+, Cu2+, Cd2+, and Cr2+ all caused a decrease in the sensor response as the metal cation amount increased. Among these species, Cd2+ was the most important interferent, as only 3% of the initial signal was observed at a 50-fold excess, and no measurable signal was observed at a 100-fold excess. Cu2+ and Cr2+ were also major interferents, with a 10-fold excess of Cu2+ leading to only 56% of the initial signal and a 10-fold excess of Cr2+ resulting in just 25% recovery of the initial signal. Interestingly, Cu2+ caused an increase in the broad peak around 0.3 V, which is typically associated with chloride anion desorption [36], suggesting that Cu2+ was co-preconcentrated with Hg(II) and then reoxidized in this potential range. No similar changes or new peaks were observed for the other metal cations in this group, indicating that they were not detected under these conditions. However, their negative interference may be explained considering that these species are reduced during the chloride desorption step which takes place at −0.8 V. While reducing, they could interfere with Cl− desorption or even interact with the amalgam, thus reducing the efficiency of the chloride desorption or hindering Hg0 reoxidation. In the second group of interferents, Ag+ and Ni2+ both enhanced the sensor response as their concentrations increased. These metal cations were likely preconcentrated and reoxidized near the Hg(II) signal. This was not a significant issue for Ag+, as its concentration in most natural samples is typically lower than 5 nmol·L−1 (a 1.25-fold excess), meaning it would not interfere at concentrations typically encountered in the environment.
| Ratio | Zn2+ | Co2+ | Pb2+ | V5+ | Cu2+ | Cd2+ | Cr2+ | Ag+ | Ni2+ |
|---|---|---|---|---|---|---|---|---|---|
| 10 | 100 | 106 | 88 | 64 | 56 | 42 | 25 | 108 | 295 |
| 50 | 101 | 74 | 69 | 62 | 32 | 3 | 16 | 119 | 307 |
| 100 | 72 | 47 | 64 | 47 | 11 | — | 16 | 118 | 210 |
| −2.7 10−3 | −5.8 10−3 | −3.4 10−3 | −3.6 10−2 | −4.4 10−2 | −5.8 10−2 | −7.5 10−2 | 1.7 10−3 | 0.195 | |
- Note: Results are expressed as a percentage of the initial signal (i.e., signal recorded for 4 nmol·L−1 Hg(II) with no metal cation in the solution) as a function of the excess ratio metal cation/Hg(II).
The obtained values for the amperometric selectivity coefficients are summarized in Table 2. These values confirm the qualitative analysis and clearly show that Ni2+ is the most important interferent toward Hg(II) trace detection.
3.4. Lifetime of the Sensor
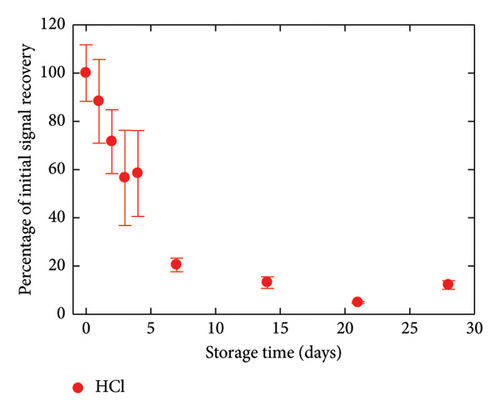
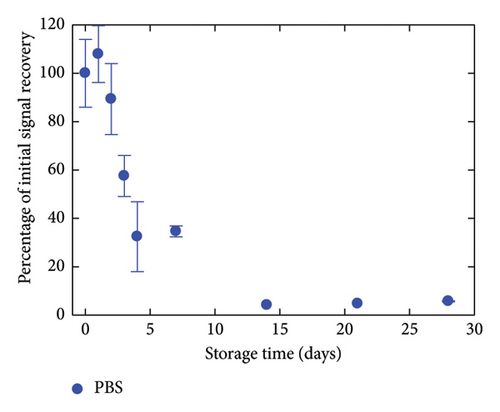
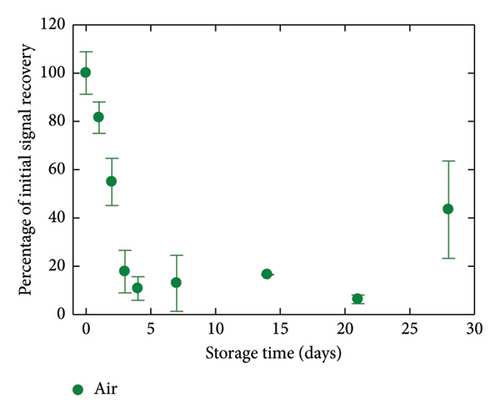
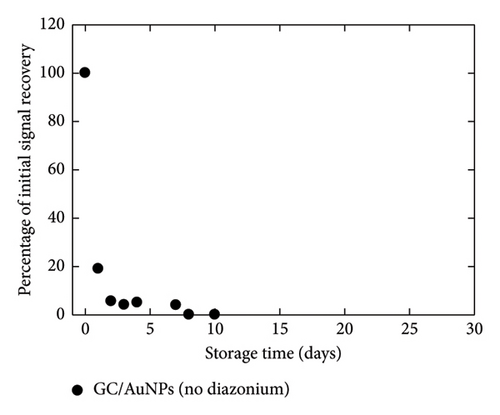
The critical matter of sensor lifespan was investigated by examining the stability of the GC/TPD/AuNPs electrode over time using different storage conditions. A first batch of three electrodes was stored in the medium used for Hg(II) analysis, that is, in a 0.01 mol·L−1 HCl solution. A second batch of three electrodes was kept in a neutral medium, namely, a 0.05 mol·L−1 (pH 7) PBS. A last batch of three electrodes was left in ambient air without special care. For the sake of comparison, GC/AuNPs electrodes (i.e., without diazonium film) were also stored in air under similar conditions as done in previous studies. The entire experiment, which involved four sets of three electrodes and various storage conditions, was repeated twice. The evolution of all the interfaces over time was monitored by recording SWASVs in a 4 nmol·L−1 Hg(II)-containing HCl solution (Figure 5). GC/AuNPs electrodes showed a rapid decrease in signal recovery, with only 5% of the initial signal measured after 2 days of storage. The signal decline slowed