Molecular Diagnosis of Thyroid Nodules Using Next-Generation Sequencing in the Chinese Population
Abstract
Background: Fine-needle aspiration (FNA) cytology remains a challenge in the diagnosis of indeterminate thyroid nodules. Molecular testing can bridge the gap left by FNA cytology and improve the diagnostic accuracy of FNA.
Methods: 786 FNA samples and 40 formalin-fixed paraffin-embedded (FFPE) specimens from thyroid nodules were enrolled in next-generation sequencing (NGS) molecular testing, which included gene mutation and gene fusion analysis. The molecular diagnostic performance was assessed by analyzing sensitivity, specificity, accuracy, negative predictive value (NPV), and positive predictive value (PPV).
Results: Among 826 thyroid nodules, 409 were NGS-positive (49.52%), with a high prevalence of BRAF V600E (36.32%, 300/826) and RAS (9.32%, 77/826) mutations, a low prevalence of TERT promoter mutations (1.69%, 14/826), and gene fusions involving RET (1.82%, 15/826), NTRK3 (0.73%, 6/826), ALK (0.24%, 2/826), and PAX8-PPARG (0.12%, 1/826). With the analysis of genetic profiles in thyroid nodules, BRAF V600E, TERT mutations, and gene fusions were included in the 6-gene test panel. The overall diagnostic performance of the 6-gene test panel, including sensitivity, specificity, accuracy, NPV, and PPV, was 84.87%, 89.61%, 86.26%, 71.13%, and 95.15%, respectively. For thyroid nodules in Bethesda III, IV, and V, the diagnostic sensitivity, specificity, accuracy, NPV, and PPV of the panel were 85.71%, 88.89%, 86.36%, 61.54%, and 96.77%, respectively.
Conclusion: The results reveal that the 6-gene test panel as a “rule in” test in a clinical setting improves the accuracy of FNA cytology, potentially assisting in the diagnosis of the thyroid nodules with indeterminate FNA cytology.
1. Introduction
Thyroid cancer is the most common endocrine malignant tumor worldwide and is rapidly increasing with an estimated occurrence of 220,000 cases in China by 2020 [1]. The most recent global cancer data indicate a significantly higher incidence of thyroid cancer in women compared to men, with an average annual increase of approximately 20%. Thyroid cancer typically manifests in patients with thyroid nodules. High-quality thyroid ultrasonography (US) and fine-needle aspiration (FNA) biopsy are widely utilized as the primary and dependable diagnostic modalities for distinguishing benign nodules from tumors. However, approximately 25% of nodules cannot be definitively excluded as cancer by FNA, posing a potential dilemma for both patients and clinicians [2]. Similar to other malignancies, the emergence and advancement of thyroid cancer are accompanied by genetic mutations, such as RAS, BRAF, or TERT mutations, as well as RET and NTRK rearrangements [3, 4]. Consequently, the incorporation of molecular testing serves as an additional method to complement the limitations of FNA cytology and assists in the identification of malignant thyroid nodules. It is recommended by the guidelines of the Chinese Society of Clinical Oncology and the American Thyroid Association [2].
Multiple genetic mutations identified in thyroid cancer are receiving increasing attention, including BRAF, RAS, TERT, PIK3CA, AKT, PTEN, RET, TP53, and gene fusions of RET and NTRK [5, 6]. Notably, BRAF V600E mutations are the most prevalent and significant, accounting for 98∼99% of all the BRAF mutations, which occur in approximately 40∼70% of papillary thyroid cancer (PTC) and 30∼40% of anaplastic thyroid cancer, especially in Chinese patients [3, 7, 8]. A study conducted on a cohort of the Chinese population revealed that the frequency of the BRAF V600E mutation in PTC exceeds 75%, which is higher than the rates observed in Indonesian and French populations [9]. The prevalence of RAS mutations is second only to BRAF mutations in differentiated thyroid cancer. RAS mutations are found not only in malignant tumors but also in benign thyroid nodules [10, 11]. The TERT gene encoding the catalytic subunit of telomerase is closely associated with tumorigenesis and development, as its activation plays a significant role in these processes [12, 13]. RET fusion and NTRK fusion are the most common rearrangements observed in thyroid cancer [14, 15]. In cases of thyroid cancer, the predominant fusion events involve RET/PTC1 and RET/PTC3, which entail the fusion of the RET gene with the CCDC6 gene and the NCOA4 gene, respectively [16]. The presence of ETV6-NTRK3 is linked to the tumor size, metastasis, and patient prognosis [17, 18].
Molecular alterations in thyroid nodules, as described above, have been identified as useful diagnostic and prognostic markers for thyroid cancer. Large-scale studies have fully elucidated the genomic and pathological changes in thyroid cancer, particularly thyroid follicular carcinoma, in Western populations [19]. Multiple gene panels for thyroid FNA samples are available in the United States, including ThyroSeq V3, Afirma GSC, and ThyraMIR [20]. The reported data indicate differences in genetic mutations associated with thyroid cancer between Chinese and Western patients. For instance, the BRAF V600E mutation exhibits higher frequencies in the Chinese population [8, 21]. However, the prevalence of RAS mutations in PTC is lower in the Chinese population compared to the data from the TGCA study (1% vs. 13%) [22, 23]. There are limited studies on the mutation profiles in thyroid nodules specific to the Chinese population.
