Visual Neuroprostheses for Impaired Human Nervous System: State-of-the-Art and Future Outlook
Abstract
Background: Visual neuroprostheses refer to devices or systems that restore the impaired visual system caused by severe degeneration or damage to the retina, optic nerve, or brain.
Current Status of the Topic: Current clinical applications encompass a wide range of areas, including various technologies such as introducing engineered living tissues or microfabricated photoreceptor arrays and implantable electronic microsystems, referred to as visual prostheses solutions to restore vision. Among them, visual neuroprosthesis harbors the most promising advantage by artificially receiving image information from the outside world and delivering it to the natural visual system, enabling the subject to obtain a meaningful perception of the image.
Problem Statement: Although the classification and the implementation of visual neuroprostheses have been discussed in several articles, very limited works have focused on the electrode-based, up-to-date approaches incorporated into these devices.
Aim of the Study: This review discusses four main types of visual neuroprosthesis approaches, including retinal approaches, lateral geniculate nucleus (LGN) approaches, optic nerve approaches, and cortical approaches, along with their constituent electrodes, metals, and stimulation patterns. Using existing neuroscience knowledge, the prospects of these devices are also highlighted.
1. Introduction
Currently, around 30 million visually handicraft people suffer from severe retina, optic nerve, visual cortex, or brain degeneration due to several factors ranging from traumatic injury to neurodegenerative diseases [1, 2]. One of the most common causes of visual impairment is age-related macular degeneration (AMD), which occurs due to the rapid and gradual degradation of photoreceptor cone cells that leads to damage of the macula inside the retina [3]. Other photoreceptor cells in the retina, called rod cells, are also affected by the retinitis pigmentosa (RP) disease because of the faulty encoding genes [4]. Although, in recent years, the advancement of molecular biology and molecular genetics enabled the identification and character of numerous genes responsible for visual disease, very few successful approaches have been seen to restore human vision problems. On the other hand, some proportion of advancement has been suggested to develop an artificial vision by the combined approach of molecule biology and computational biology; these approaches still require more improvement to receive a successful output [5–9].
In this case, neuroprostheses for visual implants offer a significant solution to restoring normal visual ability. Visual neuroprostheses are medical devices that aim to provide some degree of vision by substituting impaired motor, sensory, or cognitive body organs [10, 11]. Most of them are constructed by incorporating common components, one or more cameras, dedicated neuroprosthetic devices that provide patterns of electrical simulations, and microelectrodes. Cameras are located outside the patient’s body to send information about the object into the neuroprosthetic devices set up inside the body. This information is transformed into microelectrodes through a pattern of electrical simulations. Microelectrodes are incorporated at any part of the visual pathway to visualise the object in front of the camera [12].
The visual pathway comprises the retina, optic nerves, optic chiasma, optic tracts, lateral geniculate bodies, optic radiations, and visual cortex [13]. In this visual pathway, a network of interconnected neurons processes our surroundings, beginning with the optic nerve in the eye and extending to the visual cortex in the forebrain. All information is transmitted through nerve impulses that are initiated by photosensitive chemical reactions that transpire in the retina. There are numerous parallel and distinct pathways that encode its processing at various locations throughout the nervous system [14]. The retino-geniculo-cortical pathway is one of the crucial pathways that transmits visual information from the retina to the visual cortex in the brain. Despite the existence of alternative pathways that transmit visual information to the visual cortex, the retino-geniculo-cortical pathway is the primary pathway for visual signal transmission to the cerebral cortex. Information regarding the movement and timing of objects within a visual scene, as well as their brightness, color, and contrast, is transmitted through the retino-geniculo-cortical pathway [15]. This pathway initiates the processing of visual information at the retina, where light interacts with photoreceptors. Those signals travel via the optic nerve to the lateral geniculate nucleus (LGN) in the thalamus, which then sends the processed information to the primary visual cortex in the occipital lobe. Then, the brain assembles the final image [16]. Based on the neuroanatomy and visual pathway, the main development approaches of visual neuroprostheses are of four categories: retinal approaches, LGN, optic nerve, and cortical approaches [12].
This paper focuses on the recently developed neuroprostheses covering these four visual approaches. The natural and synthetic biomaterials used to construct these neuroprosthetic devices are discussed. Before introducing the neuroprosthetic devices and their constituent biomaterials [17, 18], the visual pathway and their impairment causes are explained.
2. Neural Activity in the Visual Nervous System
The human visual system is composed of the sensory organ (the eye) and the central nervous system (CNS) (retina, the optic nerve, the LGN, and the visual cortex) (Figure 1). The image that is received by the lens of the eye is converted into neural signals that are transmitted to the LGN and visual cortex of the brain through the optic nerve for visual reorganization [19].
