Synthesis of Binary Nanocomposite Conductive Polypyrrole and Reduced Graphene Oxide as Electrode Materials for High-Performance Supercapacitor
Abstract
In this study, a polypyrrole/reduced graphene oxide (PPy/rGO) composite material was synthesized through a chemical oxidative process, to tackle the persistent challenge of developing cost-effective and scalable electrode materials with high energy density and excellent charge storage capabilities for supercapacitor applications by integrating polypyrrole with rGO. The rGO was introduced into the polymerization process of polypyrrole. The composite underwent detailed investigation using different characterization techniques. Subsequently, analysis of the electrochemical properties included galvanostatic charge–discharge (GCD), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS). Nickel foam was used as the substrate material for the electrode. The PPy/rGO composite exhibited a remarkable specific capacitance of 365.1 F/g at a scan rate of 10 mV/s, as evidenced by CV, highlighting its excellent electrochemical properties. Moreover, the specific capacitance derived from GCD measurements surpassed expectations, reaching an impressive value of 375 F/g at a current density of 2 A/g. The corresponding energy density and power density for the specific capacitance of 375 F/g are 46.875 Wh/kg and 1140.20 W/kg, respectively. This further highlights the exceptional potential of this composite material for energy storage purposes. These findings highlight the PPy/rGO composite’s remarkable electrochemical characteristics and demonstrate its effective synthesis and thorough characterization. The simplicity of the synthesis process coupled with the outstanding performance of the composite underscores its viability for various energy storage and conversion devices.
1. Introduction
The global demand for energy has been steadily increasing, and this growing consumption presents significant challenges as current energy resources are insufficient to meet future needs [1]. This trajectory could lead to potential energy crises, further compounded by the depletion of fossil fuels, environmental concerns related to greenhouse gas emissions, and rising energy needs across various sectors [2, 3]. To address this challenge, there is an urgent need to develop energy alternatives that are both efficient and sustainable.
Supercapacitors, with their unique characteristics, stand out as promising solutions for energy storage. Their advantages include long life cycles, rapid charging and discharging capabilities, high power density, and notable energy density, making them essential complements to traditional energy storage devices such as fuel cells and batteries [4, 5]. Since their introduction by Nippon Electric Company (NEC), supercapacitors have found wide-ranging applications in industries such as electronics, transportation, aerospace, military, and sensor technologies [6]. Their continued development holds great potential for addressing global energy challenges.
Among materials explored for supercapacitor applications, graphene has attracted significant attention due to its exceptional properties, including high electrical conductivity, low density, large surface area (2670 m2/g), mechanical strength, and chemical stability [7, 8]. Studies have shown that graphene can achieve gravimetric capacitance values of up to 135 F/g in aqueous electrolytes and 99 F/g in organic electrolytes [9]. For instance, Wang et al. utilized hydrazine to reduce graphene oxide (GO) and achieved a gravimetric capacitance of 205 F/g [10]. However, variations in reported capacitance values, ranging from 59 to 169.3 F/g, often stem from factors such as graphene purity and the composition of the electrolyte [11, 12].
Conducting polymers such as polypyrrole (PPy) have also emerged as promising materials for pseudocapacitors due to their ability to deliver high specific capacitance through reversible redox reactions. Among these, PPy has gained prominence due to its excellent conductivity, environmental stability, and mechanical flexibility [13, 14]. Despite these advantages, the challenge lies in effectively integrating PPy with graphene to create a composite material that maintains the high conductivity of graphene while leveraging the electrochemical properties of PPy. This has led to increased interest in developing composite materials that can overcome these limitations. The combination of PPy with carbon-based nanomaterials has been shown to enhance supercapacitor performance. Biswas et al. synthesized a graphene/PPy composite material that achieved a gravimetric capacitance of 165 F/g at a current density of 1 A/g [15], while Parl et al. demonstrated a specific capacitance of 400 F/g with a graphite/PPy composite in a three-electrode system [16].