In the present study, the 22-gene mutation assay (AKT1, APC, ATM, BRAF, CTNNB1, EIF1AX, GNAS, HRAS, KRAS, NRAS, PIK3CA, PTEN, RET, TG, TP53, TSHR, TERT, TTN, as well as gene fusions involving NTRK1, NTRK3, ALK, PPARG, and RET) was utilized to detect gene mutations and fusions associated with thyroid cancer in a substantial cohort of 826 samples obtained from FNA samples and surgical formalin-fixed paraffin-embedded (FFPE) specimens. This study aimed to delineate the genetic landscape of thyroid nodules in China, providing deep insights into disease formation and helping to stratify patients according to their risk level for malignancy. Furthermore, it was intended to evaluate the diagnostic and screening performance of NGS assays for thyroid cancer in FNA samples, particularly in the case of indeterminate nodules and suspicious malignancy (SUSP) nodules.
2. Materials and Methods
2.1. Patients and Clinical Specimens
A total of 826 patients with thyroid nodules were enrolled in the study conducted from May 2022 to July 2023. Of these, 736 samples were collected from The First Hospital of Putian City, and another 90 samples were from Zhongshan Hospital. All participants in the study provided informed consent, and the research protocol was approved by the Institutional Ethics Committee of The First Hospital of Putian City (No 2022-032) and the Institutional Ethics Committee of Zhongshan Hospital (No B2022-609R). Written informed consent was obtained from each individual who participated in the study. The sample types used for molecular testing included 786 FNA samples and 40 FFPE specimens. The FNA procedure was conducted under ultrasound guidance by an experienced thyroid surgeon or endocrinologist/radiologist, following a standardized protocol of each institution.
2.2. Nucleic Acid Isolation
Genomic DNA/RNA of FNA samples was extracted using the nucleic acid extraction kit (Shanghai Singlera, China), and FFPE samples were processed using the FFPE extraction kit (CoWin Biotech, China). The elution volumes for DNA/RNA were 30 μL. The purity of DNA/RNA was assessed using a UV spectrophotometer (NanoDrop Technologies, Thermo Fisher Scientific Inc, USA), and quantification was conducted with Qubit 3.0 (Thermo Fisher Scientific Inc, USA).
2.3. Library Preparation
The sequencing libraries were prepared using the OncoAim thyroid cancer multigene assay kit (Singlera Genomics, Inc., Shanghai, China). RNA fusion was detected based on cDNA, and SNVs/indels were detected based on DNA. After PCR and purification, the barcodes and adapters specific to GENETRON S5 based on semiconductor sequencing technology (Genetron, China) were incorporated, and the quality of the prepared libraries was assessed using the LabChip GX Touch24 (PerkinElmer, USA). The sequencing depth is above 1000X. The sequencing procedure was conducted in accordance with the reagent protocols using the GENETRON S5.
2.4. Sequencing Data Analysis
Raw reads were filtered and mapped to the reference human genome (UCSC, hg19) using the GENETRON S5 platform. Subsequently, the sequencing quality was assessed. The sequencing data quality control was considered acceptable if the average coverage was ≥ 1000, the uniformity was ≥ 70%, and the coverage of the target regions was ≥ 95%. For DNA analysis, the Freebayes (v1.0.2) was used for SNVs/indels calling. The identified variants were annotated using the variant effect predictor (VEP, v90.9) in conjunction with the dbSNP (v152) and COSMIC (v91) databases. For RNA analysis, blast (v2.2.20) was used for gene fusion calling and analysis. This 22-gene mutation assay has the capability to detect single-nucleotide variants (SNVs) and insertions/deletions (Indels) in the AKT1, APC, ATM, BRAF, CTNNB1, EIF1AX, GNAS, HRAS, KRAS, NRAS, PIK3CA, PTEN, RET, TG, TP53, TSHR, and TTN, as well as variants in the TERT promoter region (C228T, chr5:1295228C>T, and C250T, chr5:1295250C>T), the NTRK1, NTRK3, ALK, PPARG, and RET fusion variants simultaneously.
2.5. Interpretation of Molecular Testing Results
The molecular testing results were categorized into two components: gene mutation and gene fusion. Positive identification was made for the BRAF V600E gene mutation with a frequency of ≥ 0.2%, and mutation-positive status was determined for other genes (AKT1, APC, ATM, BRAF, CTNNB1, EIF1AX, GNAS, HRAS, KRAS, NRAS, PIK3CA, PTEN, RET, TG, TP53, TSHR, TERT, and TTN) with a mutation frequency of ≥ 2%. The presence of NTRK1, NTRK3, ALK, PPARG, or RET gene fusion mutations with a frequency of CP100K ≥ 500 was determined to be positive (CP100K: average number of reads of fusion gene sequences per 100 K total number of reads).
2.6. Statistics
Patient age was compared using a t-test. Patient gender and nodule size were analyzed using chi-square test or Fisher’s exact test. p < 0.05 was considered statistically significant. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated with 95% Wilson confidence intervals. The area under the curve (AUC) was computed by MEDCALC v18.2.1 (MedCalc Software, Ostend, Belgium).
