Quality Assessment of Clinical Practice Guidelines for the Management and Surgical Treatment of Female Stress Urinary Incontinence
Abstract
Clinical practice guidelines (CPGs) are tools developed to support evidence-based decision making in healthcare. However, despite the availability of CPGs for the surgical management of female stress urinary incontinence (FSUI), their methodological quality has not been evaluated. The aim of this study was to assess the methodological quality of published guidelines for the surgical management of FSUI using the AGREE II tool. A systematic search of CPGs published between 2017 and 2023 was performed in databases including MEDLINE/PubMed, LILACS, Scopus, and Trip Medical Database. Data extraction and guideline selection were performed independently by two reviewers, as was the assessment using the AGREE II instrument. Of 1459 initial records, six guidelines met the eligibility criteria. The scores for each domain evaluated were as follows: scope and purpose (45.83%; SD: 22.69), stakeholder participation (30.56%; SD: 29.03), development (48.56%; SD: 30.42), presentation clarity (58.80%; SD: 22.25), applicability (24.04%; SD: 26.36), and editorial independence (44.87%; SD: 32.88). One of the six included CPGs was rated as high quality and recommended for clinical practice. Three CPGs with modifications were recommended because there were still areas that needed improvement to enhance their quality, and two CPGs were not recommended for clinical practice because the six domains evaluated scored below 60%. According to these findings, it is essential that new CPGs developed for the surgical management of FSUI adhere to greater methodological rigor to ensure that recommendations are based on the best available evidence. Furthermore, guidelines should take into account patient values and clinical expertise to improve and facilitate effective healthcare decision making.
1. Introduction
Female stress urinary incontinence (FSUI) is a condition characterized by involuntary loss of urine during routine activities that increase intra-abdominal pressure, such as coughing, sneezing, or laughing, and has a significant impact on women’s quality of life [1]. According to the International Continence Society (ICS), 10% of women experience weekly episodes of urine leakage and between 25% and 45% of the female population report occasional episodes of urine leakage [2]. It has been suggested that FSUI is the most common form of urinary incontinence, accounting for 80% of cases in women, with an estimated prevalence of 4%–35% in middle-aged women [3]. Risk factors for FSUI include age, ethnicity, body mass index (BMI), parity, diabetes, smoking, and hysterectomy [4].
Conservative treatments for FSUI focus on lifestyle modification, pelvic floor muscle training, and electrical stimulation [3]. However, the effectiveness of these approaches depends on adherence and adequate training, which limit the long-term efficacy [3]. In addition, several pharmacological treatments have been investigated, but an effective pharmacological agent for the treatment of FSUI still remains unavailable [5].
In cases where conservative treatment fails, synthetic midurethral slings (mesh or tape) are the standard surgical treatment for FSUI worldwide [6]. Another alternative is the use of autologous fascial slings, which have shown clinical success rates to synthetic slings, but with longer operative times and graft-related complications [7]. Other available surgical options include periurethral injection therapy (“bulking agents”), Burch colposuspension (open or laparoscopic), artificial urinary sphincters, and compression systems [8].
Given the variety of surgical treatments available for FSUI, there is a large amount of scientific literature that limits clinicians’ abilities to go through all the available information and formulate appropriate recommendations [9]. However, numerous associations, societies, and scientific institutions have developed clinical practice guidelines (CPGs) to support its management. Although CPGs are essential tools to keep healthcare professionals up to date when making decisions with their patients, the methodological quality of FSUI guidelines remains unknown [10].
Several methods are currently available to assess the quality and applicability of CPGs. Among these, the Appraisal of Guidelines for Research and Evaluation II (AGREE II) tool is one of the most widely used and validated tools [11]. In light of this, the aim of this study is to systematically evaluate the quality of published guidelines for the surgical management of FSUI using the AGREE II tool.