In response to challenges, this study aims to build on previous research by synthesizing and characterizing PPy/reduced graphene oxide (rGO) composite electrodes for supercapacitor applications. By integrating rGO with PPy, the unique properties of both materials are harnessed to create a composite with enhanced electrochemical performance. Compared to previous methods, such as electrochemical polymerization and in situ chemical reduction, the chemical oxidative method used in this study is both simpler and more environmentally friendly, as it eliminates the need for hazardous chemicals and complex equipment. Additionally, it offers scalability for industrial applications, making it more cost-effective while still achieving comparable or improved electrochemical performance. rGO was incorporated during the polymerization process of PPy, resulting in a composite material with high conductivity and superior energy storage capabilities.
The central focus of this research is to investigate the electrochemical properties of the PPy/rGO composite, evaluating its synergistic effects and potential for use in efficient supercapacitors. PPy was chosen due to its widespread use and high specific capacitance (136 F/g, as determined using a three-electrode system) [17]. Additionally, this study presents an environmentally friendly and cost-effective synthesis method for the composite, with an optimal oxidant-to-pyrrole (O/py) ratio of 4, ensuring the highest conductivity [18].
Our results demonstrate that the PPy/rGO composite exhibits an impressive specific capacitance of 365.1 F/g at a scan rate of 10 mV/s, as determined by cyclic voltammetry (CV). Furthermore, galvanostatic charge–discharge (GCD) measurements revealed a specific capacitance of 375 F/g at a current density of 2 A/g, significantly surpassing the 165 F/g reported by Biswas et al. [19]. These findings highlight the superior performance of the PPy/rGO composite and its potential for large-scale energy storage applications.
2. Methodology and Materials
2.1. Synthesis of rGO
The modified Hummer method stands as a commonly utilized technique for synthesizing GO and rGO from graphite. Through this method, graphite undergoes oxidation using potent oxidizing agents such as sodium nitrate, potassium permanganate, and sulfuric acid to yield GO. During the oxidation process, graphene layers acquire oxygen-containing functional groups. This makes them capable of interacting with water and dispersing in it. In this method, a mixture comprising 5 g of graphite fine powder and 150 mL of concentrated sulfuric acid is stirred for 30 min in a beaker. While stirring, 7.5 g of NaNO3 is gradually added to the mixture. The stirring continues for an additional 30 min at 10°C. Subsequently, 15 g of KMnO4 is added very slowly while maintaining the temperature of the mixture, resulting in a darkening of its color. Continuous stirring is maintained for 3 h while ensuring the temperature remains below 20°C, typically achieved using an ice bath. Following this, 500 mL of deionized (DI) water is gradually added while stirring, and the temperature is maintained at 95°C. Then, 10 mL of hydrogen peroxide (H2O2) is slowly added, and the stirring continues for another 30 min. A noticeable color change from deep brown to yellow indicates the progression of the oxidation process. The mixture is then filtrated and washed with DI water to eliminate residual metal ions. Subsequently, 10 mL of hydrochloric acid (HCl, 37% purity) and 100 mL of DI water are added, followed by stirring for 15 min. NH3 is introduced to regulate the pH of the mixture. After filtration, the resulting solid residue is sonicated for approximately 7–8 h. The obtained GO powder is then dried in an oven to yield GO. For the preparation of rGO, the GO powder is dissolved in 100 mL of DI water and subjected to ultrasonication for 30 min. Subsequently, the GO solution is agitated, and 1 mL of hydrazine monohydrate is introduced as the reducing agent. The solution is subjected to thermal treatment at a temperature range of 95°C–100°C for a prolonged duration to produce rGO. This procedure facilitates the efficient amalgamation of GO and rGO, which find use in several industries including energy storage, electronics, and biomedical engineering [14, 15].
2.2. Synthesis of PPy/rGO Composite
The synthesis of PPy/rGO composite was accomplished via the chemical oxide method. Figure 1 represents the synthesis process of composites. During polymerization, rGO was introduced into the pyrrole solution. FeCl3 served as the oxidant, while CTAB acted as a surfactant. To initiate the synthesis, 0.5 M pyrrole was dissolved in 50 mL of water, followed by the addition of CTAB. Subsequently, 0.25 g of rGO was incorporated into the same solution. Meanwhile, a 2 M FeCl3 solution was prepared in 50 mL of distilled water. The pyrrole solution underwent continuous stirring, while the FeCl3 oxidant was slowly added to facilitate optimal polymerization. After 12 h of thorough stirring at room temperature, the resulting composite was subjected to multiple washes using DI water and ethanol to remove any residual impurities. Subsequently, the composite was subjected to a 12-h heating process at 80°C to enhance its structural integrity. The synthesized composite was then utilized for various characterizations and analyses to evaluate its electrochemical properties [16, 17].