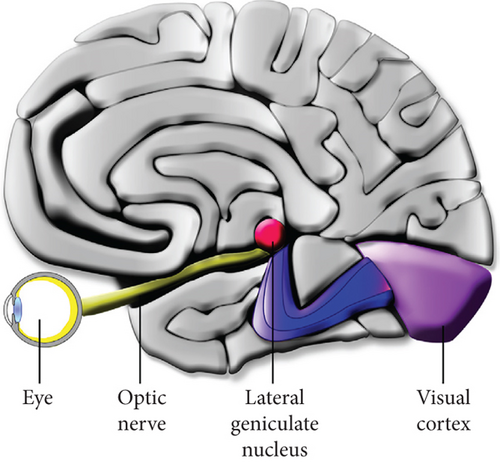
The human eye is approximately spherical in structure with about 2.4 cm in diameter. The first layer of the human optic system is made up of a cornea and a lens [19] (Figure 2). The cornea is an immune-privileged, dome-shaped, and transparent membrane that makes contact with transparent aqueous humour located just behind the cornea. The special membrane structure of the cornea allows it to interface between air and liquid environments on the eyeball’s surface, prohibiting foreign materials from entering the membrane [21]. Besides serving as a principal barrier to fluid and pathogens, the corneal endothelium membrane significantly enhances the precision of light passing through the cornea [22]. The high fidelity of outer lights is then forwarded towards the pupil, the opening recenter of the iris, to allow the light to focus on the retina. Iris aids in changing the diameter of the pupil from about 2 to 8 mm, allowing the retina to absorb the appropriate amount of light for the processing of sight. Moreover, by changing the lens’s shape and the lens shape’s focal distance, the eye’s focal distance can be adjusted, which allows for the absorption of more focused light on the retina [19].
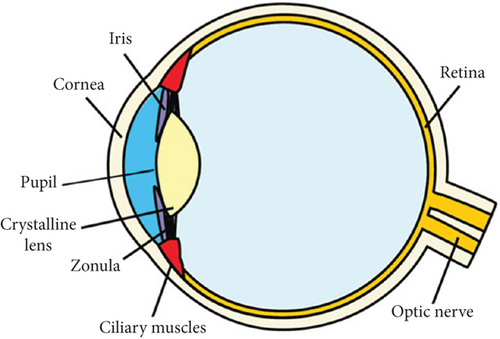
The retina is a part of the CNS, which is often referred to as a “window to the brain” [23]. It comprises different types of cells and thousands of neurons that accomplish the neural activity of the visual pathway. Two types of photoreceptors, the rod and the cone photoreceptor cell, are found inside the retinal structure stimulated by the light through the retinal pigment epithelium membrane. Cone cells are located at the center of retinal anatomy, which detect the image’s color and detail in daytime vision, whereas rod cells are found at the peripheral site, contributing to nighttime vision in poor lighting. The retina’s external portion contains a special disc membrane called the opsin protein that absorbs photons. When this opsin protein combines with pigment in retinal cone cells, it converts into rhodopsin to play a central role in phototransduction [24, 25]. Other branches of cells, such as bipolar cells, Muller cells, and amacrine cells, propagate signals to retinal ganglion cells. Signal outputs are further sent by the ganglion cells as a bundle of signal output axons to the optic nerve. Multiple neurotransmitters such as glutamate, glycine, GABA, and dopamine transmit signals from the retinal portion to the LGN and visual cortex [25].
In the human visual pathway, the optic nerves from both retinas of the eyeballs join together at a point called optic chiasm (Figure 3) that aims to assist humans with in-depth perception [26]. This optic chiasm divides into optic tracts, which consist of bundles of nerve fibers or axons originating from the retinal ganglion cells that carry visual signals to the left and right lateral geniculate bodies [27].
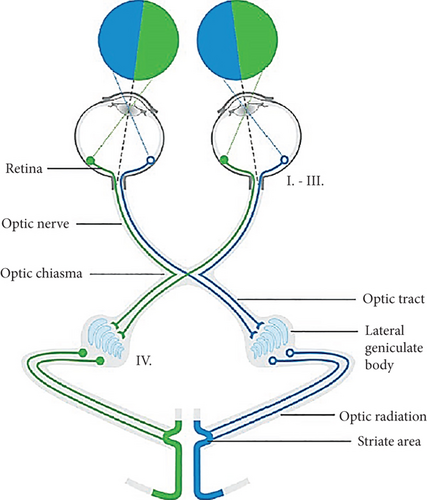
The lateral geniculate body, also known as the LGN, is organized into several positions of the extrastriate cortex, including the optic radiation and striate area. The main objective of these structures is to collect information from retinal ganglion signals and attend to visual processing [28].