3. Results
3.1. Basic Information on Samples
This study involved the analysis of 786 FNA samples and 40 FFPE specimens through gene sequencing, comprising 684 samples obtained from female patients and 142 samples from male patients (Table 1). In this cohort, the ratio of females to males was 4.82, and the average age at diagnosis was 48.98 years (with a range of 15–82 years). The overall incidence is higher in women than in men, consistent with previous reports [24]. The patients’ ages were categorized into youth (14–47 years), middle-aged (48–63 years), and old (> 64 years). Cytologically, 198 (23.97%) patients were not obtained for FNA results, categorized as no cytology. 45 samples were confirmed as Bethesda I, primarily due to insufficient or inadequate tissue (Table 2). Additionally, 250 (30.27%) samples were diagnosed as benign, 187 (22.64%) were classified as indeterminate, 129 (15.62%) were deemed suspicious for malignancy, and 17 (2.06%) were identified as malignant. A total of 524 surgical histopathological specimens were not collected as a result of a decision without surgery, or surgical scheduling. 77 specimens were surgically determined to be benign, while 225 specimens were confirmed to be malignant tumors.
| Category | Number of samples (n = 826) | |
|---|---|---|
| Sex | Male | 142 |
| Female | 684 | |
| Age | 15∼47 | 360 |
| 48∼63 | 358 | |
| ≥ 64 | 108 | |
| Cytology | Number of samples (n = 826) | Surgical pathology | ||
|---|---|---|---|---|
| Bethesda | Benign | Malignant | Unknown | |
| I | 45 | 0 | 2 | 43 |
| II | 250 | 4 | 3 | 243 |
| III | 157 | 15 | 29 | 113 |
| IV | 30 | 3 | 1 | 26 |
| V | 129 | 0 | 40 | 89 |
| VI | 17 | 0 | 7 | 10 |
| No cytology | 198 | 55 | 143 | 0 |
3.2. The Landscape of Molecular Alterations in NGS Results
The 22-gene mutation assay was performed to investigate the genetic alterations in the thyroid nodules. Molecular testing for RNA and DNA was conducted simultaneously on all 826 samples that contained a sufficient amount of both DNA and RNA. The results of the testing indicated that 417 samples were categorized as negative, while 409 samples exhibited genetic variants within the detectable range, resulting in a positive rate of 49.52% (Figure 1 and Tables 3 and 4). The NGS-positive patients were significantly younger than the NGS-negative patients (47.56 vs. 50.37 years, p < 0.01). No significant relationship was observed with respect to gender (p = 0.770) (Table 3). The most prevalent mutation observed was BRAF V600E, with 300 (73.35%) samples classified as positive, and two samples carried the rare BRAF K601E mutation. The BRAF V600E-positive patients were also significantly younger than the BRAF V600E-negative patients (46.95 vs. 50.13 years, p < 0.001). Similarly, no significant relationship was observed regarding gender (p = 0.785) (Table 3). RAS mutations were the second most common mutations, with a prevalence of 18.83% (77/409). NRAS mutations were detected in 53 cases, occupying the most prevalence of RAS mutations, followed by HRAS mutations (n = 16), and the least by KRAS mutations (n = 8) (Table 4). Moreover, RAS mutations were found in patients with malignant tumors (n = 15) and benign tumors (n = 9). 14 samples exhibited TERT mutations (1295228C>T, 1295250C>T), with a prevalence of 3.42%. The TERT promoter mutation-positive patients tended to be older than the mutation-negative patients (62.07 vs. 48.75 years, p < 0.01), with no significant relationship observed concerning gender (p = 0.075) (Table 3). Gene fusions emerged as another common type of mutation, with 15 (3.67%) samples confirmed to exhibit RET/PTC fusion (CCDC6-RET and NCOA4-RET), followed by NTRK3 fusion (ETV6-NTRK3 and EML4-NTRK3) with 6 (1.47%) samples. Some rare gene fusions were found in this cohort, including STRN-ALK (n = 2) and PAX8-PPARG (n = 1). Additionally, the gene fusion-positive patients tended to be younger than the gene fusion-negative patients (43.79 vs. 49.13 years, p < 0.05), with no significant relationship noted with respect to gender (p = 0.784) (Table 3). No statistically significant differences were observed in mean nodule size between the nodules with genetic alterations and those without, including BRAF V600E mutations, TERT promoter mutations, and gene fusions (Table S1). Four samples carried PTEN mutations, with two having PTEN mutations only and two having BRAF V600E and PTEN mutations both. Four samples with TSHR mutations (two with TSHR only and two with BRAF V600E/TSHR) were confirmed to be one malignant tumor, one benign tumor, and two lack of surgical pathology. TTN S3536N mutations were detected in three patients, but no surgical pathology was performed. Two samples with PIK3CA mutations (one with PIK3CA only and one with BRAF V600E/PIK3CA) were confirmed to be one malignant tumor and one benign tumor. Two samples carried only EIF1AX mutations.
