Probiotics′ Effects on Depression, High-Sensitivity C-Reactive Protein (hs-CRP), and Oxidative Stress in Patients With Multiple Sclerosis: A Meta-Analysis
Abstract
Objectives: Major depressive disorder (MDD) is highly prevalent in patients with multiple sclerosis (MS). There is evidence that the gut microbiota affect the inflammatory responses and oxidative status in MS and may affect depression signs and symptoms in these patients. Based on this fact, this updated meta-analysis assessed the effect of probiotic supplementation on the Beck Depression Inventory (BDI), high-sensitivity C-reactive protein (hs-CRP), total antioxidant capacity (TAC), and malondialdehyde (MDA) in patients with MS.
Methods: A systematic literature search was done for English literature through PubMed/Medline, Cochrane Library, Google Scholar, and ScienceDirect from 1 January 2011 to 1 June 2024. This study followed the PRISMA guidelines. Based on the heterogeneity, the random-effects or fixed-effects models were used in the present meta-analysis.
Results: After checking for eligibility, four RCTs were included in the final analysis. There was an improvement in BDI-II score, hs-CRP level, MDA, and TAC in patients with MS (BDI-II score: MD = −2.78; 95% CI, −3.32 to −2.24; Z = 10.09; p < 0.00001; I2 = 57%; p = 0.1; hs-CRP level: MD = −1.69; 95% CI, −1.98 to −1.40; Z = 11.38; p < 0.00001; I2 = 0%; p = 0.96; MDA: MD = −0.37; 95% CI, −0.52 to −0.23; Z = 5.00; p < 0.00001; I2 = 90%; p < 0.0001; and TAC: MD = 5.48; 95% CI, −6.71 to 17.66; Z = 0.88; p = 0.38; I2 = 93%; p < 0.00001).
Conclusion: This meta-analysis suggests that probiotic supplementation has improved BDI scores and inflammatory and oxidative status. This finding promises that probiotic supplementation can be considered as a therapeutic option for depression symptoms and inflammatory status in patients with MS. However, more interventional studies are required to assess the complex relationship between MS, depression, and the gut microbiome. PROSPERO Registration ID: CRD42024552648.
1. Introduction
Multiple sclerosis (MS) is a progressive autoimmune disease that is characterized by demyelination of the central nervous system (CNS) [1]. It is one of the most important reasons for disability in young adults with a higher prevalence in women [2]. The worldwide prevalence of patients with MS is 2.8 million, and its prevalence has increased since 2013 [3].
T helper 1 cells (Th1) and T helper 17 cells (Th17) and proinflammatory cytokines have an important role in the pathophysiology of MS [4].
Major depressive disorder (MDD) is highly prevalent (up to 54%) in MS. Depression leads to worsening of MS symptoms and a significant reduction in quality of life [5]. Inflammatory processes and the gut–brain axis (GBA) are important factors in the pathophysiology of depression [6]. GBA is the physiological idea that consists of all endocrine, immune, metabolic, neural, and molecular pathways that have roles in signaling between the gastrointestinal tract and the CNS [7]. It is a link between the gut microbiome and the CNS called GBA. There is a direct relationship between the CNS and the intestine. Two factors affect this relationship: the autonomic nervous system and the gut microbiome [8]. The gut microbiome composition is affected by the enteric nervous system and changes in the intestinal environment (like pH, motility, or permeability) [9–11].
The topic of probiotics explains that their use is growing in many fields. Recently introduced compounds such as postbiotics [12] and paraprobiotics [13] showed promising results in many fields of medicine. There is evidence that probiotic supplements or fecal microbiota transplantation (FMT) impacts microbiome composition in patients with MS and the inflammatory responses [14, 15]. This will change the microbiota composition and function, improving inflammatory processes and immune function in patients with autoimmune diseases like MS [16–18].