Thus, the human visual process combines a series of cells and signal transduction pathways that transmit visual information from the outside environment to the brain for processing [29]. Each organ related to this process is prone to potential injuries by several factors, such as chemical burns, traumatic injury, contact abrasion, glaucoma, or neurodegenerative diseases [21, 30]. Besides various surgical and biomedical approaches, visual neuroprosthetic treatment has been substantially used over the last few years to restore the impaired visual system. The next section of this paper will review the approaches implemented for visual neuroprosthetics.
3. Visual Neuroprosthesis
In this section, different types of visual neuroprosthetic devices will be discussed based on four approaches—retinal approaches, LGN approaches, optic nerve approaches, and cortical approaches (Figure 4) [12].
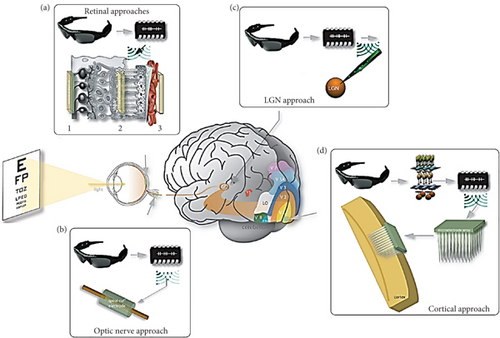
3.1. Retinal Approaches
Retinal approach–based neuroprosthetic devices (Table 1) are one of the FDA-approved treatment options for visually disabled patients [41]. In this approach, retinal implants are used as a form of neuroprosthetic device that aids in manipulating the neuronal signals of the brain by simultaneously receiving stimuli from the environment and controlling the specific part of the human brain. Here, the retina is responsible for computing visual senses and sending the output to the cortex for further computation. All this visual information must be processed by the brain within a millisecond of the period [42]. These retinal-based neuroprosthetic implants can be categorized based on two distinctive criteria—their position concerning the retina or their functional principle. Epiretinal and subretinal are the two kinds of retinal implants that have been suggested based on their position concerning the retina. In terms of configuration property, epiretinal prostheses are constructed to be placed on the surface of the impaired neurosensory retina, directly attached to the optic nerve fibers and retinal ganglion cell layers. In contrast, the subretinal implants are placed behind the retina at the position of photoreceptors.
| SL | Approach | Advantage | Reference |
|---|---|---|---|
| 01 | Intelligent Retinal Implant System (IRIS) II | Capable of providing required functional electrically induced stimulation | Hornig et al. [31] |
| 02 | EPIRET3 retinal implant | Low infection risk and better connectivity with the ganglion cells | Mokwa et al. [32] and Lin et al. [33] |
| 03 | The Boston Retinal Implant | Lower risk of tissue damage, balanced charge transfer, and biphasic creation | Kelly and Rizzo [34] and Shire et al. [35] |
| 04 | Photovoltaic retinal implant | Low risk of tissue damage and easy-to-insert techniques | Mathieson et al. [36]; Chen et al. [37]; and Chenais, Leccardi, and Ghezzi Chenais et al. [38] |
| 05 | Suprachoroidal prostheses | Does not require transversal surgery, easy to maintain, and simple to replace | Ayton et al. [39] and Bloch, Luo, and da Cruz Bloch et al. [40] |
On the other hand, based on the criterion of their functional principle, retinal prostheses can be classified as microelectrode arrays (MEAs) or microphotodiode arrays (MPDAs). Both devices have an electrode array implanted and are physically attached to a multichannel stimulator. A digital camera is incorporated with these prosthetics for image collection from the surroundings. The inbuilt image-processing unit of these implants can process the images and generate stimulation patterns as an output that are then transmitted to the implanted chip through a wireless transmission process. These retinal approach–based neuroprostheses encompass a variety of implants that offer some degree of advantages to restore visual impairment. In this sector, we will discuss the currently used retinal approach–based devices.
3.1.1. Intelligent Retinal Implant System (IRIS) II
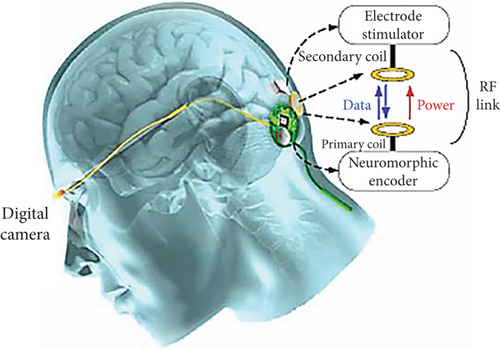
The IRIS II aids in receiving visual information from the outside area, transmitting it to the processing unit, and stimulating the degenerated retina and remaining neurons to evoke visual percepts [43]. This system (Figure 5) consists of visual interface devices and a pocket processor, where the visual interface includes a camera, energy transmitter, and data, and the pocket processor contains image processing software. When the system is activated, the camera collects and records the images. The camera-recorded data and energy are transmitted to the visual interface via cable. The visual interface sends them to the pocket processor for image processing and interpretation. In the feedback loop, these processed data are transmitted back to the visual interface via the same cable [31].