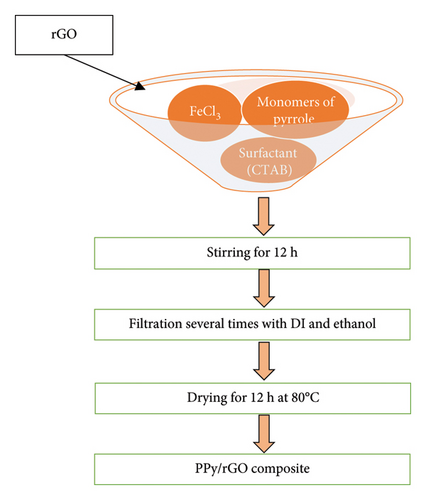
2.3. Electrode Synthesis
Electrochemical measurements were performed using a three-electrode system to evaluate the electrochemical performance of the synthesized composites. In this setup, the working electrode was prepared by depositing the synthesized material PPy/rGO onto nickel foam. The reference electrode used was an Ag/AgCl electrode, providing a stable and constant reference potential throughout the experiments. A platinum wire served as the counter electrode, ensuring reliable current conduction during the measurements. The electrolyte used in all tests was 3 M KOH.
The electrode fabrication process for the PPy/rGO composite involved the deposition of the PPy/rGO composite as an active material onto the surface of nickel foam by using a micropipette. Nickel foam of area 1 × 1 cm2 was used for deposition. This entailed preparing a slurry consisting of 40 mg of active material, 5 mg of acetylene black, and 5 mg of PVDF binder in an 8:1:1 ratio, along with the addition of 3 mL of N-methyl pyrrolidone (NMP). The slurry underwent ultrasonication for 6 h and was heated at a controlled temperature of 50°C for 8 h to ensure thorough dispersion of the materials. Subsequently, the slurry was carefully deposited onto the nickel foam surface using a micropipette. The mass loading of the material was 1 mg. Before slurry deposition, the nickel foam was activated (treating the foam to enhance its surface properties, making it more suitable for electrochemical applications) by washing it sequentially with DI water and HCl, followed by acetone and ethanol rinses. The deposited slurry-coated nickel foam was then dried for 7–8 h at temperatures ranging from 70°C to 80°C to ensure proper adhesion and stability of the electrode structure. To evaluate supercapacitor applications electrode’s performance can be monitored. The electrode system utilized nickel foam covered with the nanomaterial as the working electrode, while a 3 M KOH solution served as the electrolyte for all electrochemical tests [18]. The choice of electrolyte was based on its widespread usage in electrochemical research, owing to its broad electrochemical window and excellent ionic conductivity, facilitating the observation of various redox processes and ensuring optimal performance of the supercapacitor system. Considering all things, the choice of electrolyte and the method of electrode fabrication help to create high-performance supercapacitors with improved electrochemical properties [19, 20].
2.4. Characterization Techniques
X-ray diffraction (XRD) analysis of the powdered samples of the synthesized PPy/rGO composite was performed using a BRUKER diffractometer. This technique allowed us to investigate the crystallographic structure of the composite. The surface morphology of the composites was examined using a JEOL scanning electron microscope (SEM), which was equipped with an electron dispersive X-ray spectroscopy (EDS) system featuring a BRUKER nanodetector. This setup facilitated the analysis of the morphological features and elemental composition of the materials. Fourier transform infrared spectroscopy (FTIR) spectra were obtained with a PerkinElmer infrared spectrophotometer, providing insights into the functional groups present in the composite. For electrochemical experiments, the METHROM NOVA software was utilized, enabling precise control and analysis of the electrochemical measurements conducted in our study.