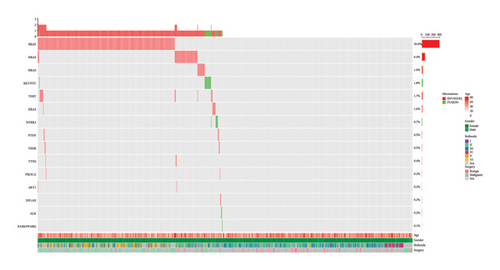
| Genetic alterations | p value | BRAF V600E | p value | TERT promoter mutations | p value | Gene fusions | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |||||
| Number of patients | 409 (49.52%) | 417 (50.48%) | 300 (36.32%) | 526 (63.68%) | 14 (1.69%) | 812 (98.31%) | 24 (2.91%) | 802 (97.09%) | ||||
| Gender | 0.770 | 0.785 | 0.075 | 0.784 | ||||||||
| Female | 341 | 343 | 247 | 437 | 9 | 675 | 21 | 663 | ||||
| Male | 68 | 74 | 53 | 89 | 5 | 137 | 3 | 139 | ||||
| Age (years) | < 0.01 | < 0.001 | < 0.01 | < 0.05 | ||||||||
| Mean ± SD | 47.56 ± 12.28 | 50.37 ± 12.78 | 46.95 ± 11.99 | 50.13 ± 12.81 | 62.07 ± 12.40 | 48.75 ± 12.50 | 43.79 ± 10.20 | 49.13 ± 12.65 | ||||
| 95% CI | 46.37 − 48.75 | 49.14 − 51.60 | 45.59 − 48.31 | 49.03 − 51.23 | 55.57 − 68.57 | 47.89 − 49.61 | 39.71 − 47.87 | 48.25 − 50.01 | ||||
| Surgical pathology | Cytology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Malignant | Benign | Unknown | I | II | III | IV | V | VI | No cytology | |
| BRAF V600E | 281 | 147 | 6 | 128 | 5 | 30 | 49 | 0 | 95 | 11 | 91 |
| BRAF V600E/TERT C228T | 6 | 4 | / | 2 | / | 1 | / | / | 1 | 1 | 3 |
| BRAF V600E/TERT C250T | 2 | 2 | / | / | / | / | 1 | / | / | / | 1 |
| BRAF V600E/CCDC6-RET | 1 | / | / | 1 | / | / | / | / | 1 | / | / |
| BRAF V600E/NRAS Q61R | 1 | 1 | / | / | / | / | 1 | / | / | / | / |
| BRAF V600E/NRAS Q61R/AKT1 | 1 | 1 | / | / | / | / | 1 | / | / | / | / |
| BRAF V600E/NRAS Q61K | 1 | / | / | 1 | / | / | / | / | / | 1 | / |
| BRAF V600E/TTN S3536N | 1 | / | / | 1 | / | / | / | / | / | 1 | / |
| BRAFV600E/PIK3CA H1047R | 1 | 1 | / | / | / | / | / | / | / | 1 | / |
| BRAF V600E/PTEN 802 2AD>T | 2 | 1 | / | 1 | / | / | / | / | / | 1 | 1 |
| BRAF V600E/TSHR R450H | 2 | 1 | / | 1 | / | / | / | / | 1 | / | 1 |
| BRAF V600E/KRAS GQ61GK | 1 | / | / | 1 | / | / | / | / | 1 | / | / |
| TERT C228T | 2 | 1 | / | 1 | / | 1 | / | / | / | / | 1 |
| TERT C228T/ETV6-NTRK3 | 1 | / | 1 | / | / | / | / | / | / | / | 1 |
| CCDC6-RET | 12 | 6 | / | 6 | / | 2 | 4 | / | 3 | / | 3 |
| NCOA4-RET | 2 | 1 | 1 | / | / | / | / | / | / | / | 2 |
| ETV6-NTRK3 | 4 | 1 | / | 3 | 1 | / | 2 | / | / | / | 1 |
| TERT C228T/NRAS Q61R | 1 | 1 | / | / | / | / | / | / | / | / | 1 |
| TERT C228T/TERT C250T/NRAS Q61R | 1 | 1 | / | / | / | / | / | / | // | 1 | |
| TERT C228T/ HRAS Q61K | 1 | / | / | 1 | / | / | / | 1 | / | / | / |
| NRAS Q61R | 40 | 3 | 5 | 32 | 2 | 19 | 6 | 1 | 6 | / | 6 |
| NRAS Q61K | 7 | / | / | 7 | / | 7 | / | / | / | / | / |
| TTN S3536N | 2 | / | / | 2 | / | 1 | / | / | 1 | / | / |
| PIK3CA H1047R | 1 | / | 1 | / | / | / | / | / | / | / | 1 |
| BRAF K601E | 2 | / | 1 | 1 | / | / | 1 | / | / | / | 1 |
| PTEN 802-2A>T | 2 | / | / | 2 | / | / | 2 | / | / | / | / |
| HRAS Q61R | 12 | 5 | 3 | 4 | / | 2 | 3 | / | 1 | / | 6 |
| HRAS Q61K | 3 | / | 1 | 2 | / | 2 | / | / | / | / | 1 |
| KRAS Q61R | 4 | 3 | / | 1 | / | 1 | 1 | / | 1 | / | 1 |
| KRAS G12V | 1 | / | / | 1 | / | 1 | / | / | / | / | / |
| TSHR R450H | 2 | 1 | 1 | / | 1 | / | / | / | / | 1 | |
| NRAS Q61R/AKT1 | 1 | / | / | 1 | / | / | 1 | / | / | / | / |
| EIF1AX 338-1G>C | 1 | / | / | 1 | / | 1 | / | / | / | / | / |
| EIF1AX G8R | 1 | / | / | 1 | / | 1 | / | / | / | / | / |
| KRAS GQ61GK | 2 | / | / | 2 | / | 2 | / | / | / | / | / |
| STRN-ALK | 2 | 2 | / | / | / | / | / | / | / | / | 2 |
| EML4-NTRK3 | 1 | 1 | / | / | / | / | / | / | / | / | 1 |
| PAX8/PPARG | 1 | / | / | 1 | / | / | / | 1 | / | / | / |
- Note: No distinction in sample types (FNA samples or FFPE samples).