2. Methods
A systematic review was conducted to assess the methodological quality of CPGs on surgical management of FSUI published between 2017 and 2023, using the AGREE II tool [12]. This study followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for reporting systematic reviews [13]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42023420042 [14].
2.1. Research Question
The research question was structured using the PICO framework (population, intervention, comparison, outcome). The study population included patients diagnosed with FSUI without restrictions on age or ethnicity. The intervention focused on the surgical treatment of FSUI. All comparators were included, and the primary outcome of this study was to assess the methodological quality of guidelines for the surgical treatment of FSUI.
2.2. Search Strategy
The search strategy was developed based on the PICO question. Keywords used were “clinical practice guidelines,” “stress urinary incontinence,” “female,” “bladder control problems,” and “pelvic floor dysfunction.” The search was carried out on the MEDLINE/PubMed, LILACS, Scopus, and Trip Medical Database and was limited to publications between 2017 and 2023 in English and Spanish. In addition, a manual search was conducted on the websites of guideline developers, such as the National Institute for Health and Care Excellence (NICE) and the Scottish Intercollegiate Guidelines Network (SIGN), as well as in registers and journals of relevant scientific societies and professional organizations. The full search strategy is detailed in Appendix 1 in the Supporting Information.
2.3. Eligibility Criteria
CPGs that provide recommendations for the surgical management of FSUI of any etiology in women of any age were included, provided they reported their search strategy and methodology for developing recommendations.
CPGs that only provided recommendations for pharmacological management, secondary research publications (such as systematic reviews with and without meta-analysis), commentaries, abstracts, or articles developed from other CPGs on FSUI were excluded.
2.4. Selection of Guidelines
The selection of CPGs was carried out in two stages. In the first stage, three reviewers (MVP, ASR, and JMPV) independently screened the title and abstract of the identified publications following the eligibility criteria. Guidelines that met the inclusion criteria were retrieved for full-text review. In the second stage, the same reviewers (MVP, ASR, and JMPV) independently assessed the full text of the preselected CPGs. Disagreements in both stages were resolved by discussion and consensus, with a fourth reviewer (DSR) intervening when necessary. The CPG screening process was carried out using Rayyan web application (Rayyan Systems Inc.) [15]. The entire process was documented using the PRISMA flowchart presented in the results section.
2.5. Data Collection and Extraction
Data extraction was performed independently by three reviewers (MVP, ASR, and JMPV) using a standardized XLSX format in Microsoft Excel. The data included title, year of publication, organization developing the guideline, funding sources, methods used to assess the quality and validity of the evidence, methods used to formulate recommendations, language, country of origin, and main recommendations of each guideline.
2.6. Quality Assessment
The methodological quality of CPGs was assessed using the AGREE II instrument, which is a validated tool that includes 23 items divided into six domains: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence [16]. Two reviewers (JMPV and CMG) independently assessed each item in the six domains, scoring each item on a scale from 1 (strongly disagree) to 7 (strongly agree), along with an overall recommendation item for the included guidelines. Disagreements were resolved by consensus, and where disagreements persisted, a third reviewer (DSR) was consulted.
2.7. Statistical Analysis
A descriptive analysis of the guidelines was performed based on the general characteristics obtained from data extraction. To calculate and analyze the quality of the guidelines, standardized percentages were used according to the number of reviewers, as described in the AGREE II manual [17, 18]. Guidelines were considered high quality if they scored more than 60% in three or more domains, including the rigor of the development domain [16, 18, 19]. Data analysis was performed using SPSS v25.0 and Microsoft Excel. For visual representation of the results, a box plot was created to show the distribution of the standardized scores for each assessed domain, and a hexagonal radar chart was used to compare the mean scores between domains. Both graphs were developed using Microsoft Excel.
