Exploring Prognostic Markers for Patients With COVID-19 in a Low-Resource Setting: A Cross-Sectional Study
Abstract
Aim: Triaging patients based on prognostic biomarkers may contribute to better management of at-risk patients in resource-constrained settings. This study aimed to explore readily available and cost-effective predictors of mortality among COVID-19 patients.
Methods: This cross-sectional study, conducted across multiple centers, involved patients with COVID-19 admitted to four hospitals in Bangladesh. The analysis encompassed demographic information, clinical features, laboratory findings, and in-hospital outcomes. Logistic regression was utilized to identify factors contributing to mortality risk.
Results: Among 442 patients, 55 (12.44%) experienced mortality. The patients’ mean was 60 ± 14 years. DM (76% vs. 62%), IHD (42% vs. 19%), CKD (35% vs. 15%), and COPD (24% vs. 11%) were the most prevalent comorbidities in nonsurvivors. Compared to the survivor group, median NLR (7 vs. 4.1; p = 0.005), ferritin (507 vs. 328; p = 0.21), and D-dimer (900 vs. 567; p = 0.12) were higher in the nonsurvivor group. Higher age (OR = 1.05; 95% CI = 1.02–1.08), coexisting CKD (OR = 2.59; 95% CI = 1.27–5.29), leukocytosis (OR = 2.52; 95% CI = 1.21–5.28), thrombocytopenia (OR = 0.27; 95% CI = 0.12–0.61), lower SpO2 upon admission (OR = 0.92; 95% CI = 0.89–0.95), and more extensive lung involvement on CT (OR = 1.01; 95% CI = 1.001–1.03) were significant risk factors of in-hospital death.
Summary
- •
Reporting method:
- ◦
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).
- ◦
1. Introduction
Emerged in December 2019, the global prevalence of SARS-CoV-2 continues to pose a significant public health challenge, with approximately 6.9 million reported deaths as of the latest data [1]. Unlike influenza, individuals afflicted with COVID-19 face a substantially heightened risk of mortality during hospitalization [2]. As of February 8, 2024, the COVID-19 vaccine has been administered to approximately 5.63 billion individuals worldwide, with 88% of Bangladesh’s population (151.5 million) receiving vaccination [3]. Despite the extensive vaccination efforts, the emergence of novel SARS-CoV-2 variants has impacted the trajectory of COVID-19 infections and associated in-hospital fatalities [4, 5]. Furthermore, documented cases of breakthrough infections among vaccinated individuals present new challenges [6]. While Emergency Use Authorization (EUA) has been granted for several monoclonal antibodies for COVID-19 treatment, their utilization remains limited due to cost constraints and restricted indications. Moreover, existing evidence indicates a decline in the effectiveness of immunity acquired through both natural infection and vaccination against SARS-CoV-2 over time [7, 8].
Given these complexities, COVID-19-related mortality remains a significant concern for global and national policymakers. In the absence of curative treatments, identifying risk factors contributing to mortality becomes imperative to prioritize the management of high-risk patients. Numerous risk factors associated with COVID-19 mortality have been identified, yet many aspects remain unclear, especially concerning emerging variants of the virus. Existing evidence highlights age, male sex, obesity, hypertension, diabetes, and various laboratory parameters such as elevated white cell count, liver enzymes (ALT and AST), lactate dehydrogenase, interleukin-6, procalcitonin, fibrinogen, and albumin as significant contributors to in-hospital mortality due to COVID-19. Conversely, lymphocyte count and albumin levels have been inversely correlated with mortality [9–11].
However, it is crucial to recognize that risk factors may vary depending on population characteristics, geographical location, demographics, socioeconomic development, healthcare delivery systems, and other factors, many of which remain obscure [12–14]. In Bangladesh, the first COVID-19 case was detected on March 8, 2020, and since then, a total of 2 million people have tested positive for COVID-19, with 29,475 reported deaths [1].
In resource-limited settings such as Bangladesh, the imperative lies in discerning individuals at heightened risk, a task critical for early prognostication and optimal resource allocation toward those most vulnerable. Hence, there exists a pressing urgency to unravel the determinants underpinning mortality risk in COVID-19 patients, especially within tertiary care facilities tasked with managing the bulk of cases. The primary objective of this investigation is to scrutinize the diverse characteristics of hospitalized COVID-19 patients in Bangladesh, with the aim of identifying predictors indicative of unfavorable outcomes throughout their hospitalization period.