3. Results and Discussion
3.1. Morphological Characterization
The morphologies of the prepared composite PPy/rGO are summarized in Figure 2.


Field emission scanning electron microscopy (FESEM) images reveal the sponge-like porous structure of the composite. Due to strong interactions between PPy and rGO, including stacking, hydrogen bonding, van der Waals force, and physical force, rGO appears to be encircled by PPy [10, 21]. An abundance of pores on the electrode surface can improve the PPy/rGO electrode’s capacitance and charge–discharge rate. The PPy/rGO electrode’s specific capacitance should increase as a result of more active materials coming into contact with the electrolyte.
3.2. Structural and Surface Characterizations
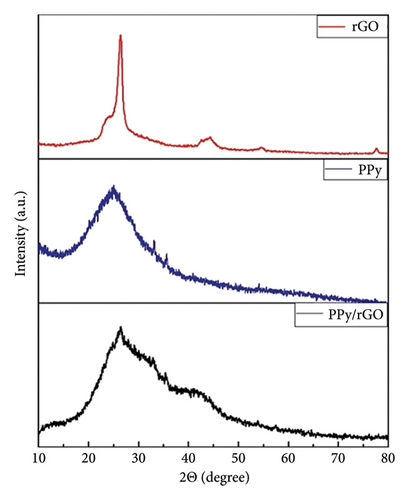
Figure 3 shows XRD patterns that represent the distinctive features of rGO, PPy, and the composite of PPy/rGO. In Figure 3, the discernible diffraction peak at theta = 26.33° is attributed to rGO. The interlayer spacing of rGO is measured at 3.38 Å. XRD of PPy shows a broad peak in the region 20°–30°, showing the amorphous nature of PPy. The XRD pattern for PPy/rGO reveals a distinct diffraction peak within the 20°–30° range [22]. This observation suggests the complete deposition of PPy onto the carbon matrix. However, the peak lacks sharpness, indicating the disruption of the regular stacking of graphene sheets. The characteristic broadness of this peak suggests that PPy exists in an amorphous form. The XRD analysis highlights the presence of rGO and the successful deposition of PPy on the carbon matrix in the PPy/rGO composite. The observed characteristics of the diffraction peaks provide insights into the structural features of these materials, with the amorphous nature of PPy being particularly notable in the XRD pattern [23].
3.3. Spectroscopic Characterization
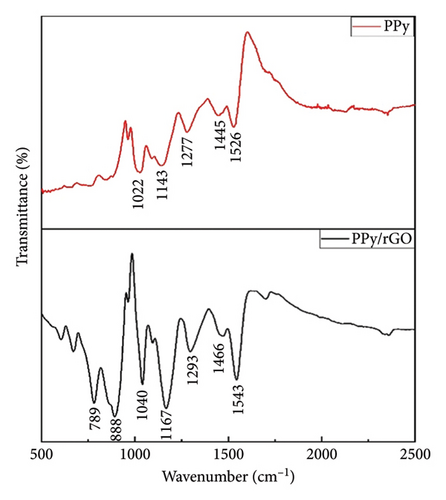
Figure 4 illustrates the FTIR spectrum of the synthesized PPy/rGO composite, with transmittance (%) plotted against wavenumber (cm−1). The spectrum reveals several prominent peaks characteristic of PPy, accompanied by subtle shifts that suggest potential π-π interactions between the rGO layers and the PPy rings. A peak observed at 1040 cm−1 is associated with C–H in-plane bending vibrations, which are indicative of the PPy framework. Another distinct peak at 1166 cm−1 corresponds to C–N stretching vibrations, confirming the successful polymerization of pyrrole. The band near 1293 cm−1 is attributed to C–H deformation within the polymer backbone, highlighting the structural integrity of the PPy. Additional peaks at 1466 and 1543 cm−1 represent the C=C stretching vibrations within the pyrrole rings, further validating the presence of the conjugated backbone typical of PPy. These vibrational modes are reflective of the inherent electronic properties of the polymer, which contribute to its potential for energy storage applications. The FTIR spectrum also shows characteristic peaks at 789 cm−1 and 888 cm−1, which are associated with bipolaron ring deformations and polaron symmetric C–H in-plane bending vibrations. These features provide evidence of charge carriers within the polymer matrix, underscoring the conductive nature of PPy in the composite. In the case of rGO, the spectrum reveals a broad band around 1620 cm−1, which is attributed to C=O stretching vibrations originating from residual oxygen groups within the rGO. This indicates that some oxygenated functionalities remain after the reduction process, likely enhancing the interaction between rGO and PPy and contributing to the composite’s structural stability. Additionally, a peak near 1050 cm−1 corresponds to C–O stretching vibrations, further indicating the presence of oxygen-containing groups in the rGO [4]. These vibrational modes confirm the successful integration of PPy and rGO in the composite, highlighting their strong interactions and the unique structural characteristics of the PPy/rGO material. This detailed FTIR analysis provides valuable insight into the chemical composition and bonding interactions within the composite, further supporting its potential for applications requiring enhanced electronic and structural properties [24–26].