3. Results
3.1. Study Characteristics
Through the initial search, 1459 records were identified. After removing 310 duplicates, 1149 titles and abstracts were screened. 15 documents met the criteria for full-text review. After applying the eligibility criteria, six CPGs were included for data extraction [20–25] (Table 1). The CPG selection process is summarized in the PRISMA flowchart in Figure 1.
| Clinical practice guideline | First author | Publication year | Organization | Country/region | Language | Methods to assess the quality and certainty of the evidence | Reference |
|---|---|---|---|---|---|---|---|
| Surgical treatment of female stress urinary incontinence: AUA/SUFU guideline (published 2017; amended 2023) | Kobashi | 2023 | American Urological Association (AUA) | United States | English | AUA nomenclature | [20] |
| Guidelines for the evaluation and treatment of recurrent urinary incontinence following pelvic floor surgery—No. 248 | Lovatsis | 2017 | Society of Obstetricians and Gynecologists of Canada | Canada | English | Canadian Task Force on Preventive Health Care | [21] |
| European Association of Urology guidelines on the diagnosis and management of female non-neurogenic lower urinary tract symptoms. Part 1: Diagnostics, overactive bladder, stress urinary incontinence, and mixed urinary incontinence | Nambiar | 2022 | European Association of Urology | Europe | English | Modified GRADE | [25] |
| Urinary incontinence and pelvic organ prolapse in women: Management. NICE guideline | NICE | 2019 | National Institute for Health and Care Excellence (NICE) | United Kingdom | English | NICE | [22] |
| Urogynecology section of the Polish Society of Gynecologists and Obstetricians guidelines on the management of stress urinary incontinence in women | Stangel-Wojcikiewicz | 2021 | Polish Society of Gynecologists and Obstetricians | Poland | English | Not specified; mentions that it is based on the recommendation grade of available data sources | [23] |
| Clinical guidelines for female lower urinary tract symptoms (second edition) | Takahashi | 2021 | Japanese Continence Society | Japan | Japanese/English | Own classification scheme | [24] |
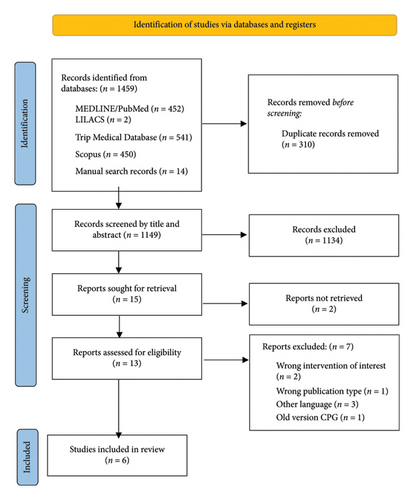
The included CPGs were published between September 2017 and March 2023. Out of the six included CPGs, one was developed in the United States, one in the United Kingdom, one in Japan, one in Poland, one in Canada, and one was regionally developed in Europe. The organizations that developed the six guidelines include the European Association of Urology, the Japanese Continence Society, the Polish Society of Gynecologists and Obstetricians, the National Institute for Health and Care Excellence (NICE), the Society of Obstetricians and Gynecologists of Canada, and the American Urological Association. All the guidelines were published in English. The characteristics of the guidelines are summarized in Table 1.
3.2. Quality Assessment
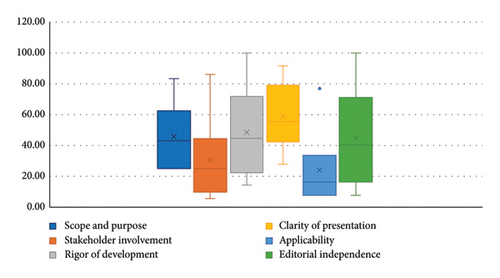
Out of the six analyzed CPGs, five (83.3%) were classified as having low quality [20, 21, 23–25], and only one (16.7%) as having high quality [22]. Figure 2 shows a box plot summarizing the statistical analysis of the standardized scores for each of the domains assessed using the AGREE II tool. In addition, Table 1 shows the standardized scores for all the evaluated domains, their total score, and the overall recommendation for each guideline.
