Effect of Iron Accumulation on Bone Mineral Density in Patients Diagnosed With Transfusion-Dependent Thalassemia
Abstract
Introduction: Transfusion-dependent thalassemia (TDT) is most commonly caused by defects in beta globin chain production. Iron overload, hypogonadism, vitamin D deficiency, adverse effects of desferrioxamine treatment, and delayed puberty due to loss of bone mass are the main complications of TDT. In the present study, we aimed to investigate bone complications and precursor markers in TDT patients.
Materials and Methods: Our retrospective study included 93 patients, aged between 18 and 45, who were followed up in a tertiary care institution and did not have any disease other than TDT. The patients’ BMD values and biochemical parameters were compared.
Results: Osteoporosis was observed in 33 of 93 patients, and the mean transferrin saturation of patients with osteoporosis was 60%, and the mean transferrin saturation of patients without osteoporosis was 42%. Patients with osteoporosis had transferrin saturation that was observed to be considerably higher than that of the group without osteoporosis (p = 0.006), with an average of 60%.
Conclusion: Our investigation has demonstrated that in patients with TDT, elevated transferrin saturation may be a sign of osteoporosis.
1. Introduction
Hemoglobinopathies, known as thalassemia syndromes, affect globin chain production. In these syndromes, there may be a defect in the αβ or δβ globin chain in the hemoglobin structure and therefore thalassemia syndromes can be categorized depending on the transfusion dependency that occurs [1]. Thalassemia is classified as transfusion-dependent thalassemia (TDT) and non-transfusion-dependent thalassemia, depending on clinical symptoms, the degree of impairment in globin chain production, and the patient’s transfusion dependency. TDT most commonly occurs due to problems in beta globin chain production [2]. TDT is characterized by impaired tissue oxygenation capacity, hemolytic anemia, and ineffective erythropoiesis [3]. Although the life expectancy of patients diagnosed with TDT is extended by chelator therapy to suppress accumulated ferritin levels and regular erythrocyte replacement, the bone marrow cavity expands and the mass of the cortical and tubular parts of the bone tissue decreases due to increased ineffective erythropoiesis [4].
Since TDT is a hemoglobinopathy, globin chain production is affected. This disease, caused by globin chain deficiency, results in loss of bone mass due to iron overload, hypogonadism, vitamin D deficiency, adverse effects of desferrioxamine therapy, and delayed puberty [5]. Some candidate gene polymorphisms seem to have an effect on bone mineral density (BMD), but few studies support that the most common cause of osteoporosis in TDT patients is ineffective erythropoiesis [6, 7]. Therefore, many bone disorders can develop in thalassemia patients, including bone pain, bone deformities, delayed bone growth, growth retardation, rickets, scoliosis, spinal deformities, pathological fractures, osteopenia, and osteoporosis [8].
Loss of bone tissue and deterioration of the skeletal structure lead to a disease that causes decreased bone strength and an increased risk of bone fractures, known as osteoporosis [9]. Patients with thalassemia have a better prognosis when treated with transfusion and iron chelation therapy; nevertheless, frequent transfusions lead to increased iron overload and a related increased risk of osteoporosis [10]. In addition, it should be noted that blood transfusion therapy causes significant side effects on bone tissue [11]. In pediatric and adult thalassemia cases, which can be significant, bone mass is largely determined by BMD [12]. BMD is a good index of bone status and is the most important predictor of fracture risk. Dual-energy X-ray absorptiometry (DXA) is an excellent noninvasive choice for repeated measurements of any temporal changes of BMD because of its 1% precision rate and low radiation exposure [13].
Iron overload and toxicity in TDT are measured using markers such as magnetic resonance imaging (MRI), serum ferritin and transferrin saturation, the ratio of non-transferrin-bound iron to labile plasma iron, biosusceptometry, and liver biopsy. Transferrin saturation is an easily accessible, noninvasive parameter that can measure non-transferrin-bound iron and indirectly estimate iron accumulation in the liver and heart. Because it is not as easily affected by inflammation as ferritin, many guidelines recommend that transferrin saturation and ferritin be assessed together when iron accumulation is suspected [14, 15].