Previous clinical trials have shown that probiotic supplementation can affect high-sensitivity C-reactive protein (hs-CRP) as an inflammatory marker, total antioxidant capacity (TAC), and malondialdehyde (MDA) as biological markers for oxidative stress, as well as the Beck Depression Inventory (BDI) score, which assesses characteristics and symptoms of depression in patients with MS [7, 19–21]. This evidence suggests that probiotic supplementation has a significant impact on the gut microbiome composition and leads to lower inflammation and improved depression symptoms.
While prior studies suggest probiotics may reduce inflammation and depressive symptoms, many have been limited by small sample sizes and heterogeneous methodologies. Based on the previously mentioned evidence, the small number of trials, and the small sample sizes, this updated meta-analysis, due to the population studied and some significant results, can provide us with better statistical power and effect size to evaluate the effects of probiotic supplementation on depressive symptoms and inflammatory status in patients with MS [7, 19–21]. There is a link between probiotics, inflammation, depression, and MS in separate studies, and this updated meta-analysis provides us with evidence-based results for a better understanding of this link.
2. Materials and Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline for assessing the effect of probiotic supplementation on inflammatory status and depression symptoms in patients with MS [22].
The protocol of this systematic review and meta-analysis has been prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) (Registration ID: CRD42024552648).
2.1. Population, Interventions, Comparators, Outcomes, and Study Designs (PICOS)
Population: Patients with MS are included in this study.
Intervention: The effect of probiotic supplementation on inflammatory status and depression symptoms was assessed.
Comparators: We assessed the effect of the probiotics compared with placebo on BDI score, hs-CRP, TAC, and MDA in patients with MS.
Outcomes: Changes in BDI score, hs-CRP, TAC, and MDA in patients with MS were assessed.
Study designs: A systematic literature search was done for English literature through PubMed/Medline, Cochrane Library, Google Scholar (Cochrane guideline suggested it as gray literature), and ScienceDirect from 1 January 2011 to 1 June 2024.
Search terms included multiple sclerosis, probiotics, depression, BDI, hs-CRP, TAC, and MDA. Moreover, for articles not found in four databases, investigation was done from reviews and reference lists of relevant manuscripts. Abstracts and conference proceedings are not included. Piloted forms are used for data extraction.
The primary endpoints were BDI score and hs-CRP level and secondary endpoints were TAC and MDA. In original papers when analysis of covariance (ANCOVA) was done, the variables were adjusted for baseline measures (including age, sex, education, marriage status, living arrangements, time since MS diagnosis, and BMI).
All the relevant studies that have reported mean ± SD changes for BDI-II, hs-CRP, TAC, and MDA for the association of probiotic supplementation with depression and inflammatory status in patients with MS were included. Only English-language studies were included, and animal studies were excluded. Furthermore, we have excluded all studies that had the wrong design (review, letter, guideline, and paper without primary clinical data) and those that did not assess the required data for meta-analysis.
We used these MeSH terms (but not limited to these words): MS (multiple sclerosis); probiotics; C-Reactive Protein; Depression; Depressive Disorder; Inflammation; randomized controlled trial.
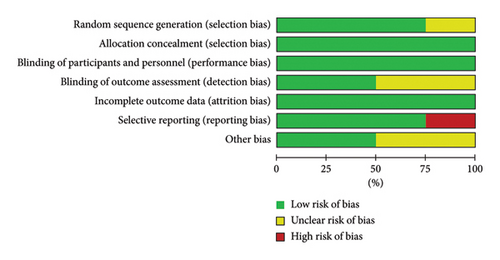
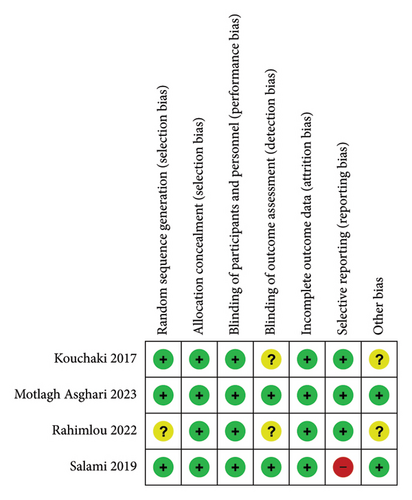
The Cochrane Collaboration risk of bias tool [23] was used to assess the risk of bias for randomized clinical trials (RCTs). Review Manager Version 5.4 software was used for evaluating the risk of bias [24]. Seven items have been evaluated with the Cochrane Collaboration risk of bias tool, which includes random sequence generation for selection bias, allocation concealment for selection bias, blinding of participants and personnel for performance bias, blinding of outcome assessment for detection bias, incomplete outcome data for attrition bias, selective reporting for reporting bias, and other bias.