3.2.1. Domain 1: Scope and Purpose
The scope and purpose domain, which assesses the overall aim of the guideline, the clinical questions, and the target population, had a mean score of 45.83% (SD: 22.69). Only one of the evaluated CPGs scored above 60% in this domain (Table 1).
3.2.2. Domain 2: Stakeholder Involvement
The mean score for stakeholder involvement was 30.56% (SD: 29.03). This domain examines the extent to which users are involved in the development of CPGs. One guideline achieved an outstanding score of 86.11% in this domain, while 83.33% of the evaluated guidelines did not describe stakeholder involvement (Table 2).
| Clinical practice guideline | Scope and purpose | Stakeholder involvement | Rigor of development | Clarity of presentation | Applicability | Editorial independence | Overall | Quality | General recommendation |
|---|---|---|---|---|---|---|---|---|---|
| Surgical treatment of female stress urinary incontinence: AUA/SUFU guideline (published 2017; amended 2023) [20] | 52.78 | 11.11 | 62.50 | 75.00 | 19.23 | 42.31 | 5 | Low | Recommended with modifications |
| Guidelines for the evaluation and treatment of recurrent urinary incontinence following pelvic floor surgery—No. 248 [21] | 33.33 | 19.44 | 25.00 | 52.78 | 7.69 | 7.69 | 3 | Low | Not recommended |
| European Association of Urology guidelines on the diagnosis and management of female non-neurogenic lower urinary tract symptoms. Part 1: Diagnostics, overactive bladder, stress urinary incontinence, and mixed urinary incontinence [25] | 25.00 | 30.56 | 49.04 | 47.22 | 15.38 | 61.54 | 4.5 | Low | Recommended with modifications |
| Urinary incontinence and pelvic organ prolapse in women: Management. NICE guideline [22] | 83.33 | 86.11 | 100.00 | 91.67 | 76.92 | 100.00 | 6.00 | High | Recommended |
| Urogynecology section of the Polish Society of Gynecologists and Obstetricians guidelines on the management of stress urinary incontinence in women [23] | 25.00 | 5.56 | 14.42 | 27.78 | 7.69 | 19.23 | 2 | Low | Not recommended |
| Clinical guidelines for female lower urinary tract symptoms (second edition) [24] | 55.56 | 30.56 | 40.38 | 58.33 | 17.31 | 38.46 | 4.5 | Low | Recommended with modifications |
3.2.3. Domain 3: Rigor of Development
The domain of development rigor, which assesses evidence search, synthesis process, and the formulation of recommendations, had a mean score of 48.56% (SD: 30.42). The guideline developed by NICE achieved the maximum score of 100% in this domain (Table 2).
3.2.4. Domain 4: Clarity of Presentation
The mean score of clarity of presentation was 58.80% (SD: 22.25); this domain assesses the language, structure, and the guideline’s format. Two of the six evaluated CPGs scored above 60% (Table 2).
3.2.5. Domain 5: Applicability
The applicability domain received the lowest mean score, which was 24.04% (SD: 26.36). This domain analyzes barriers and facilitators to guideline implementation, implementation strategies, and resource implications. Scores in this domain ranged from 7.69% to 76.92% (Table 3).