Although many studies have been conducted on pediatric TDT patients and their bone densitometry, the number of studies examining treatment-related bone densitometry results in adult patients diagnosed with TDT is limited and regional, and the results are variable. In the present study, we aimed to retrospectively investigate the relationship between screening bone densitometry and previously measured ferritin and transferrin saturation values in patients diagnosed with TDT who were followed up in five different centers in Turkey.
2. Materials and Methods
2.1. Study Methods
Our retrospective study included 93 patients aged 18–45 who were followed up in five tertiary healthcare institutions between 2018 and 2023. Patients over the age of 18 who had a diagnosis of TDT, no comorbidities, required regular erythrocyte replacement, received iron chelation therapy, and had a body mass index between 18.5 and 25 were included in the study. Exclusion criteria of the study were determined as growth hormone deficiency, hypothyroidism or parathyroid gland dysfunction, nutritional deficiency, renal failure, hepatic failure, additional disorders related to calcium mechanism, and lifestyle-associated parameters such as smoking, rapid fluctuation in body mass index, vitamin D or calcium-vitamin D use, and alcohol use. Patients with additional diseases or nutritional problems that could lead to osteoporosis or low bone mass were not included in the study. The ECLIA (electrochemiluminescence) method was used to measure serum ferritin values, and the average of annual measurements of patients’ ferritin and transferrin saturation values was taken into account. Since transferrin saturation is affected by nutritional fluctuations and malnutrition, patients with low body mass index or sudden increases or decreases in body mass index were not included in the study. This method is known for its high sensitivity and specificity, making it a reliable choice for such measurements [16]. Patients participating in the study were informed in accordance with the principles stated in the Declaration of Helsinki, and their written consent was obtained. The study was approved by the Harran University Clinical Ethics Committee; the decision number for this approval was HRÜ/23.08.03.
2.2. BMD Assessment
Bone densitometry was performed for each patient. The lumbar spine (L1–L4) and femoral neck were assessed for BMD using the DXA technique (Hologic, Inc., Bedford, MA, USA). According to the World Health Organization (WHO) osteoporosis guidelines, T and Z scores are used instead of BMD to interpret DXA results for osteoporosis. The T score is used to diagnose osteoporosis in postmenopausal women and men over the age of 50, while the Z score is used to diagnose osteoporosis in premenopausal women and men under the age of 50. A Z score of −2 SD and below indicates “lower bone mass than expected for chronological age, significant risk of osteoporosis,” while a score above −2 indicates “normal bone mass for chronological age.” In patients, BMD Z scores for the lumbar spine and hip were categorized accordingly and recorded into two subgroups: osteoporosis or reduced bone mass (Z score < −2.0) and normal (Z score > −2.0) [17].
2.3. Statistical Analyses
Statistical analyses were performed using “IBM SPSS Statistics for Windows Version 25.0 (Statistical Package for the Social Sciences, IBM Corp., Armonk, NY, USA).” Descriptive statistics are presented as mean ± SD or median (IQR) for continuous variables. The data were examined with the Kolmogorov–Smirnov test for normal distribution, and since p was found to be < 0.05, Mann–Whitney U test, one of the nonparametric tests, was used for pairwise group comparisons with continuous variables. The Spearman correlation test was used to look at the link between continuous variables, and a value of p < 0.05 was deemed statistically significant.
3. Results
The average age of the 93 patients included in the study was 30.80 ± 11.31/18–45. Fifty-four of the patients were female (58.1%) and 39 were male (41.9%). Osteoporosis (low BMD) (35.5%) was detected in 33 patients. The average ferritin value of the patients was determined as 2242.06 ± 2104.57 ng/mL. TDT patients without osteoporosis had a mean ferritin value of 1618 ng/mL, whereas patients with osteoporosis had a mean ferritin value of 1124 ng/mL. There was no statistically significant difference between the two groups (p = 0.438). The lowest ferritin value was 370 ng/mL in patients, while the highest ferritin value was 9243 ng/mL. There were large differences between ferritin values. Transferrin saturation was 60% in patients with reduced bone mass, while it was 42% in patients with normal bone mass. Transferrin saturation was observed to be significantly higher in patients with osteoporosis than in the group without osteoporosis (p = 0.006). The mean Z score of the patients in the L1–4 vertebrae was calculated as −2.15 ± 1.06, and the mean Z score of the femoral head was calculated as −1.50 ± 0.98. Some sociodemographic and clinical data about the patients are presented in Table 1.