The fixed-effects or random-effects analysis models with the inverse-variance method (with Review Manager Version 5.4 software) were used to evaluate the effects of probiotic supplementation versus placebo on the BDI scores, hs-CRP, TAC, and MDA. The model selection was based on the heterogeneity for each outcome.
A p value < 0.05 was considered statistically significant, and effect size was considered as 95% confidence interval (95% CI) in the analysis.
For evaluating between-study heterogeneity, I2 and chi-square tests were used. I2 was stratified into three categories for heterogeneity: low (0%–50%), moderate (51%–75%), or high (> 75%). If the number of included studies is sufficient, funnel plots and Egger regression asymmetry analysis will be done for assessing publication bias [25].
3. Results
3.1. General Characteristics of Studies
The characteristics (mean age, intervention interval, probiotic strains, probiotic dose, and sample size (placebo group/probiotic group)) of the included manuscripts in this study are demonstrated in Table 1. After eligibility evaluation, four manuscripts with 235 patients were included in the final meta-analyses [7, 19–21]. The flow diagram for the selection of manuscripts is demonstrated in Figure 1.
| Study (first author, year) | Mean ± SD age (years) (placebo group/probiotic group) | Intervention interval | Dosage (CFU/g) | Type of probiotic strains | Sample size (placebo group/probiotic group) |
|---|---|---|---|---|---|
| Kouchaki, et al. 2017 [19] | 33.8 ± 8.9/34.4 ± 9.2 | 12 weeks | 2 × 109 CFU/capsule (one capsule daily) | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum | 30/30 |
| Salami, et al. 2019 [20] | 36.54 ± 1.44/34.79 ± 1.06 | 16 weeks | 2 × 109 CFU/capsule (one capsule daily) | Bifidobacterium infantis, Bifidobacterium lactis, Lactobacillus reuteri, Lactobacillus casei, Lactobacillus plantarum, and Lactobacillus fermentum | 33/32 |
| Rahimlou, et al. 2022 [21] | 39.9 ± 8.76/42.15 ± 11.98 | 24 weeks | 2 × 109 CFU/capsule (two capsules daily) | Bacillus subtilis PXN 21, Bifidobacterium bifidum PXN 23, Bifidobacterium breve PXN 25, Bifidobacterium infantis PXN 27, Bifidobacterium longum PXN 30, Lactobacillus acidophilus PXN 35, Lactobacillus delbrueckii ssp. bulgaricus PXN 39, Lactobacillus casei PXN 37, Lactobacillus plantarum PXN 47, Lactobacillus rhamnosus PXN 54, Lactobacillus helveticus PXN 45, Lactobacillus salivarius PXN 57, Lactococcus lactis ssp. lactis PXN 63, Streptococcus thermophilus PXN 66 | 35/35 |
| Asghari, et al. 2023 [7] | 34.95 ± 7.03/33.80 ± 1.37 | 16 weeks | 1 × 1010 CFU/capsule (one capsule daily) | Saccharomyces boulardii | 20/20 |
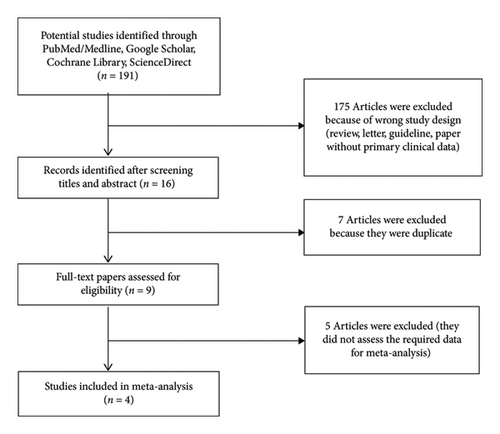
3.2. Meta-Analysis
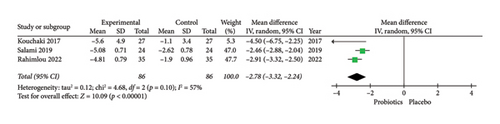