| Recommendations | Clinical practice guideline | ||||||
|---|---|---|---|---|---|---|---|
| Clinical guidelines for female lower urinary tract symptoms [24] | Surgical treatment of female stress urinary incontinence: AUA/SUFU guideline (published 2017; amended 2023) [20] | Urogynecology section of the Polish Society of Gynecologists and Obstetricians guidelines on the management of stress urinary incontinence in women [23] | European Association of Urology guidelines on the diagnosis and management of female non-neurogenic lower urinary tract symptoms. Part 1: Diagnostics, overactive bladder, stress urinary incontinence, and mixed urinary incontinence [25] | Guidelines for the evaluation and treatment of recurrent urinary incontinence following pelvic floor surgery—No. 248 [21] | Urinary incontinence and pelvic organ prolapse in women: Management. NICE guideline [22] | ||
| Midurethral slings | Tension-free vaginal tape (TVT) technique | X | X | X | X | ||
| Transobturator tape (TOT) | X | X | X | ||||
| Single incision sling | X | X | X | X | X | X | |
| Traditional suburethral sling surgery | X | ||||||
| Pubovaginal sling (rectus fascia) | X | X | X | ||||
| Open colposuspension | X | X | X | ||||
| Laparoscopic colposuspension | X | X | X | X | X | ||
| Anterior colporrhaphy | X | X | |||||
| Vesical neck needle suspension | X | X | X | ||||
| Artificial urinary sphincter | X | X | X | X | X | ||
| Repair of paravaginal defects | X | X | |||||
| Periurethral injection of bulking agents | X | X | X | X | X | ||
| Urinary diversion | X | ||||||
| Marshall–Marchetti–Krantz procedure | X | ||||||
| Adjustable continence therapy | X | ||||||
| Urethral catheterization | X | ||||||
3.2.6. Domain 6: Editorial Independence
The editorial independence domain, which assesses the influence of conflicts of interest on recommendations, had a mean score of 44.87% (SD: 32.88). Two of the six CPGs evaluated scored above 60%, with scores ranging from 7.69% to 100% (Table 2).
3.2.7. Comparative Assessment of the Domains
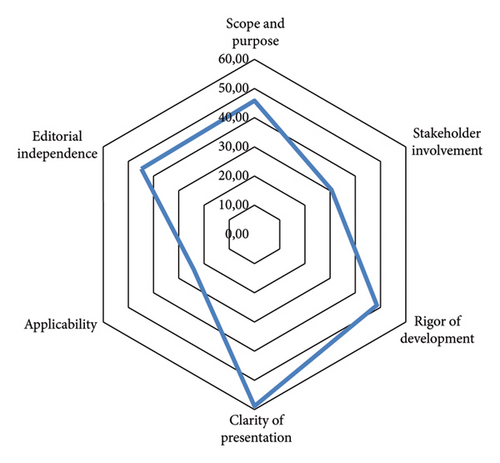
To compare the scores of all the evaluated domains across guidelines, a hexagonal radar chart was created, with the maximum score for each domain corresponding to each vertex of the inner hexagon (Figure 3). The “clarity of presentation” domain had the highest score among all the guidelines for the surgical management of FSUI. The “scope and purpose,” “editorial independence,” and “rigor” domains showed similar scores; however, the “applicability” domain showed a significantly lower standardized mean score across all the evaluated guidelines.
3.2.8. Overall Rating
One of the six CPGs evaluated (16.7%) was “recommended by reviewers” [22], three CPGs (50%) were “recommended with modifications” [20, 24, 25], and two CPGs (33.3%) were “not recommended” [21, 23]. The recommended guideline [22] was the only one to meet the criterion of scoring above 60% in three or more domains, including rigor of development. This guideline was developed by NICE in the United Kingdom (Table 2).
3.3. Recommendations
In terms of key surgical recommendations, all guidelines advocated for the use of midurethral slings, particularly single incision slings. In addition, laparoscopic colposuspension and artificial urinary sphincters were recommended when appropriate. Periurethral injection of bulking agents was also widely recommended in all guidelines, in contrast to other surgical treatments such as adjustable continence therapy, urethral catheterization, and the Marshall–Marchetti–Krantz procedure, which were mentioned in only one guideline (Table 3).
4. Discussion
CPGs have been designed to support healthcare professionals on making evidence-based decisions and they are essential to bridge the gap between the growing scientific literature and daily clinical practice [26]. Several CPGs have been published in the fields of gynecology, obstetrics, and urology, highlighting the need for a rigorous evaluation of their quality to ensure their appropriate clinical application. This study focused on the systematic evaluation of the quality of guidelines for surgical management of FSUI. Six CPGs met the inclusion criteria [20–25].