| Variables | n | % |
|---|---|---|
| Age | ||
| Mean ± SD | 30.80 ± 11.31 | |
| Median (min–max) | 28 (18–76) | |
| Gender | ||
| Female | 54 | 58.1 |
| Male | 39 | 41.9 |
| Osteoporosis (low bone mineral density) | ||
| None | 60 | 64.5 |
| Yes | 33 | 35.5 |
| Parameters | Mean ± SD | |
| Ferritin | 2242.06 ± 2104.57 | |
| Number of annual transfusions | 18.02 ± 6.09 | |
| Transferrin saturation | 49.00 ± 19.74 | |
| L1 Z | −1.78 ± 1.08 | |
| L2 Z | −2.06 ± 1.12 | |
| L3 Z | −2.15 ± 1.11 | |
| L4 Z | −2.35 ± 1.15 | |
| L1-4 Z | −2.15 ± 1.06 | |
| Femur T | −1.63 ± 1.00 | |
| Femur Z | −1.50 ± 0.98 | |
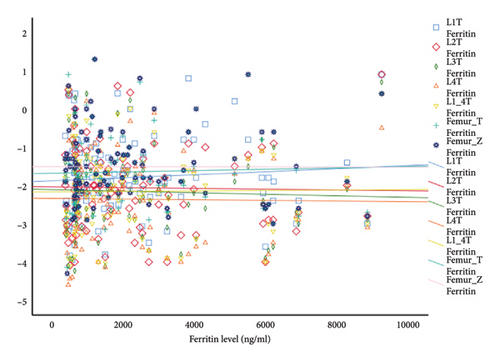
Of the 33 patients with osteoporosis, 14 were male (42%) and 19 were female (58%), and no statistically significant difference was found between the group with osteoporosis and the group without osteoporosis (p > 0.05). The mean age of the group with osteoporosis was 31.60 ± 9.21/20–44, and no significant difference was found compared to the group without osteoporosis (p > 0.05). The variability in ferritin readings among patients is believed to be associated with the irregular application of iron chelator therapy in individuals with TDT. These fluctuating ferritin values and the high number of TDT patients without osteoporosis may have affected the statistical distribution, and this situation is seen in Figure 1. However, since transferrin saturation is more stable in the case of iron overload, it may have been detected statistically significantly higher in patients with low BMD. Table 2 shows the ferritin and transferrin saturation values of the patients.
| Variables | Osteoporosis (low bone mineral density) | p | |
|---|---|---|---|
None N = 60 Median (IQR) |
Yes N = 33 Median (IQR) |
||
| Ferritin | 1618.0 (2113.2) | 1124.0 (3197.0) | 0.438 |
| Transferrin saturation | 42.0 (24.0) | 60.0 (26.2) | 0.006 |
- Note: The correlation between ferritin levels and transferrin saturation in individuals with osteoporosis (reduced bone mineral density). Bold values indicate statistical significance.
As seen in Table 3, a statistically significant negative correlation was also detected between transferrin saturation values and L3-Z score (r = −0.358, p = 0.005), L4-Z score (r = −0.317, p = 0.013), L1-4 Z score (r = −0.313, p = 0.014), femur-T score (r = −0.308, p = 0.016), and femur-Z score (r = −0.336, p = 0.007).