For the assessment of probiotic supplementation on depression, three RCTs included 172 patients with MS in the probiotics, and the control groups were included. Due to the moderate heterogeneity among the included manuscripts (Figure 2), a random-effects model was used. The results of the meta-analysis suggested a significant difference between the probiotics and placebo groups in improving depression based on BDI-II score in patients with MS (BDI-II score: MD = −2.78; 95% CI, −3.32 to −2.24; Z = 10.09; p < 0.00001; I2 = 57%; p = 0.1). The related forest plot of the analysis is shown in Figure 2.
For the assessment of probiotic supplementation on inflammatory status, three RCTs that included 148 patients with MS in the probiotics and control groups were included. Due to the low heterogeneity among the included manuscripts for hs-CRP level (Figure 3), a fixed-effect model was used.
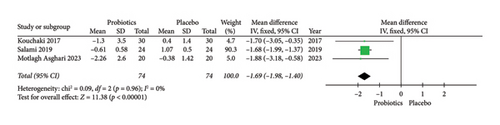
The results of the meta-analysis suggested a significant difference between the probiotic supplementation and placebo groups in improving inflammatory status based on hs-CRP level in patients with MS (MD = −1.69; 95% CI, −1.98 to −1.40; Z = 11.38; p < 0.00001; I2 = 0%; p = 0.96). The related forest plot of the analysis is shown in Figure 3.
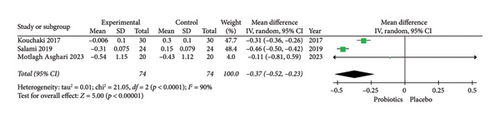
Furthermore, the results of the meta-analysis suggested a significant difference between the probiotics and placebo groups in improving one of the biological markers for oxidative stress (MDA) in patients with MS (MDA: MD = −0.37; 95% CI, −0.52 to −0.23; Z = 5.00; p < 0.00001; I2 = 90%; p < 0.0001; and TAC: MD = 5.48; 95% CI, −6.71 to 17.66; Z = 0.88; p = 0.38; I2 = 93%; p < 0.00001). Due to the high heterogeneity among the included manuscripts for MDA and TAC (as biological markers for oxidative stress) (Figures 4(a) and 4(b)), a random-effects model was used. The related forest plot of the analysis is shown in Figures 4(a) and 4(b).

Review authors’ judgments about the risk of bias in the included studies are shown in Figure 5. The following categories have a low risk of bias in all of the included studies: allocation concealment for selection bias, blinding of participants and personnel for performance bias, and incomplete outcome data for attrition bias. We used risk of bias assessments in our evidence synthesis based on Cochrane Methods guidelines, representing each included study and how strong and valid it is across several quality criteria. It helps to ensure transparency and clarity in evidence synthesis findings [26]. For assessing reliability and sensitivity analysis, each study was excluded separately. We did not find any significant changes in our pooled results. Assessing publication bias with funnel plot (as visual analysis) and Egger’s intercept test was impossible due to the insufficient number of studies [25].