3.3. Genetic Features in Different Subtypes of Thyroid Carcinomas
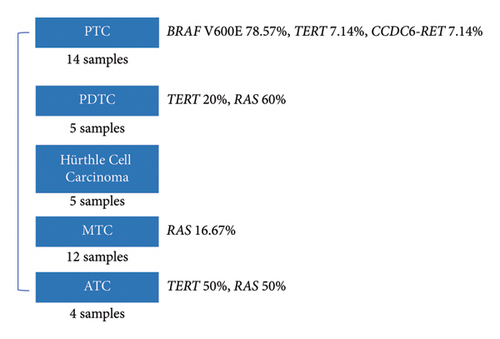
40 FFPE samples included 14 identified as papillary thyroid carcinomas (PTCs), 5 poorly differentiated thyroid carcinomas (PDTCs), 5 Hurthle cell tumors, 4 anaplastic thyroid carcinomas (ATCs), and 12 medullary thyroid carcinomas (MTCs). The results of the NGS testing indicated that 20 samples were categorized as positive. The detailed sequencing results of the whole cohort are described in supporting information (Table S2). Different gene mutations were observed in different subtypes of thyroid carcinomas (Figure 2). PTC is the most common subtype, accounting for approximately 35% of the FFPE cases. 11 samples exhibited BRAF V600E, with a prevalence of 78.57% in PTC. Mutations were not detected in 2 PTC samples. The most prevalent mutation observed in PDTC was RAS, with a prevalence of 60%. Moreover, one carried TERT C228T, and two PDTCs were categorized as negative. However, genetic alterations were not detected in 5 Hurthle cell tumors. Another subtype at a low mutation frequency was MTC, with only 2 samples carrying RAS mutations. In 4 ATCs, the prevalence of TERT mutation and RAS mutation was 50%.
3.4. Samples With Two or More Mutations
The NGS profiles of thyroid nodules indicated that the majority of NGS-positive cases exhibited single-gene mutations (n = 385), whereas a few cases had multiple genetic alterations (n = 24). 24 samples exhibited the presence of two or three mutations. Eight samples were found to carry the BRAF V600E and TERT co-mutations. 6 cases exhibited the BRAF V600E and TERT C228T co-mutations, while two cases showed the BRAF V600E and TERT C250T co-mutations. Six of the samples were confirmed to be malignant tumors through surgical pathology and were specifically identified as PTC (Table 5). The other two cases were categorized by FNA cytology as Bethesda II and V, respectively. However, they lack the pathological results and follow-up. Patient FNA-0002 exhibited the BRAF V600E mutation combined with CCDC6-RET fusion and was categorized as Bethesda V based on FNA cytology. She also lacked the results of surgical pathology. Patient FNA-0480 also presented with the TERT C228T mutation and ETV6-NTRK3 fusion, as confirmed by surgical pathology indicating benign conditions. Three samples were identified as carrying BRAF V600E and RAS mutations (two with BRAF V600E/NRAS and one with BRAF V600E/KRAS), and of them, patient FNA-0692, with BRAF V600E and NRAS Q61R, was confirmed as PTC by surgical pathology. In addition to the BRAF V600E mutation, TERT mutations were the second most common among multiple mutations. Two samples carried the TERT C228T and RAS mutations, and one (FFPE-026) was confirmed as PDTC with the invasion of nerve bundles by histopathology, and the other was categorized as Bethesda IV by cytology. According to sequencing analysis, AKT1 mutations were significantly associated with the presence of NRAS mutations (one with BRAF V600E/NRAS Q61R/AKT1 and one with NRAS Q61R/AKT1). Patient FNA-0330, who has three mutations (BRAF V600E/NRAS Q61R/AKT1), was confirmed as follicular thyroid carcinoma (FTC) with lymph node metastasis. Patient FFPE-035 with three mutations (TERT C228T/TERT C250T/NRAS Q61R) was confirmed as FTC.
| Sample | Sex | Age | Bethesda | Pathology | Molecular testing |
|---|---|---|---|---|---|
| FNA-0042 | Male | 74 | / | PTC | BRAF V600E/TERT C228T |
| FNA-0297 | Female | 57 | / | PTC | BRAF V600E/TERT C228T |
| FNA-0318 | Female | 72 | II | / | BRAF V600E/TERT C228T |
| FNA-0677 | Female | 38 | V | / | BRAF V600E/TERT C228T |
| FNA-0771 | Male | 60 | VI | PTC | BRAF V600E/TERT C228T |
| FFPE-002 | Female | 56 | / | PTC | BRAF V600E/TERT C228T |
| FNA-0036 | Female | 69 | / | PTC | BRAF V600E/TERT C250T |
| FNA-0068 | Female | 53 | III | PTC | BRAF V600E/TERT C250T |
| FNA-0002 | Female | 46 | V | / | BRAF V600E/CCDC6 |
| FNA-0692 | Female | 50 | III | PTC | BRAF V600E/NRAS Q61R |
| FNA-0807 | Female | 65 | VI | / | BRAF V600E/NRAS Q61K |
| FNA-0755 | Female | 29 | VI | / | BRAF V600E/TTN S3536N |
| FNA-0118 | Female | 37 | VI | PTC | BRAF V600E/PIK3CA H1047R |
| FNA-0420 | Female | 52 | VI | / | BRAF V600E/PTEN 802-2A>T |
| FNA-0830 | Female | 55 | / | PTC | BRAF V600E/PTEN 802-2A>T |
| FNA-0084 | Male | 36 | / | PTC | BRAF V600E/TSHR R450H |
| FNA-0184 | Female | 51 | V | / | BRAF V600E/TSHR R450H |
| FNA-0855 | Male | 31 | V | / | BRAF V600E/KRAS GQ61GK |
| FNA-0480 | Female | 56 | / | Benign | TERT C228T/ETV6-NTRK3 |
| FFPE-026 | Female | 82 | / | PDTC | TERT C228T/NRAS Q61R |
| FNA-0774 | Male | 53 | IV | / | TERT C228T/HRAS Q61K |
| FNA-0198 | Female | 45 | III | / | NRAS Q61R/AKT1 |
| FFPE-035 | Male | 81 | / | ATC | TERT C228T/TERT C250T/NRAS Q61R |
| FNA-0330 | Female | 52 | III | FTC | BRAF V600E/NRAS Q61R/AKT1 |
- Abbreviations: ATC, anaplastic thyroid cancer; FTC, follicular thyroid carcinoma; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma.