afterward and the response remained nearly constant for a few days, but no more signal was detected after 8 days of storage. For the GC/TPD/AuNPs electrodes, a noticeable decrease in the sensor response was also observed during the first week, regardless of the storage conditions. However, this decrease was much slower on the GC/TPD/AuNPs electrodes, with, for instance, a percentage of initial signal recovery ranging from 55% to 89%, compared to just 5% for GC/AuNPs electrodes, after two days of storage. After one week, the best response was obtained for GC/TPD/AuNPs electrodes stored in PBS, where about 35% of the initial signal was retained. After 4 weeks, the signal recorded on the GC/TPD/AuNPs electrodes was still clear and unambiguously extracted from the background. The percentage of initial signal recovery was 6% and 12% for electrodes stored in PBS and HCl, respectively. For electrodes stored in air, a weak signal was still detectable, but the repeatability was low, and this value was considered unreliable and likely an outlier. Therefore, the presence of the organic film on the GC significantly improved the stability of the AuNPs and consequently, the sensor lifespan, extending it from 1 week to about 4 weeks. This improvement appeared to be independent of the storage conditions. The strong interactions between sulfur and Au may explain this result.
4. Conclusion
In this work, GC electrodes were functionalized for the very first time via the electrodeposition of AuNPs onto thick (ca. 4 nm) diazonium films. A critical influence of the film substituents was observed, as the electrodeposition process was facilitated by two key factors: (i) Au(III) reduction primarily occurs via an outer-sphere mechanism and (ii) the thiol groups in the organic film promote the approach of Au(III). In contrast, when the substituents on the organic film were nitro groups instead of thiol groups, the electrodeposition process was still possible but more challenging due to electrostatic repulsion. Once the first nuclei were formed, electron transfer across the organic layer was recovered, and the AuNPs continued to grow according to the classical nucleation/growth mechanism, with no further influence of the film substituents. The resulting AuNPs deposits exhibited markedly different densities and average diameters, depending on whether they were electrodeposited on GC/NBD or GC/TPD, with the latter providing much denser and more homogeneous deposits. The GC/TPD/AuNPs electrode was then evaluated for Hg(II) trace detection, showing a linear response in the range 1.0–9.9 nmol·L−1, with a normalized sensitivity of 0.03 μA·L·nmol−1·min−1. This sensitivity value was lower than that observed in previous studies where no organic layers were used, reflecting the negative effect of the film on the sensor performance. However, the film significantly improved the sensor lifetime, extending it up to 4 weeks. The interactions between the SH groups of the film and the AuNPs may be invoked to account for this improvement, which results from the stabilization of the AuNPs on the interface.
Future studies will focus on testing the GC/TPD/AuNPs electrode for Hg(II) trace detection in natural water samples. Further work will also concern the investigation of new films that could further enhance the sensor lifetime, potentially extending it to several months, while preserving the sensitivity provided by the AuNPs.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was funded by the Agence Nationale de la Recherche, ANR-15-CE04-0010 and ANR-10-INBS-04.
Supporting Information
S1: Detailed Experimental Section including references.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