3.4. Electrochemical Characteristics
3.4.1. CV
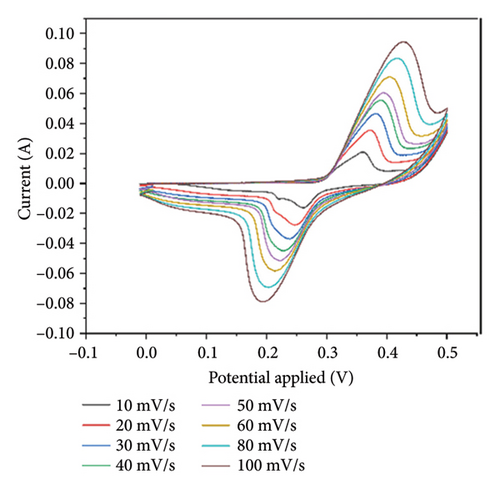
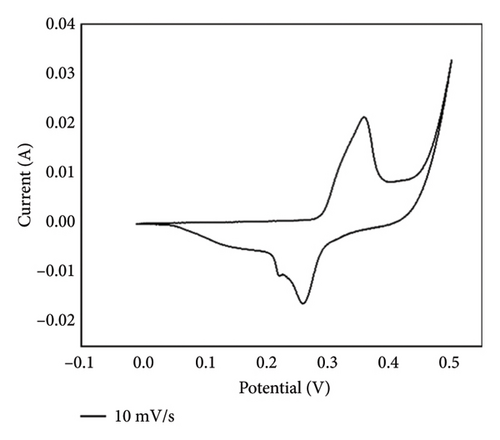
Cyclic voltammograms of PPy/rGO were recorded over a potential range of 0 V to 0.5 V at various scan rates (10, 20, 30, 40, 50, 60, 80, and 100 mV/s), as illustrated in Figure 5. The findings unveiled unique redox peaks, which are indicative of the electrochemical characteristics of the PPy/rGO composite. The CV curves exhibited well-defined and separated oxidation and reduction peaks at lower scan rates, indicating efficient charge storage and redox processes within the composite material. As the scan rate increased, the peak currents exhibited a corresponding rise, suggesting good electrochemical reversibility. The composite’s specific capacitance at a scan rate of 10 mV/s is 365.1 F/g. Specific capacitances for scan rates 20, 30, 40, 50, 60, 80, and 100 mV/s are 340.15, 306.6, 275, 240, 233.3, 225, and 210 F/g, respectively. In summary, the outcomes from CV underscore the appropriateness of PPy/rGO composites for electrochemical energy storage applications, showcasing their robust electrochemical performance and potential for use in supercapacitors and related devices. Further analysis and optimization can be pursued for tailored applications in energy storage systems [24, 27].
3.4.2. GCD
The GCD curves for the PPy/rGO composite at various current densities are displayed in Figure 6. Figure 6(a) demonstrates the similarities among the curves of different current densities, indicating the excellent capacitive performance of the electrode material. The values of specific capacitance corresponding to the current densities of 1, 2, 4, and 6 A/g are 367.5, 375, 370, and 345 F/g, respectively. Figure 6(b) shows the nearly stable result of specific capacitance for different current densities.