| Ferritin | Transferrin saturation | ||
|---|---|---|---|
| L1-Z | r | 0.092 | −0.215 |
| p | 0.379 | 0.096 | |
| L2-Z | r | −0.042 | −0.221 |
| p | 0.689 | 0.087 | |
| L3-Z | r | −0.066 | −0.358 ∗∗ |
| p | 0.532 | 0.005 | |
| L4-Z | r | −0.013 | −0.317 ∗ |
| p | 0.898 | 0.013 | |
| L1-4Z | r | 0.012 | −0.313 ∗ |
| p | 0.909 | 0.014 | |
| Femur-T | r | 0.065 | −0.308 ∗ |
| p | 0.533 | 0.016 | |
| Femur-Z | r | 0.015 | −0.336 ∗∗ |
| p | 0.887 | 0.008 | |
- Note: Bold values indicate statistical significance.
- ∗Correlation is significant at the 0.05 level (Spearman correlation test).
- ∗∗Correlation is significant at the 0.01 level (Spearman correlation test).
4. Discussion
The present study did not show a correlation between blood ferritin levels and lumbar and femoral bone density. Certainly, ferritin is indeed an acute phase reactant, meaning that its levels can be affected by various factors such as inflammation, infection, and chronic diseases. This variability can make it difficult to use ferritin levels as a sole indicator of iron status or bone density. Transferrin saturation can be easily affected, especially in cases of malnutrition. To avoid this, patients with a body mass index below normal or with a significant decrease in body mass index between two examinations were excluded from the study. When assessing transferrin saturation, nutritional assessment should definitely be considered [14].
A statistically significant correlation was found between bone densitometry of the femoral and lumbar regions and transferrin saturation. This finding suggests that information about BMD can be obtained from transferrin saturation values. Previous studies have shown that iron accumulation may contribute to bone deterioration. It has been stated that transferrin saturation is an indirect indicator of non-transferrin-bound iron and may be an indicator of iron accumulation in some tissues. MRI, serum ferritin and transferrin saturation, the ratio of non-transferrin-bound iron to labile plasma iron, biosusceptometry, and liver biopsy can be used to demonstrate iron accumulation. However, the ratio of non-transferrin-bound iron to labile plasma iron is not widely used and is still quite labile. MRI cannot be used continuously because it indirectly shows liver iron load, requires continuous calibration, and requires a special technician, biosusceptometry cannot be used continuously due to access difficulties, and liver biopsy cannot be used continuously due to its invasiveness and risk of complications [18].
Ferritin level measurement and transferrin saturation alone may not be sufficient to demonstrate iron accumulation in TDT patients. In TDT patients, it is very valuable to follow iron accumulation with liver biopsy and then T2- and R2-weighted MRI. However, access to these examinations is difficult in many centers that follow TDT. The present study suggests that serum transferrin saturation in patients may be a suitable indicator of bone density or pathological fractures in terms of demonstrating iron accumulation in tissues. It may be important to evaluate patients with increased transferrin saturation for osteoporosis. There are many risk factors that can lead to osteoporosis. While sedentary lifestyle, malnutrition, weight loss, smoking, and alcohol use are common causes in society, hypogonadism, chronic liver disease, inflammatory diseases, and kidney and cardiovascular diseases can also be causes of osteoporosis in TDT patients [19]. Therefore, care was taken to ensure that these etiological factors that could cause osteoporosis were not present in the patients included in the study. WHO’s guidelines for diagnosing and treating osteoporosis are indeed widely used. These guidelines use reference limits for BMD determined by the NHANES study for white women and men. According to these guidelines, the diagnostic values for BMD can also be applied to men. This is because observational studies have shown that the absolute risk of fracture is the same in men and women of the same age and BMD values [17].
Studies in the adult age group may yield inconsistent results, even though several investigations into the association between ferritin level and BMD were previously carried out in the pediatric age group. There are not many studies looking into the connection between BMD and transferrin saturation.
In the study conducted by Mahmoodi et al., 70 patients diagnosed with thalassemia were included in the study, and the average ferritin value of patients with osteoporosis was 2532 ng/mL, which was significantly higher than the present study [20]. Compared to the present study, the incidence of osteoporosis in thalassemia patients included in the study was found to be relatively high, at 68.6%. In contrast to our investigation, ferritin level and BMD were shown to be significantly correlated in this study. The patients’ inclusion in the combined pediatric and adult age groups may be the cause of the study’s inconsistent findings. Furthermore, transferrin saturation and BMD were not examined by the authors in this investigation.