2. Materials and Methods
2.1. Data Collection
This retrospective multicenter study involves analyzing data obtained from hospital records of COVID-19 patients admitted to four hospitals in Sylhet, Bangladesh, between September 8, 2020, and February 14, 2021. The study comprised a total of 442 patients aged 18 years or older, diagnosed with COVID-19, either through positive reverse transcription-quantitative polymerase chain reaction (RT-qPCR) tests or characteristic chest computed tomography (CT) findings, was enrolled in the study. Data pertaining to the demographics, clinical profiles, laboratory results, respiratory assistance provided, radiographic features, and inpatient prognoses of the enrolled subjects were compiled. Patients with incomplete data records or unknown outcomes were excluded from the study.
2.2. Study Variables
The outcome variable under consideration was mortality during hospitalization, which was dichotomized into survivors and nonsurvivors. This binary variable was assigned a value of “1” for COVID-19 patients who passed away during hospitalization (nonsurvivors) and “0” for those who survived.
The demographic variables analyzed encompassed age, gender, and duration of hospitalization (in days). Clinical attributes considered consisted of initial symptoms such as fever (yes or no), sore throat (yes or no), loss of smell (yes or no), cough (yes or no), shortness of breath (yes or no), fatigue (yes or no), diarrhea (yes or no), loss of appetite (yes or no), chest pain (yes or no), vomiting (yes or no), headache (yes or no), and runny nose (yes or no); the existence of underlying health conditions like diabetes mellitus (DM) (yes or no), hypertension (yes or no), chronic obstructive pulmonary disease (COPD) (yes or no), chronic kidney disease (CKD) (yes or no), ischemic heart disease (IHD) (yes or no), cerebrovascular disease (CVD) (yes or no), and dyslipidemia (yes or no); various forms of respiratory assistance required encompassing ventilator support, noninvasive ventilation (NIV), high-flow nasal cannula (HFNC), low-flow oxygen, and no supplemental oxygen (No O2).
2.3. Statistical Analysis
Descriptive statistics were utilized for data description. The demographic profiles, clinical attributes, and laboratory results of both survivors and nonsurvivors were examined and compared. The normality of continuous variables was examined using the Shapiro–Wilk test.
Continuous variables following a normal distribution are reported using the mean and standard deviation (SD), while skewed data are depicted using the median and interquartile range (IQR). Categorical variables were depicted through frequency distributions and percentages.
To determine the mean disparity between the two groups (survivors vs. nonsurvivors) in a continuous variable, the study utilized a two-sample independent t-test for data that followed a normal distribution and the nonparametric Mann–Whitney U test for data that were not normally distributed.
The correlation analysis between comorbidities, admission SpO2 levels, neutrophil-to-lymphocyte ratio (NLR), and D-dimer levels among COVID-19 patients was conducted to explore potential associations. Specifically, Spearman’s rank correlation coefficient was employed to assess the strength and significance of relationships between continuous variables, while point-biserial correlation and chi-square tests of association were utilized for examining associations between continuous and binary variables, as well as between categorical variables, respectively.
A multivariable logistic regression model was utilized to identify factors predicting in-hospital mortality. The potential variables for inclusion in the multivariable model were determined via univariable analysis, taking into account their significant predictive power (p < 0.05). Variables that exhibited high correlation or association with each other were excluded from the model to address multicollinearity concerns. The model’s findings were reported with odds ratios (OR) and their respective 95% confidence intervals (CI), with statistical significance defined as a p value less than 0.05. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated to evaluate the discriminatory performance of the multivariable logistic regression model. Statistical analyses were conducted utilizing R software.
3. Results
3.1. Demographics and Baseline Characteristics of Patients
This study enrolled 442 hospitalized patients with COVID-19, among whom 387 (87.6%) survived and 55 (12.4%) succumbed to the illness. Table 1 presents the distribution of patients based on demographic information and clinical attributes. The mean age of patients was 60 ± 14 years, with a significant difference noted between survivor (59 ± 14 years) and nonsurvivor groups (69 ± 13 years) (p < 0.001). Male patients constituted approximately two-thirds of the hospitalized population (66%). The most prevalent comorbidities were hypertension (70%) and DM (64%), followed by IHD (22%), CKD (18%), and COPD (12%).