In addition to the samples with a lack of surgical pathology, 90% of patients with two or three mutations developed malignant thyroid cancer. Hence, an increased number of mutations may indicate a greater probability of malignancy. However, the sample size was insufficient to facilitate a comprehensive analysis and to establish more definitive conclusions.
3.5. Diagnostic Performance of the NGS Testing for Thyroid Nodules
The diagnostic performance of the 22-gene mutation assay was calculated. Among the 786 FNA samples, the positive rates of the 22-gene mutation assay were 17.78% (8/45) in nondiagnostic cytology (Bethesda I), 29.20% (73/250) in benign cytology (Bethesda II), 40.11% (75/187) in indeterminate cytology (Bethesda III & IV), 85.27% (110/129) in SUSP cytology (Bethesda V), and 94.12% (16/17) in malignant cytology (Bethesda VI). The positive rate of the 22-gene mutation assay was found to increase in correlation with the risk of cytologic malignancy. Surgical pathology was taken as the gold standard for diagnosing malignancy. In this cohort, 262 FNA samples underwent surgical resections, of which 185 were histologically confirmed as malignant, and 77 samples were identified as benign (Table 6). The diagnostic results of the 22-gene mutation assay revealed a positive rate of 69.85% (183/262) and a negative rate of 30.15% (79/262). The diagnostic performance of 22-gene mutation assay in thyroid nodules revealed 88.11% sensitivity, 74.03% specificity, 72.15% NPV, 89.07% PPV, and an overall accuracy of 83.97% (Table 6). Limitations of several gene mutations for diagnosing thyroid nodules were identified, including the observation of RAS mutations in benign thyroid nodules. With the analysis of sequencing results, 147 FNA samples with BRAF V600E mutation were confirmed with malignant tumors by surgical pathology and 6 samples were benign. Five FNA samples (83.33%, 5/6) with TERT mutations were identified as malignant tumors, while one was benign. 10 FNA samples (83.33%, 10/12) with gene fusions were confirmed with malignant tumors, while two were benign nodules. Therefore, the targets widely recognized and recommended by scholars at home and abroad were selected to compose a 6-gene test panel for thyroid nodule diagnosis, including BRAF V600E mutation, TERT mutation, and gene fusions (CCDC6-RET, NCOA4-RET, ETV6-NTRK3, EML4-NTRK3, STRN-ALK, and PAX8/PPARG). The diagnostic performance of the 6-gene test panel in detecting thyroid nodules revealed a sensitivity of 84.87%, specificity of 89.61%, NPV of 71.13%, PPV of 95.15%, and an overall accuracy of 86.26% (Table 6).
| Surgical pathology | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) | ||
|---|---|---|---|---|---|---|---|
| Malignant | Benign | ||||||
| 22-gene positive | 163 | 20 | 88.11 | 74.03 | 83.97 | 89.07 | 72.15 |
| 22-gene negative | 22 | 57 | |||||
| 6-gene positive | 157 | 8 | 84.87 | 89.61 | 86.26 | 95.15 | 71.13 |
| 6-gene negative | 28 | 69 | |||||
- Note: 6-gene test panel includes BRAF V600E mutation, TERT mutation, and gene fusions.
The major objective of this molecular testing was to distinguish histopathological malignant nodules from cytologically indeterminate nodules, which causes a dilemma in clinical management. In this cohort, the prevalence of indeterminate nodules (Bethesda III, IV) was found to be 23.79% (187/786), of which only 48 samples underwent surgical resections. Upon further analysis of the surgical pathology results in Bethesda V, it was found that only 31.01% (40/129) of patients opted for surgery, with a high malignant detection rate of 100%. Consequently, there was a high demand for molecular testing results for samples categorized as suspicious for malignancy (Bethesda V) and indeterminate nodules, to assist patients in making informed decisions regarding subsequent treatment. When relying on the presence of the 6-gene test panel, 26 negative samples and 62 positive samples were detected (Table 7). The performance of the 6-gene test panel in diagnosing thyroid nodules (Bethesda III, IV, and V) demonstrated a sensitivity of 85.71%, specificity of 88.89%, NPV of 61.54%, PPV of 96.77%, and an accuracy of 86.36%.
| Surgical pathology | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) | ||
|---|---|---|---|---|---|---|---|
| Malignant | Benign | ||||||
| 6-gene positive | 60 | 2 | 85.71 | 88.89 | 86.36 | 96.77 | 61.54 |
| 6-gene negative | 10 | 16 | |||||
4. Discussion
In this study, the 22-gene mutation assay demonstrated the ability to detect gene mutations and gene fusions with minimal amounts of DNA and RNA, thereby contributing to the diagnostic process of thyroid cancer. Our results suggested that genetic alterations, including BRAF V600E and gene fusions, were associated with younger age, while TERT promoter mutations were related to older age. There was no significant association between gender or nodule size and gene mutations. This finding is consistent with the results of Yang et al. regarding the prevalence of TERT promoter mutations and their clinicopathological associations in 2092 Korean patients [25]. Chen et al. reported that no significant correlation was observed between average nodule size and BRAF V600E mutations [26]. However, some reported literature correlating mutations with gender or age were conflict with our findings. For instance, Sun et al. demonstrated that BRAF V600E mutations were associated with older age (p < 0.001) [27]. This discrepancy may arise because our findings are based on a population of thyroid nodules. Additionally, a meta-analysis reported an association between the BRAF mutation and male gender [28].