Karimi et al. evaluated 107 pediatric patients diagnosed with TDT [21]. In this study, osteoporosis was not observed in thalassemia patients, but BMD values were found to be significantly lower than the control group. In this study, the relationship between BMD value and age, gender, chelator selection, and ferritin was compared and, as in the present study, no correlation was found between ferritin and BMD. However, unlike the present study, transferrin saturation of the individuals participating in the study was not evaluated in this study. The main difference between this study and the present study is that the average age of the individuals participating in the study was thirteen.
In the study conducted by Jensen et al., 82 TDT patients, with an average age of 27 and including the pediatric age group, were included in the study [12]. In this study, the average serum ferritin level during the study was calculated as 2733 ng/mL, and osteoporosis was observed in 52% of the patients. Although there was a significant relationship between gender (male) and the severity of low bone mass, no significant relationship was shown between ferritin levels and BMD in the patient group compared to the control group, similar to the present study. Unlike the present study, only three patients with normal BMD were detected in this study, and patients with additional diseases were included in the study.
In the study conducted by Ekbote et al. in India, 208 patients were included in the study and the average age of these patients was found to be 12.9 ± 5.4 years [22]. The average ferritin value of the patients was found to be 2256 ng/mL, and osteoporosis was observed in 26% of the patients participating in the study. The BMD of these patients in the lumbar vertebra and femoral head, determined by the DXA method, was compared with ferritin and liver iron load. Unlike the present study, this study, which manually investigated whether the DXA method could detect liver iron load retention, found a significant relationship between high ferritin and low BMD. The reason for this difference may be that the study was conducted in the pediatric age group.
The link between bone densitometry, ferritin and transferrin saturation, and sociodemographic information of sixty patients with thalassemia was compared in the study by Atmakusuma et al. [23]. Similar to the present study, patients above the age of 18 were enrolled in this one, with an average participant age of 25. According to the study, 68% of the patients had low BMD, and the average ferritin level was found to be 3881 ng/mL. However, in this study, the number of patients diagnosed with TDT was 19. As in our investigation, no meaningful correlation between ferritin levels and BMD was found. Similar to the present study, a correlation between transferrin saturation and BMD was found, and those with transferrin saturation levels higher than 89% were shown to have substantial osteoporosis. The authors of this study concluded that there is a weak but significant correlation between transferrin saturation and BMD. Nonetheless, our investigation revealed a statistically significant correlation between patients’ BMD values and transferrin saturation. Although a cutoff value was determined for the present study, a correlation was discovered between the prevalence of osteoporosis and increasing transferrin saturation percentage.
There may be some possible limitations to this study. The wide range of ferritin values of the patients makes it difficult to evaluate the patients statistically. In addition, although patients with a normal body mass index and no low nutritional values were included in the study, it is very difficult to fully determine the nutritional habits of all patients.
5. Conclusion
Osteoporosis is uncommon in youth in Turkey, but after age 50, it becomes much more common, with an average prevalence of 25% [24]. Similar to other studies, the present study found that 35.5% of young patients with TDT had osteoporosis and low bone mass. Unlike other studies, the present study excluded participants with comorbid medical conditions such as kidney and bone disorders. The geographical area of the study was expanded by conducting the current study in five different regions and geographical locations. Tests such as liver biopsy, followed by T2- and R2-weighted MRI, are very successful in detecting iron overload in patients with TDT, but these tests are not available in every center. Although high ferritin level is a priority in the follow-up of patients diagnosed with TDT, monitoring transferrin saturation in patients is also valuable in terms of evaluating iron load. Our investigation has demonstrated that in patients with TDT, elevated transferrin saturation may be a sign of osteoporosis. Such patients should have their bone health evaluated, and appropriate testing and medication for bone preservation should be administered as needed.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors indicate no financial support.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