| Variables | Total n = 442 | Nonsurvivor n = 55 | Survivor n = 387 | p value |
|---|---|---|---|---|
| Age, mean (±SD) | 60 ± 14 | 69 ± 13 | 59 ± 14 | < 0.001 |
| Sex | ||||
| Male | 291 (65.84%) | 34 (61.8%) | 257 (66.4%) | 0.63 |
| Female | 151 (34.16%) | 21 (38.2%) | 130 (33.6%) | 0.603 |
| Comorbidity | ||||
| Hypertension | 311 (70.36%) | 41 (74.5%) | 270 (69.8%) | 0.57 |
| DM | 281 (63.57%) | 42 (76.4%) | 239 (61.8%) | 0.05 |
| IHD | 98 (22.17%) | 23 (41.8%) | 75 (19.4%) | < 0.001 |
| CKD | 78 (17.65%) | 19 (34.5%) | 59 (15.2%) | < 0.001 |
| COPD | 54 (12.22%) | 13 (23.6%) | 41 (10.6%) | 0.011 |
| CVD | 20 (4.52%) | 5 (9.1%) | 15 (3.9%) | 0.163 |
| Hypothyroidism | 10 (2.26%) | 0 (0%) | 10 (2.6%) | 0.471 |
| Dyslipidemia | 8 (1.81%) | 0 (0%) | 8 (2.1%) | 0.592 |
| Clinical characteristics | ||||
| Fever | 399 (90.27%) | 52 (94.5%) | 353 (91.2%) | 0.566 |
| Cough | 322 (72.85%) | 36 (65.5%) | 286 (73.9%) | 0.248 |
| SOB | 294 (66.52%) | 42 (76.4%) | 252 (65.1%) | 0.133 |
| Fatigability | 246 (55.66%) | 33 (60%) | 213 (55%) | 0.584 |
| Loss of smell | 87 (19.68%) | 9 (16.4%) | 78 (20.2%) | 0.631 |
| Diarrhea | 71 (16.06%) | 11 (20%) | 60 (15.5%) | 0.513 |
| Sore throat | 47 (10.63%) | 13 (23.6%) | 34 (8.8%) | 0.002 |
| Anorexia | 13 (2.94%) | 2 (3.6%) | 11 (2.8%) | 1 |
| Chest pain | 9 (2.04%) | 0 (0%) | 9 (2.3%) | 0.527 |
| Vomiting | 4 (0.90%) | 0 (0%) | 4 (1%) | 1 |
| Headache | 3 (0.68%) | 1 (1.8%) | 2 (0.5%) | 0.824 |
| Runny nose | 1 (0.23%) | 0 (0%) | 1 (0.3%) | 1 |
| Admission SpO2 | 92 (88–95) | 84 (73–93) | 93 (89–96) | < 0.001 |
| LOS, median (IQR) | 7 (6–10) | 9 (6–14) | 7 (6–10) | 0.0001 |
- Abbreviations: CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, CVD = cerebrovascular disease, DM = diabetes mellitus, IHD = ischemic heart disease, LOS = length of stay, and SOB = shortness of breath, SpO2 = peripheral capillary oxygen saturation.
Nonsurvivors exhibited a significantly higher prevalence of DM (76% vs. 62%; p = 0.05), IHD (42% vs. 19%; p < 0.001), COPD (24% vs. 11%; p = 0.01), and CKD (35% vs. 15%; p < 0.001) compared to survivors. Upon admission, fever was observed in 90% of patients, while approximately 73% presented with cough, 67% with shortness of breath, and 56% with fatigue. Other symptoms included loss of smell (20%), diarrhea (16%), and sore throat (11%). Nonsurvivors had a substantially lower median peripheral capillary oxygen saturation (SpO2) level at admission compared to survivors (84 vs. 93; p < 0.001). The duration of hospitalization was notably longer for nonsurvivors compared to survivors (median (IQR): 9 (6–14) vs. 7 (6–10); p = 0.0001).
3.2. Laboratory Findings
Laboratory results are summarized in Table 2. One-third of patients had a white blood cell (WBC) count above the reference range, while more than half had a lymphocyte count below the reference range. Nonsurvivors exhibited significantly higher median levels of WBC cells (10.8 vs. 8.1 × 109/L; p = 0.003), neutrophils (8.8 vs. 6.1 × 109/L; p < 0.001), and NLR (7.0 vs. 4.1; p < 0.005) compared to survivors. Conversely, median lymphocyte count (1.18 vs. 1.4 × 109/L; p = 0.12) and platelet count (209 vs. 230; p = 0.05) were lower in nonsurvivors. Leukocytosis (52.7% vs. 32%, p = 0.004) and lymphocytopenia (72.7% vs. 51.9%, p = 0.006) were significantly more prevalent in nonsurvivors.