In this study, the frequency of the BRAF V600E mutation in malignant thyroid nodules was 70.22% (158/225), which was consistent with the results reported in the Chinese population (63.6∼87.7%) and higher than that in the Western population (30∼70%) [8, 29, 30]. Six cases with BRAF mutations were confirmed as benign nodules, possibly because the patients were in the early stages of the disease. A prospective longitudinal study has reported that 39 patients with BRAF mutations confirmed as benign nodules at the beginning were all identified as malignant after 3 years of follow-up [31]. This demonstrates that the BRAF gene can predict the emergence of malignancy in benign thyroid nodules with high specificity. The high prevalence of BRAF V600E in the Chinese population may be attributed to abnormal iodine intake in the daily diet. An epidemiological study in China has demonstrated that the frequency of BRAF V600E mutations is higher among individuals with high iodine intake compared to those with normal iodine intake [32]. This suggests that iodine intake could be a risk factor for the occurrence of BRAF V600E mutations and the development of PTC. The population in this study primarily resides along the southeast coast of China, where iodine intake is relatively high, potentially contributing to the increased frequency of BRAF V600E mutations. A similar study conducted in Korea found that both low and excessive iodine intake may serve as significant risk factors for the development of BRAF mutations in the thyroid, thereby posing risks for the development of PTC in iodine-adequate areas [33]. In the context of iodine deficiency, there is an accelerated proliferation of follicular cells in response to elevated serum TSH levels, rendering them more susceptible to genetic alterations [34, 35]. Furthermore, retrospective studies have demonstrated that iodine intake may not have an impact on BRAF mutations in the thyroid and thus is not considered a risk factor for the development of PTC [36–38]. Consequently, further exploration is needed to understand the molecular mechanisms underlying the varying prevalence of BRAF V600E mutations among different populations.
The overall incidence of TERT mutations in this study was only 1.69% (14/826), which is lower than the rates reported in previous studies [21]. Eight (57.14%) patients exhibited concurrent BRAF V600E and TERT mutations. A similar association was observed in Chinese PTC patients, with a higher prevalence (94.7%) of coexisting mutations [27]. A meta-study reported that patients with coexisting mutations exhibit more clinical aggressiveness and have a poorer prognosis compared to those with BRAF or TERT mutations alone [39]. The potential mechanism of the synergistic effect of BRAF V600E and TERT promoter mutations on PTC invasion and prognosis may be attributed to the upregulation of several ETS transcription factors induced by BRAF, leading to increased TERT expression [40].
RAS mutations (NRAS, HRAS, and KRAS) with a prevalence of 9.32% (77/826) were the second most common mutations in this study, followed by BRAF V600E. Notably, RAS gene mutations have limited diagnostic value, as they can be found in adenomas, low-grade malignancies, and highly malignant tumors. Medici et al. addressed that five cases of RAS-positive thyroid nodules were observed with no significant nodule growth or other suspicious sonographic features during the mean 5-year follow-up period [10]. A similar phenomenon of some other gene mutations has been noted in recent literature. For instance, with an average follow-up period of 1.77 years, it is found that thyroid nodules with PTEN mutation are mostly benign and unlikely to exhibit rapid growth [41]. In addition, TSHR, TTN, PIK3CA, and EIF1AX mutations in thyroid nodules categorized as indeterminate are uncommon and are typically verified as benign [42].
Consistent with the previous report, the prevalence of RET fusion in thyroid cancer is 3.11% (7/225), while the occurrence of NTRK3 fusion in thyroid cancer is infrequent, with a frequency of 0.95% (2/225) in our study. The RET fusion has been reported as the most prevalent mutation in pediatric PTC, exhibiting a significant difference from the mutation profile observed in adult cases [43]. The fusion of ETV6-NTRK3 has been documented to have a strong correlation with the prognosis and recurrence of thyroid tumor [44]. Recently, testing for NTRK fusion genes has gained increasing attention because of their potential for therapeutic targeting. Tumors with NTRK fusion genes have shown sensitivity to TRK inhibitors, such as larotrectinib and entrectinib, which have demonstrated good tolerability and effectiveness [18].
Considering the diagnostic value of genetic mutations, BRAF V600E, TERT, and gene fusions have been incorporated into the 6-gene test panel to assist in the diagnosis of benign and malignant nodules. Compared to the 22-gene mutation assay analysis, the diagnostic performance of the 6-gene test panel demonstrated superior diagnostic value with higher specificity (from 74.03% to 89.61%), PPV (from 89.07% to 95.15%), and accuracy (from 83.97% to 86.26%) (Table 6). In this study, 23.79% (187/786) FNA samples were categorized as indeterminate, and only 25.67% (48/187) of the patients underwent surgical treatment, of which 62.50% (30/48) were categorized as malignant based on surgical pathology. A significant proportion of patients (about 74.33%) with indeterminate nodules choose not to undergo surgery. Han et al. reported that an 18-gene panel used in the evaluation of indeterminate thyroid nodules had a sensitivity of 76.2% and specificity of 66.7% for diagnostic performance [21]. In this cohort, the diagnostic performance of the 6-gene test panel for indeterminate thyroid nodules was better than what is reported in the literature. The sensitivity, specificity, NPV, PPV, and accuracy of the panel were 83.33%, 88.89%, 76.19%, 92.59%, and 85.42%, respectively (Table S3). Compared to the DNA-RNA classifier (sensitivity of 88.4%, specificity of 53.8%, PPV of 84.4%, and NPV of 61.5%), the sensitivity, specificity, PPV, and NPV of the 6-gene test panel were higher [45]. Nevertheless, the PPV and specificity of the panel were higher, and the NPV was lower than those of commercial ThyroSeq v3 (sensitivity of 96.9%, specificity of 35.7%, PPV of 63.3%, and NPV of 90.9%) [46]. The discrepancy in the diagnostic performance may be due to differences in usage scenarios. ThyroSeq v3 was used to rule out malignancy in indeterminate thyroid nodules due to the high sensitivity and NPV [47]. More importantly, with the higher PPV and the specificity, the 6-gene test panel as “rule in” tests was used to identify malignancy in indeterminate nodules and aid in making decision for surgery.