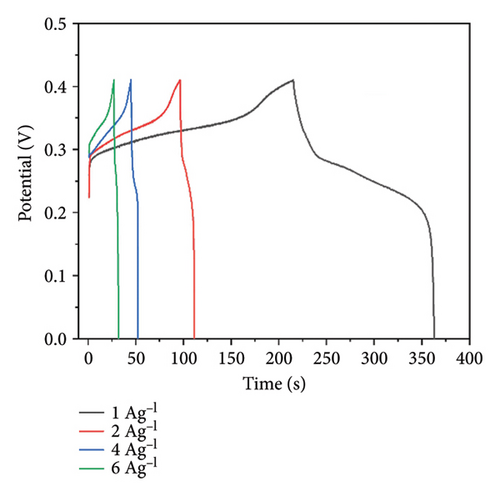

The energy density and power density values determined based on the specific capacitance of 375 F/g are 46.875 Wh/kg and 1140.20 W/kg, respectively.
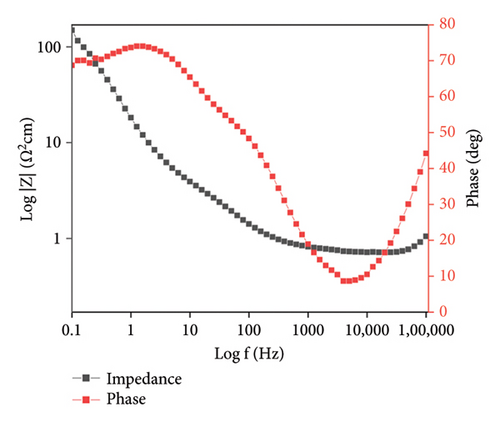
3.4.3. Electrochemical Impedance Spectroscopy (EIS)
An effective experimental way for determining a material’s impedance characteristics is EIS, which uses a low-amplitude alternating current (AC) signal. Using a range of frequencies to measure the system’s impedance, this method creates an impedance spectrum for the electrochemical material being investigated [28]. The equivalent circuit was formed by using ZSimp software.
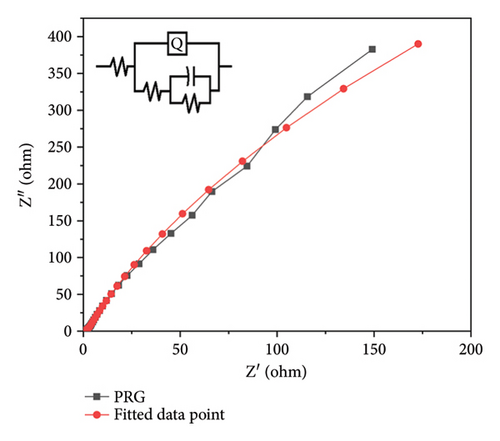
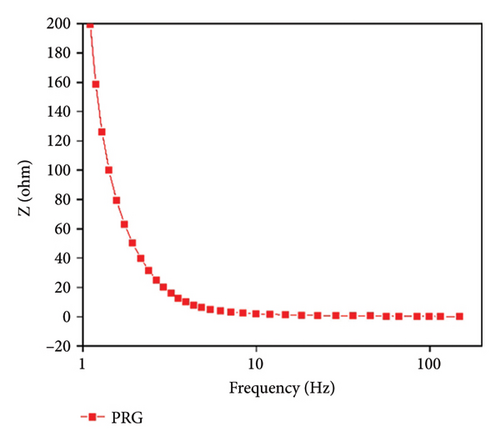
PPy/rGO was subjected to EIS in the frequency range of 105 to 10−1 in 3 M KOH solution. The Nyquist plot illustrated in Figure 7(a) displays a minor semicircle in the high-frequency range, indicating the presence of solution resistance and charge transfer resistance. Furthermore, a linear line observed in the high-frequency region indicates the presence of Warburg resistance [13]. Essentially, in Figure 7(b) the features observed in the impedance spectrum represent the material’s electrical response to varying frequency inputs. Figure 7(c) represents the Bode phase plot; in the low-frequency region, impedance is relatively high, representing the capacitive behavior of the material. In the higher frequency region, impedance is relatively low, showing the good conductivity of the material at higher frequencies. The material presents a higher resistance to the passage of electrical current at lower frequencies. This could be due to factors such as internal resistances, surface effects, or slower charge transfer processes within the material. Conversely, as the frequency of the applied signal rises, the impedance steadily decreases. This phenomenon often indicates an improved ability of the material to conduct electricity efficiently. The material becomes more responsive to the AC at these higher frequencies, allowing for easier movement of charges and a lower overall impedance [29]. Table 1 represents parameter values from fitted impedance equivalent circuits. Table 1 has the symbols “Q,” C, and “R” to stand for certain elements. R stands for the electrochemical system’s resistive elements, which may consist of resistance to solutions (Rs). The resistance connected to the transfer of charge across the electrode/electrolyte interface during a redox reaction is known as charge transfer resistance or Rct. Q (constant phase element [CPE]) helps to explain why capacitive behavior is not perfect. C represents the capacitive element. The second peak observed in the Bode plot’s phase angle curve at higher frequencies signifies the capacitive behavior of the PPy/rGO composite. As the phase angle approaches 