| Variables | Reference range | Total n = 442 | Nonsurvivor n = 55 | Survivor n = 387 | p value |
|---|---|---|---|---|---|
| TC WBC (×109/L) | 4–10 | 8.5 (6–12) | 10.8 (6.80–14) | 8.1 (6–11.3) | 0.003 |
| > 10 | 153 (34.62%) | 29 (52.7%) | 124 (32%) | 0.004 | |
| < 4 | 11 (2.49%) | 0.0% | 11 (2.8%) | 0.422 | |
| Neutrophil (×109/L) | 2.0–7.0 | 6.3 (4.2–9.57) | 8.8 (4.84–12.59) | 6.1 (4.15–9.37) | < 0.001 |
| Lymphocyte (×109/L) | 0.8–4.5 | 1.4 (0.98–2.01) | 1.18 (0.75–1.8) | 1.4 (1.02–2.04) | 0.122 |
| < 0.8 | 241 (54.52%) | 40 (72.7%) | 201 (51.9%) | 0.006 | |
| Platelet (×109/L) | 150–350 | 220 (180–300) | 209 (154–254) | 230 (180–300) | 0.05 |
| < 150 | 49 (11.09%) | 10 (18.2%) | 39 (10.1%) | 0.118 | |
| > 350 | 73 (16.52%) | 8 (14.5%) | 65 (16.8%) | 0.821 | |
| NLR | 4.2 (2.56–7.73) | 7.0 (3.85–11.12) | 4.1 (2.48–7.08) | 0.005 | |
| D-dimer (ng/L) | 0–500 | 605 (318.4–1246) | 900 (420–1411) | 567 (300–1230) | 0.12 |
| S. ferritin (μg/L) | 20–300 | 345.5 (169.85–790) | 507 (181–981.32) | 328 (169–748) | 0.21 |
| RBS (mg/dL) | 4.4–7.2 | 9.6 (7.7–13.4) | 12 (8.9–14.7) | 9.4 (7.6–13) | 0.005 |
| Percentage of lung involvement on HRCT | 38 (25–53) | 50 (24–65) | 36 (24–50) | 0.004 | |
| HRCT findings | |||||
| Consolidation | 55 (12.44%) | 6 (10.9%) | 49 (12.7%) | 0.875 | |
| GGO | 274 (61.99%) | 29 (52.7%) | 245 (63.3%) | 0.177 | |
| GGO + cons | 113 (25.57%) | 20 (36.4%) | 93 (24%) | 0.074 | |
| Lung involvement on CT (%) | |||||
| Bilateral | 395 (89.4%) | 48 (87.3%) | 346 (89.6%) | 0.766 | |
| Unilateral | 47 (10.6%) | 7 (12.7%) | 40 (10.4%) | 0.766 | |
| Type of respiratory support | |||||
| Ventilator | 22 (4.98%) | 18 (32.7%) | 4 (1%) | < 0.001 | |
| NIV | 29 (6.56%) | 24 (43.6%) | 5 (1.3%) | < 0.001 | |
| HFNC | 44 (9.95%) | 9 (16.4%) | 35 (9%) | 0.193 | |
| Low flow | 302 (68.33%) | 4 (7.3%) | 298 (77%) | < 0.001 | |
| No O2 support | 45 (10.18%) | 0 (0%) | 45 (11.6%) | 0.016 | |
- Note: Cons = consolidation, NIV = noninvasive ventilation, and No O2 = no oxygen required.
- Abbreviations: GGO = ground-glass opacity, HFNC = high-flow nasal cannula, NLR = neutrophil-to-lymphocyte ratio, RBS = random blood sugar, and TC WBC = total count of white blood cells.
Patients who died also exhibited elevated levels of D-dimer and ferritin. Regarding respiratory support, deceased patients required higher usage of HFNC (16.4% vs. 9%; p = 0.19), NIV (43.6% vs. 1.3%; p < 0.001), and ventilator support (32.7% vs. 1%; p < 0.001).