According to the latest Bethesda Thyroid Cytopathology Reporting System, samples in Bethesda V are also recommended for molecular testing. For the samples in Bethesda III, IV, and V, the diagnostic performance of the 6-gene test panel exhibited better than the 22-gene mutation assay analysis in terms of specificity (increasing from 77.78% to 88.89%) and PPV (increasing from 94.12% to 96.77%) (Table S4). Approximately 155 patients in Bethesda III, IV, and V will require surgery based on diagnostic results and the PPV of the 6-gene test panel, which is higher than the number that have undergone surgery. This is the value of the “rule in” test. The 6-gene test panel showed an acceptable ability to discriminate malignant among thyroid nodules, with an AUC of 0.87. Reducing the number of targets to test could effectively decrease DNA/RNA amount and sequencing data size required, thereby lowering the cost of NGS testing and improving diagnostic efficiency. Moreover, minimization of the testing could even facilitate the development of other detection methods such as qPCR assays, which may be more flexible and advantageous in specific scenarios considering health economics, report turnaround time (TAT), and other requirements.
Twenty two NGS-negative cases in FNA samples were subsequently diagnosed as malignant cancer after surgery. The disparity in results between NGS testing and surgical pathology may be attributed to sampling experience. If the sampling area is inaccurate or if too few tumor cells are collected, particularly in the case of tiny thyroid nodules, the accuracy of the NGS results might be affected. In a clinical setting, the selection of surgery based on ultrasound characteristics, FNA cytology, clinical symptoms, and patient willingness is not sufficient. Molecular testing may provide valuable assistance in the decision-making process.
However, there are certain limitations to this study. The insufficient number of samples, particularly for indeterminate nodules with both cytologic and surgical pathological results, as well as the lack of follow-up data for some NGS-positive samples, led to bias in evaluating the diagnostic value of NGS testing. The thyroid cancer FNA samples included in this study primarily consist of PTC, while rare subtypes such as FTC, PDTC, MTC and ATC are absent. Additionally, there are deficiencies in the collection of clinical information regarding the samples, such as iodine intake. These limitations hinder the ability to conduct in-depth research on the factors influencing the development of thyroid cancer and to determine whether these factors are associated with genetic mutations.
In the future, we will continue to follow these patients for 3∼5 years, monitor the prognosis, and update the data to analyze the relationship between gene mutations and the development and prognosis of thyroid cancer. Currently, the samples are collected from the southeastern coastal regions, and we plan to conduct multicenter clinical trials in the future that will encompass different regions and include a broader range of thyroid cancer subtypes. This approach will allow for a more comprehensive evaluation of the performance of the NGS panel. Throughout this process, promising novel molecular markers, including gene mutations, gene fusions, methylation, etc., may be identified, which could enhance the sensitivity and specificity of diagnosis. Ultimately, the research aims to provide clinical practice with valuable tools for the entire continuum of care, from diagnosis and treatment to monitoring and prognosis.
5. Conclusion
In summary, a validated molecular test based on a comprehensive panel of DNA mutations and RNA fusions is utilized to distinguish histopathological malignant nodules from cytologically indeterminate nodules. The 22-gene mutation assay showed a genetic profile of thyroid nodules in the Chinese populations with a high frequency of BRAF and RAS mutations. The results of the 6-gene test panel (BRAF V600E, TERT mutations, and gene fusions) demonstrated excellent diagnostic performance with high specificity and PPV in diagnosing thyroid nodules. For the diagnosis of the indeterminate nodules and SUSP nodules, the 6-gene test panel as a “rule in” test showed better clinical value with a higher PPV. Nevertheless, additional large-scale validation, particularly in prospective cohorts, is necessary to enhance the accuracy of diagnosing and treating thyroid nodules.
Conflicts of Interest
Hui Chen, Wei Liu, Yan Chen, Zhengzeng Jiang, Hongfeng Zhang, and Yuan Ji declare no conflicts of interest. Yuanyuan Ren, Jiajia Wu, Rui Liu, and Min Zhu are employed by Singlera Genomics (Shanghai) Ltd.
Author Contributions
Hui Chen: resources, funding acquisition, and writing – original draft. Wei Liu: formal analysis and writing – original draft. Yan Chen: visualization and writing – original draft. Zhengzeng Jiang: investigation and software. Yuanyuan Ren: investigation and data curation. Jiajia Wu: investigation. Rui Liu: writing – reviewing and Editing. Min Zhu: conceptualization and writing – reviewing and editing. Hongfeng Zhang: supervision and writing – reviewing and editing. Yuan Ji: resources, supervision, and writing – reviewing and editing. Hui Chen, Wei Liu, and Yan Chen contributed equally to this work.
Funding
This work was supported by grants from the Science Foundation of Putian University (2022094).
Acknowledgments
We gratefully acknowledge all the participants in this study.
Supporting Information
Table S1. Relationship between genetic mutations with nodule size.
Table S2. Distribution of gene alterations in the FFPE samples.
Table S3. Diagnostic performance of the 6-gene test panel in Bethesda III and IV (indeterminate thyroid nodules).
Table S4. Diagnostic performance of 22-gene mutation assay in Bethesda III, IV, and V.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.