According to this study, most CPGs were of poor quality, showing the need to improve the methodological rigor in the development of these clinical documents. Only one CPG was considered of high quality [22], as it had a comprehensive methodological approach and it was recommended by the reviewers. Among the other guidelines, three CPGs were recommended with modifications [20, 24, 25] and two were not recommended [21, 23].
Examination of the different domains assessed by the AGREE II tool revealed certain areas that require greater attention and improvement. For example, the applicability domain received the lowest mean score, suggesting that CPGs lack clear approaches and effective strategies to facilitate their practical use and overcome potential barriers in clinical care. This lack of applicability could limit the effectiveness of the recommendations and their impact on daily clinical practice. The NICE guideline, which was of high quality, could serve as a model for implementation and to consider resource implications when developing future CPGs.
Similarly, most of the evaluated CPGs had a poor performance in terms of stakeholder involvement. The lack of explicit descriptions on the involvement of different stakeholders and potential users in the development process may affect the representativeness and acceptability of the recommendations. As FSUI has a significant impact on quality of life, it is important to consider patient preferences when making surgical treatment decisions.
Among other domains, clarity of presentation achieved the highest score among the evaluated CPGs, while rigor of development had large variability among guidelines, with only one guideline achieving the maximum score. The latter is particularly important because it directly influences the reliability of surgical recommendations. Furthermore, the heterogeneity in the methodological quality may explain some differences in the recommendations between guidelines. However, despite differences in methodological quality, guidelines consistently recommend midurethral slings as first-line surgical treatment.
A strength of this study was the use of the AGREE II tool to analyze the quality of CPG, which provided a robust and standardized basis for comparing and measuring different aspects of CPGs, thereby increasing the objectivity and reliability of the results. Additionally, this is the first study to systematically evaluate the methodological quality of guidelines for the surgical management of FSUI.
On the other hand, a limitation of this study was that the included guidelines were only published in English, which does not provide us with information on the quality of CPGs that might be published in other languages. In addition, since this study only focused on the quality of CPGs, future research is recommended to assess the clinical relevance of the recommendations. Additionally, studies are needed to examine specific barriers to the implementation of surgical recommendations in different health settings.
It is essential that future CPGs in this area improve their methodological rigor and consider practical aspects of implementation. Systematic inclusion of patient perspectives and consideration of local resources and barriers could improve the applicability and acceptability of recommendations. The development of specific implementation tools and the evaluation of the impact of guidelines on clinical practice should also be priorities for future research.
5. Conclusions
This study provides insight into the current status of guidelines recommending surgical management of FSUI. The findings suggest that the quality of these guidelines needs to be improved. Guidelines need to be clearer, more precise, and based on the best available evidence. They should consider patient preferences and clinical expertise. New CPGs should focus on greater methodological rigor to ensure that recommendations are based on robust evidence, reflecting patient preferences, and incorporating clinical expertise.
Furthermore, future guidelines for the surgical management of FSUI should be developed by multidisciplinary teams comprising a diverse group of experts in the field of FSUI, including gynecologists, urologists, physiotherapists, and patients. Patient involvement will ensure that recommendations consider the needs and preferences of those affected by the condition, thus increasing their applicability, acceptability, and the utility of recommendations. Similarly, groups responsible for developing these guidelines should establish clear parameters for the disclosure and management of potential conflicts of interest among group members. This approach emphasizes transparency in guideline development and reduces recommendations’ risk of bias.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors declare that they have self-funded this research.
Supporting Information
Supporting Information includes detailed information about each database’s search strategy.
Open Research
Data Availability Statement
The data supporting this review come from previously reported studies and datasets that have been cited. The processed data are available on request from the corresponding author.