3.3. Correlation Analysis
Table 3 provides a comprehensive correlation matrix illustrating the relationships among various demographic, clinical, and laboratory parameters investigated in this study. The analysis revealed several noteworthy associations, shedding light on the interconnections between different aspects of COVID-19 patients’ health profiles.
| DM | Hypertension | IHD | CKD | COPD | Admission SpO2 | NLR | D-dimer | |
|---|---|---|---|---|---|---|---|---|
| DM | 1 | |||||||
| Hypertension | 45.847 (p = 0.001) | 1 | ||||||
| IHD | 13.950 (p = 0.001) | 16.186 (p = 0.001) | 1 | |||||
| CKD | 7.288 (p = 0.007) | 27.284 (p = 0.001) | 25.178 (p = 0.001) | 1 | ||||
| COPD | 9.722 (p = 0.002) | 2.534 (p = 0.151) | 9.962 (p = 0.003) | 13.020 (p = 0.001) | 1 | |||
| Admission SpO2 | 0.069 (p = 0.145) | −0.064 (p = 0.182) | 0.101 (p = 0.033) | 0.044 (p = 0.351) | 0.079 (p = 0.097) | 1 | ||
| NLR | −0.075 (p = 0.117) | −0.021 (p = 0.654) | −0.217 (p = 0.001) | −0.042 (p = 0.379) | −0.115 (p = 0.015) | −0.286 (p = 0.001) | 1 | |
| D-dimer | −0.004 (p = 0.936) | −0.078 (p = 0.099) | −0.137 (p = 0.004) | −0.214 (p = 0.001) | −0.127 (p = 0.007) | −0.152 (p = 0.001) | 0.227 (p = 0.001) | 1 |
- Note: SpO2 = peripheral capillary oxygen saturation.
- Abbreviations: CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, IHD = ischemic heart disease, and NLR = neutrophil-to-lymphocyte ratio.
Firstly, a strong positive correlation was evident between hypertension and DM (r = 0.847, p < 0.001), indicating a significant tendency for these two comorbid conditions to co-occur within the study population. This finding underscores the importance of considering the potential clustering of metabolic disorders in COVID-19 patients, which may have implications for disease management and prognosis.
Similarly, IHD exhibited significant positive correlations with both hypertension (r = 0.950, p < 0.001) and DM (r = 0.686, p < 0.001). This suggests a clustering of cardiovascular risk factors, wherein individuals with one cardiovascular condition may be more predisposed to developing others. Furthermore, CKD demonstrated significant positive correlations with hypertension (r = 0.288, p = 0.007) and IHD (r = 0.284, p < 0.001), indicating potential links between renal dysfunction and cardiovascular comorbidities.
Moreover, a moderate positive correlation was observed between COPD and IHD (r = 0.220, p = 0.003), suggesting potential interactions between pulmonary and cardiovascular disorders in COVID-19 patients.
In terms of physiological parameters, admission SpO2 levels displayed weak positive correlations with IHD (r = 0.101, p = 0.033) and COPD (r = 0.079, p = 0.097). These findings suggest potential influences of respiratory conditions on oxygen saturation levels at hospital admission, highlighting the importance of assessing and managing respiratory status in COVID-19 patients, particularly those with underlying cardiovascular disease.
The NLR exhibited a significant negative correlation with admission SpO2 levels (r = −0.075, p = 0.117), implying a potential link between systemic inflammation, as reflected by NLR, and oxygenation status in COVID-19 patients.
Regarding D-dimer levels, a weak positive correlation was observed with CKD (r = 0.214, p < 0.001), suggesting a potential association between coagulation abnormalities and renal dysfunction in COVID-19 patients.
3.4. Risk Factor Analysis
The results of the simple logistic regression analysis, as outlined in Table 4, revealed significant associations between in-hospital mortality among COVID-19 patients and various variables, including age, IHD, DM, CKD, COPD, total WBC count, neutrophil count, lymphocyte count, platelet count, NLR, random blood sugar (RBS) levels, initial peripheral capillary oxygen saturation (SpO2) level, and percentage of lung involvement.
| Univariable | Multivariablea | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p value | OR | 95% CI | p value |
| Age | 1.05 | 1.03–1.08 | 0.001 | 1.05 | 1.02–1.08 | 0.001 |
| Sex (male) | 0.89 | 0.46–1.49 | 0.502 | |||
| DM | 2.00 | 1.06–3.99 | 0.038 | |||
| Hypertension | 1.27 | 0.68–2.49 | 0.469 | |||
| CKD | 2.93 | 1.55–5.42 | 0.0006 | 2.59 | 1.27–5.29 | 0.01 |
| COPD | 2.61 | 1.25–5.16 | 0.007 | |||
| IHD | 2.99 | 1.64–5.39 | 0.0002 | |||
| CVD | 2.48 | 0.77–6.71 | 0.09 | |||
| WBC | 2.48 | 1.34–4.58 | 0.003 | 2.52 | 1.21–5.28 | 0.01 |
| Neutrophil | 2.47 | 1.47–4.14 | 0.0006 | |||
| Lymphocyte | 0.60 | 0.38–0.97 | 0.03 | |||
| Platelet | 0.48 | 0.24–0.96 | 0.03 | 0.27 | 0.12–0.61 | 0.001 |
| NLR | 2.05 | 1.45–2.91 | 0.001 | |||
| D-dimer | 1.26 | 0.98–1.62 | 0.06 | |||
| Ferritin | 1.27 | 0.98–1.65 | 0.06 | |||
| RBS | 2.63 | 1.32–5.22 | 0.005 | |||
| Initial SpO2 | 0.91 | 0.88–0.93 | 0.001 | 0.92 | 0.89–0.95 | 0.001 |
| Lung involvement on CT (%) | 1.02 | 1.007–1.035 | 0.002 | 1.01 | 1.001–1.03 | 0.02 |
- Note: CVD = cerebrovascular disease and SpO2 = peripheral capillary oxygen saturation. The bold values indicate statistical significance at p < 0.05.