45°, the material transitions into a capacitive-dominant state, indicating efficient charge storage and release. This peak highlights the material’s ability to maintain capacitive properties over a wide frequency range, crucial for supercapacitor applications. Additionally, the impedance behavior at higher frequencies suggests the influence of resistive components, such as internal and contact resistance, as the capacitive response diminishes at very high frequencies. The EIS results indicate that the series resistance (Rs) of the PPy/rGO composite is 15 Ω, representing the combined resistance of the electrolyte, electrodes, and contact points. Additionally, the Rct, corresponding to the Faradaic processes at the electrode/electrolyte interface, was found to be 130 Ω, indicating good charge transfer kinetics within the system. Table 2 compares PPy/rGO with previously reported composites.
| Sample | R | Q | R | C | R |
|---|---|---|---|---|---|
| PPy/rGO | 0.9013 | 0.003564 | 7.371 | 2.85E−06 | 1.13E+04 |
| % error | 6.931 | 12.52 | 5.45E+04 | 2.04E+04 | 598.4 |
| Electrode material | Galvanic charge–discharge/scan rate | Electrolyte | Specific capacitance (Fg−1) | Reference |
|---|---|---|---|---|
| PPy/GO/ZnO | 1 Ag−1 | 1 M Na2SO4 | 94.6 | [30] |
| CNT/PPy/MnO2 | 20 mVs−1 | 1 M Na2SO4 | 281 | [31] |
| PEDOT-GO/CNTs | 10 mVs−1 | 1 M KCl | 104 | [16] |
| PPy/GO/CNTs | 10 mVs−1 | 1 M KCl | 143.6 | [16] |
| PPy/rGO | 10 mVs−1 | 3 M KOH | 365.1 | Present work |
4. Conclusion
In conclusion, we introduce a straightforward, scalable, and environmentally friendly approach for fabricating binary composite electrodes composed of conductive PPy and rGO, tailored for supercapacitor applications. Formation of the composite was performed by using the chemical oxidative method, and the synthesis process allows for the uniform distribution of PPy within the rGO matrix, leading to a highly porous structure that improves the accessibility of electrolytes and enhances charge storage capacity. The controlled synthesis parameters can be adjusted to tailor the composite’s morphology, resulting in optimized electrochemical performance. Electrochemical characterizations showed that the binary PPy/rGO composite electrode delivers 365.1 F/g specific capacitance at a scan rate of 10 mV/s in CV measurement, while in galvanic charge–discharge analysis the specific capacitance was 375 Fg−1 for 2 Ag−1. These findings validate the potential of employing this straightforward synthesis approach to create composite electrode materials featuring a blend of reduced graphene and conductive polymers. This approach holds promise for designing and customizing the characteristics of advanced supercapacitors.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding is required.
Open Research
Data Availability Statement
The data will be available upon request.