4. Discussion
This meta-analysis has shown that probiotic supplementation is associated with a significant reduction in depression symptoms and inflammatory status in patients with MS. Furthermore, for oxidative stress, there was a significant reduction in MDA after probiotic supplementation. However, for TAC (as a biological marker for oxidative stress), the results were not significant after probiotic supplementation. All of the included studies in this meta-analysis were randomized controlled trials. There was evidence of moderate heterogeneity for the BDI score, low heterogeneity for hs-CRP, and high heterogeneity for MDA and TAC in the meta-analysis.
The topic of probiotics explains that their use is growing in many fields. Recently introduced compounds such as postbiotics [12] and paraprobiotics [13] showed promising results in many areas of medicine. In the recent decade, the gut microbiome has been considered an important link to health and disease based on its effects on the immune system and neuroinflammation.
Previous studies have shown that gut microbiome composition is different when compared to patients with MS and healthy subjects. There is a reduction in the genera Lactobacillus, Bacteroides, Prevotella, and Parabacteroides and also a higher level of Blautia, Akkermansia, and Ruminococcus in the patients with MS [25]. MS symptoms could be affected by changes in the gut microbiome composition, and the gut microbiome is affected by the enteric nervous system and changes in the intestinal environment (like pH, motility, or permeability) [7]. There is evidence that probiotic supplements or FMT impacts microbiome composition in patients with MS and the inflammatory responses [8, 9]. This will change the gut microbiome composition and function, improving inflammatory processes and immune function in patients with autoimmune diseases like MS [10–12]. Based on the previous evidence, this meta-analysis assumes that probiotic supplementation can change the gut microbiome composition and function and improve dysbiosis. This improvement in dysbiosis will enhance the inflammatory status and interactions between the gut and the brain.
Furthermore, two meta-analyses that assess the effects of probiotic supplementation on inflammatory markers in patients with neurological disorders and opioid-related disorder have shown that taking probiotics had beneficial effects on CRP levels, MDA, and TAC in these patients [27, 28]. These results are similar to our results that have shown probiotics lead to a reduction in inflammatory markers in patients with MS in related ways. In another meta-analysis when the researchers assessed the effects of probiotic supplementation on patients with psychiatric disorders, the results showed that probiotic supplementation did not affect BDI scores in these patients [29]. This difference with our results may be due to the main role of inflammatory processes in patients with MS. The results of our meta-analysis are similar to previous RCTs that have shown probiotics improve BDI scores [14–16]. There are previous studies that have shown an increase in inflammatory status in depression [6, 27]. In autoimmune diseases like MS, a higher state of inflammation and gut microbiome composition has a direct impact on it [11]. This connection can justify the results of this meta-analysis, which shows a significant reduction in BDI score after probiotic supplementation. Furthermore, this meta-analysis shows a significant reduction in hs-CRP levels after probiotic supplementation. There may be a link between lower inflammatory status and depressive symptoms. This result is the same as previous evidence that gut microbiome composition is an important factor for inflammatory status [10–12, 28]. Besides hs-CRP, some microbial metabolic pathways affect the inflammatory cytokine responses like TNFα and IFNγ [28]. The changes in the gut microbiota also affect anti-inflammatory cytokines (modulate cytokine levels) and host immune systems by increasing Treg cells [7]. Furthermore, probiotics can modulate 5-hydroxytryptamine receptors and dopamine and lead to neurotransmitter changes and effects on GBA [30]. This effect can explain the effects of probiotics on depression. Besides these effects, an increase in short-chain fatty acid (SCFA) production may contribute to the beneficial effects of probiotics on GBA [30]. This evidence can explain the results of this meta-analysis and its probable connection to the changes in gut microbiome composition.
On the other hand, in this meta-analysis regarding oxidative stress, there was a significant reduction of MDA as a biological marker for oxidative stress in patients with MS after probiotic supplementation. One of the most important items considered in the pathogenesis of these patients is oxidative stress and higher production of reactive oxygen species (ROS). MDA is a marker that shows lipid peroxidation. Based on the previous evidence, there is a higher level of MDA in patients with MS, especially those who are not taking interferon (IFN-β) or during relapses [29]. The results of this meta-analysis suggest that manipulation of the gut microbiome leads to oxidative stress reduction. These changes are in line with the lower inflammatory status after probiotic supplementation in patients with MS. Similarly, the results of this meta-analysis show an increase in serum TAC after probiotic supplementation. However, the result was nonsignificant.