- Abbreviations: CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, IHD = ischemic heart disease, NLR = neutrophil-to-lymphocyte ratio, and RBS = random blood sugar.
- aVariables that were significant in univariable analysis (p < 0.05) were included in the multivariable analysis.
The probability of in-hospital mortality was elevated for individuals of older age (OR = 1.05; 95% CI = 1.03–1.08), those with DM (OR = 2.00; 95% CI = 1.06–3.99), CKD (OR = 2.93; 95% CI = 1.55–5.42), COPD (OR = 2.61; 95% CI = 1.25–5.16), IHD (OR = 2.99; 95% CI = 1.64–5.39), higher WBC count (OR = 2.48; 95% CI = 1.34–4.58), elevated neutrophil count (OR = 2.47; 95% CI = 1.47–4.14), reduced lymphocyte count (OR = 0.60; 95% CI = 0.38–0.97), decreased platelet count (OR = 0.48; 95% CI = 0.24–0.96), elevated NLR (OR = 2.05; 95% CI = 1.45–2.91), higher RBS levels (OR = 2.63; 95% CI = 1.32–5.22), lower SpO2 (OR = 0.91; 95% CI = 0.88–0.93), and increased extent of lung involvement (OR = 1.02; 95% CI = 1.00–1.03) (Table 4).
After adjustment, the multiple logistic regression model illustrated in Table 4 highlighted age, presence of CKD, WBC count, platelet count, admission SpO2, and the extent of lung involvement as notable contributors to in-hospital mortality among COVID-19 patients. With each additional year of age, the likelihood of mortality during hospitalization increased by 5% (AOR = 1.05; 95% CI = 1.02–1.08). Patients with CKD exhibited a 2.6 times greater likelihood of dying in the hospital compared to those without CKD, with a 95% CI ranging from 1.27 to 5.29. Furthermore, elevated WBC count (AOR = 2.52; 95% CI = 1.21–5.28) and greater percentage of lung involvement on CT scans (AOR = 1.01; 95% CI = 1.001–1.03) were associated with a heightened risk of in-hospital mortality. Conversely, higher platelet count (AOR = 0.27; 95% CI = 0.12–0.61) and initial SpO2 level (AOR = 0.92; 95% CI = 0.89–0.95) were linked with a decreased likelihood of in-hospital death.
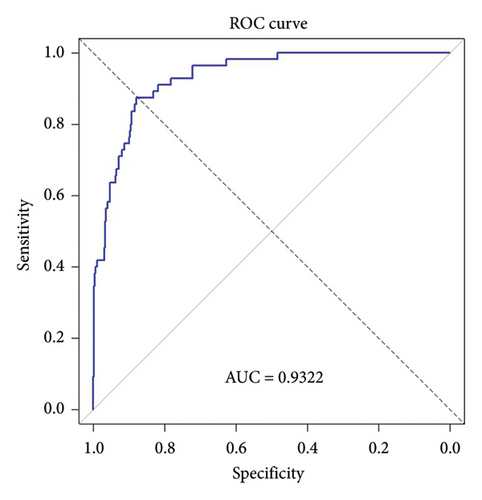
The AUC of our multivariable logistic regression model was calculated to be 0.9322, indicating a high level of discriminatory accuracy in distinguishing between individuals who survive and those who do not (Figure 1).
4. Discussion
4.1. Statement of Key Findings
This investigation delved into the demographic, clinical, and laboratory profiles of COVID-19 patients upon hospital admission, scrutinizing their correlation with in-hospital mortality. The outcomes unveiled distinct disparities between survivors and nonsurvivors, with the latter typically exhibiting advanced age, a higher prevalence of comorbidities, elevated WBC counts, diminished lymphocyte counts, and platelet counts. Additionally, nonsurvivors showcased elevated levels of D-dimer, serum ferritin, and RBS, alongside augmented lung involvement evident on CT scans. Noteworthy risk factors for in-hospital mortality encompassed older age, concurrent CKD, leukocytosis, thrombocytopenia, decreased oxygen saturation levels at admission, and heightened lung involvement.
4.2. Strengths and Limitations
The strength of this study lies in its comprehensive investigation of the multifaceted interplay between various demographic, clinical, and laboratory parameters in COVID-19 patients. By employing correlation analysis, the study offers valuable insights into the intricate relationships among comorbidities, physiological markers, and disease outcomes, thereby enhancing our understanding of COVID-19 pathophysiology. Additionally, the inclusion of a diverse patient population and rigorous statistical analysis bolsters the robustness of the findings, ensuring greater generalizability and reliability.
However, several limitations warrant consideration. Firstly, the retrospective nature of the study inherently introduces the potential for selection bias and incomplete data capture, limiting the depth of analysis and interpretation. Secondly, while correlation analysis provides valuable insights into associations between variables, it does not establish causality, necessitating caution in inferring direct causal relationships. Furthermore, the study’s reliance on existing medical records may introduce variability in data quality and consistency, potentially influencing the accuracy of the findings. Future prospective studies with larger sample sizes are warranted to validate these findings and elucidate the underlying mechanisms driving the observed correlations in COVID-19 patients.
4.3. Interpretation in the Context of the Wider Literature
Understanding the global variability in COVID-19 mortality rates is imperative for discerning the multifaceted nature of the pandemic’s impact. As highlighted in previous research [15, 16], these mortality discrepancies stem from a myriad of factors, encompassing testing rates, demographic profiles, healthcare infrastructures, and biological intricacies. The in-hospital mortality rate of 12% observed in our study serves as a reflection of this dynamic landscape.
The correlation analysis undertaken in this study offers pivotal insights into the intricate pathophysiology and clinical manifestations of COVID-19. By unraveling the complex interplay between demographic attributes, clinical parameters, and laboratory findings, this analysis illuminates the underlying mechanisms driving disease severity and prognosis.
The robust positive correlations observed between comorbidities such as hypertension, DM, and IHD underscore the clustering of metabolic and cardiovascular risk factors in COVID-19 patients. This clustering phenomenon elucidates the intricate interactions between underlying health conditions, immune dysregulation, and viral pathogenesis, thereby accentuating the heightened susceptibility to severe illness and adverse outcomes among individuals with multiple comorbidities, as extensively documented in the literature [17–19].
Moreover, the positive correlations identified between CKD and both hypertension and IHD shed light on the intricate relationship between renal dysfunction and cardiovascular morbidity in the context of COVID-19. These associations hint at shared pathophysiological mechanisms, including endothelial dysfunction, inflammation, and oxidative stress, exacerbating disease severity and mortality risk in patients with concurrent renal and cardiovascular comorbidities [20, 21].
Furthermore, the moderate positive correlation observed between COPD and IHD underscores the complex interplay between respiratory and cardiovascular conditions in COVID-19 patients. Shared risk factors, such as smoking and systemic inflammation, contribute to the heightened vulnerability of COPD patients to cardiovascular complications and adverse COVID-19 outcomes [22, 23].
Additionally, correlations between admission SpO2 levels and various comorbidities and physiological parameters provide crucial insights into disease severity and prognosis. The negative correlation between NLR and admission SpO2 levels suggests a potential association between systemic inflammation and respiratory compromise in COVID-19 patients. This underscores the role of inflammatory markers as prognostic indicators and emphasizes the importance of early risk stratification and targeted interventions to mitigate disease progression and improve clinical outcomes. Furthermore, the weak positive correlation between D-dimer levels and CKD highlights the potential link between coagulation abnormalities and renal dysfunction in COVID-19 patients. These findings underscore the intricate interplay between hemostatic derangements, endothelial dysfunction, and thrombotic complications, contributing to the heightened thromboembolic risk and adverse outcomes observed in patients with renal impairment [24, 25].