Recently, the GBA has had a potential effect on neurological diseases like MS. The changes in the gut microbiota influence the inflammatory status and immune processes in the human body that affect many diseases and conditions, such as gastrointestinal disorders, endocrine diseases, and CNS disorders. The pathogenic gut microbiome leads to a higher inflammatory status and changes the oxidative balance to oxidative stress [7, 8, 10–12, 27].
The suggested mechanism is that probiotic supplementation can alter the gut microbiome composition, and the consequence is lower inflammatory status and oxidative stress. This mechanism can explain the results of this meta-analysis, which shows a significant reduction in depressive symptoms, hs-CRP level, and MDA, as well as an increase in TAC after probiotic supplementation in patients with MS. The high heterogeneity for MDA and TAC in our meta-analysis suggests differences in study participants, sample size, and probiotic interventions that can be regarded in future studies. Based on these results, probiotic supplementation could be regarded as a nonpharmacological intervention for the improvement of MS symptoms and progression, which includes depression. It can also be considered a safe therapeutic option in MS patients since all of the included studies did not report any serious adverse effects [7, 19–21]. In one of the included studies, minor side effects consisting of nausea (10.0%), constipation (12.5%), and weight gain (12.5%) were reported [7].
The first important limitation of this study is the limited number of RCTs that have assessed the effects of probiotic supplementation on MS depressive symptoms, as well as inflammatory and oxidative status. This limited number of RCTs makes it impossible to assess publication bias with a funnel plot and Egger’s intercept test. We have potential limitations at the study level and outcome level. Factors such as selection bias, small sample size, and confounding factors exist. At the outcome level, limitations such as poorly defined outcomes, use of unreliable tools, and short follow-up periods could be considered. We also have potential limitations at the review level, such as language and database levels; however, we try to decrease these with cross-link checks and by assessing previous reviews. The strength of this updated meta-analysis is that it can give us a better statistical power and effect size to evaluate the effects of probiotic supplementation on depressive symptoms and inflammatory status in patients with MS.
Certainly, more RCTs with a larger sample size and more specific probiotic strains are needed to assess the effects of probiotic supplementation on depressive symptoms, as well as inflammatory and oxidative status in patients with MS. Especially for the future, studies that focus on more specific probiotic strains that act specifically on the GBA seem to be promising.
5. Conclusions
MS is a progressive autoimmune disease that is characterized by demyelination of the CNS. MDD is highly prevalent in MS and leads to worsening of MS symptoms and a reduction in quality of life. Inflammatory processes and the GBA are important factors in the pathophysiology of MS and, also, depression in these patients.
The results of this meta-analysis suggest that probiotic supplementation is associated with a significant reduction in depressive symptoms, hs-CRP levels, and MDA and an increase in TAC in patients with MS. These results are promising in that probiotic supplementation can be considered a safe, convenient, and inexpensive intervention for depression symptoms and inflammatory status in patients with MS. Certainly, more RCTs with a larger sample size and more specific probiotic strains are needed to assess these effects.
Ethics Statement
The authors have nothing to report.
Disclosure
An earlier version of the manuscript has been presented as conference abstract in “Current Developments in Nutrition [31].”
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
S.-A.T. designed the study, S.-A.T. and N.T. performed systematic search and selected the studies, S.-A.T. analyzed the data, S.-A.T. and N.T. prepared the manuscript and critically reviewed the manuscript, and S.-A.T. had primary responsibility for final content. All authors have read and approved the final manuscript.
Funding
The authors have not received any sources of funding and other support.
Supporting Information
PRISMA checklist was compiled.
Open Research
Data Availability Statement
The data are available from the corresponding author upon reasonable request.