The hematological system is susceptible to varied effects upon infection with SARS-CoV-2, contributing to diverse disease manifestations, disease progression, and complications. Among the commonly reported abnormalities, lymphopenia, thrombocytopenia, and neutrophilia stand out and have been consistently associated with unfavorable outcomes [26–28]. Our study aligns with these findings, as we observed a significantly higher prevalence of leukocytosis and thrombocytopenia among nonsurvivors, correlating with increased odds of death. The underlying mechanisms driving these changes are multifactorial and may involve direct viral infection of cells, interleukin-induced apoptosis, lymphoid organ atrophy, and autoimmune responses.
Moreover, COVID-19 exerts a detrimental effect on the coagulation system, precipitating arterial and venous thrombosis and directly contributing to heightened mortality rates. Notably, severe illness and fatal outcomes are characterized by elevated levels of coagulation markers, including D-dimer, serum ferritin, fibrinogen, LDH, and CRP, a pattern consistent with our study’s findings and supported by previous research [29, 30]. Our investigation revealed heightened levels of D-dimer and serum ferritin in nonsurvivors, indicative of perturbations across multiple dysregulated pathways. The aberrant expression of tissue factor (TF) in endothelial cells, triggered by proinflammatory cytokines, emerges as a key driver of thrombosis, eliciting platelet activation and fibrin deposition and ultimately culminating in a prothrombotic milieu [31].
The NLR serves as a pivotal biomarker illuminating the intricate inflammatory milieu in COVID-19 pathogenesis. Elevated NLR levels denote a robust systemic inflammatory cascade typified by heightened neutrophil activation and lymphocytic depletion. This dysregulated immune response intricately contributes to pulmonary inflammation, exacerbating lung parenchymal injury and impeding efficient viral clearance mechanisms [32]. Within the context of COVID-19, escalated NLR levels manifest a strong correlation with disease severity, progression to critical illness, and heightened mortality rates, thus underscoring its indispensable prognostic significance [33, 34]. The vigilant monitoring of NLR levels provides critical insights for precise risk stratification and informs the design of targeted therapeutic interventions, thereby facilitating more nuanced disease management strategies and optimizing clinical outcomes.
A distinguishing characteristic of COVID-19 is the occurrence of hypoxemia, often manifesting without the classical symptomatology of dyspnea, termed “Silent hypoxemia.” The degree of hypoxemia independently correlates with mortality and holds prognostic value in predicting future intensive care needs [35, 36].
Hypoxia exacerbates outcomes in COVID-19 by compromising lung function through pneumonia and acute respiratory distress syndrome, leading to respiratory failure. Pulmonary arterioles constrict in response to hypoxia, worsening ventilation–perfusion mismatch. Additionally, systemic inflammation and endothelial dysfunction disrupt oxygen transport and microvascular perfusion, exacerbating hypoxia in vital organs. Activation of hypoxia-inducible factors may further contribute to tissue injury and dysfunction. Early recognition and management of hypoxemia are essential for improving outcomes, often involving supplemental oxygen therapy, mechanical ventilation, and targeted interventions to address underlying inflammatory and microvascular pathology [37–40].
4.4. Policy, Practice, and Future Research Implications
The study’s findings have important implications for policy, practice, and future research in COVID-19 management. Policymakers should prioritize targeted interventions for high-risk populations, including those with comorbidities like hypertension and diabetes. Healthcare providers can use the insights to inform clinical decision-making and optimize patient care by monitoring key markers like admission SpO2 and NLR. Future research should focus on longitudinal studies to understand disease progression better, validate prognostic biomarkers, and elucidate underlying biological mechanisms to develop effective therapeutic interventions. Collaborative efforts are needed to translate these findings into actionable strategies to combat the pandemic effectively.
Consent
Considering the retrospective study design and the fact that data were collected from hospital records, the ethical committee waived the need for consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Md. Asaduzzaman contributed to the conception and design of the study, statistical analysis, and interpretation of analysis and wrote the final draft. Mohammad Romel Bhuia independently reanalyzed the data and checked for any inconsistency and contributed to the interpretation of analysis. Md. Asaduzzaman, Mohammad Zabed Jillul Bari, Z. H. M. Nazmul Alam, Soumitra Roy, and Goutam Talukder were involved in the acquisition of data and edited the draft manuscript. Ranjon Kumer Roy, M. M. Jahangir Alam, and Md. Shafiqul Bari contributed to the conceptualization and critical revision of the final manuscript. All authors critically reviewed the final manuscript and approved the final version. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
Our heartfelt gratitude to those patients whose data we have worked with.
Open Research
Data Availability Statement
The datasets utilized in the present study are not publicly accessible due to a lack of permission from the hospitals where the data were obtained.