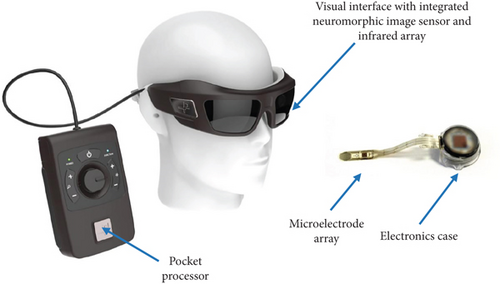
Furthermore, the visual interface sends the processed data and energy to the implanted retinal stimulator with 150 electrodes through a wireless communication system. This electrical stimulation stimulates the degenerated retina, remaining neuronal cells, and optic nerves, which in turn leads to restoring visual ability. The clinical trial data with this system demonstrates that it mitigates the problems associated with neuronal remodeling and evokes required functional electrical stimulation [44].
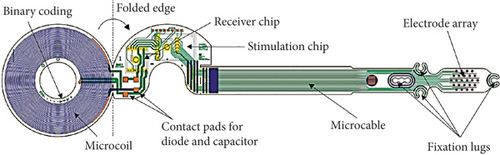
3.1.2. EPIRET3 Retinal Implant
EPIRET3 retinal implant (Figure 6) is considered to be the pioneer system of wirelessly developed retinal prosthesis that directly locates the retinal implant inside the visual organ without using any cable connection system within the ocular surface. This system contains a similar structure for constructing Argus II retinal and IRIS II, except for the location of electronic compounds such as the camera, data, and transmitter coil. Here, an artificial lens is developed on the posterior side of the eye to incorporate all the electronic compounds [32]. The main advantage of this system is the low infection evidence due to the lack of cable connection [33]. Moreover, this system ensures a 3D structure, enabling it to connect with the ganglion cells [32].

3.1.3. Boston Retinal Implant
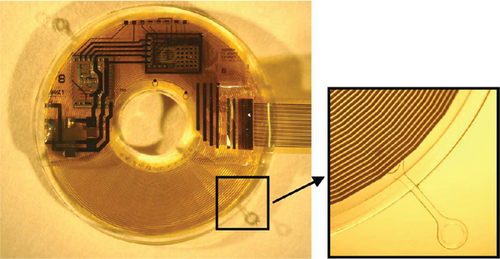
This project is implanted subretinally to restore the visual problem caused by degenerative retinal disease. The implanted device is located outside the eye, where only a multielectrode array with more than 256 stimulating channels enters the ocular site, as presented in Figure 7.
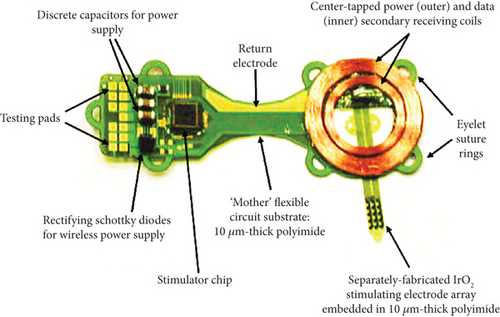

The sputtered iridium oxide film (SIROF) is used to encapsulate the electrodes that permit the transfer of a high amount of charge in each unit area. This project offers more advantages when a target-oriented integrated chip is attached to the device for decoding purposes. As clinical evidence of this device demonstrates the reduced chance of eye infection and decreased risk of erosion through the delicate tissues, it is considered a suitable implant for a retinal approach based on visual neuroprosthesis development [34]. Recently, an advanced microfabrication technique has been suggested that helps to overcome the problems associated with implant construction [35].
3.1.4. Photovoltaic Retinal Implant
In most impaired retinal diseases, blindness causes the improper function of photoreceptor cells inside the retinal structure, such as rod or cone cells. These photoreceptor cells are recruited for image collection from the outside area. When retinal approach–based neuroprosthetic devices are constructed, there is a conventional tradition of stimulating the neurons with multistructured implants. Recently, some evidence suggested that as the implants consist of different metallic elements, this leads to tissue damage. Also, critical surgical procedures need to be implemented to insert these electrodes. To mitigate these challenges, a photovoltaic retinal implant (Figure 8) offers an easy-to-insert solution. Silicon photodiodes collect data and power via neuronal electric stimulation and IR illumination [36].
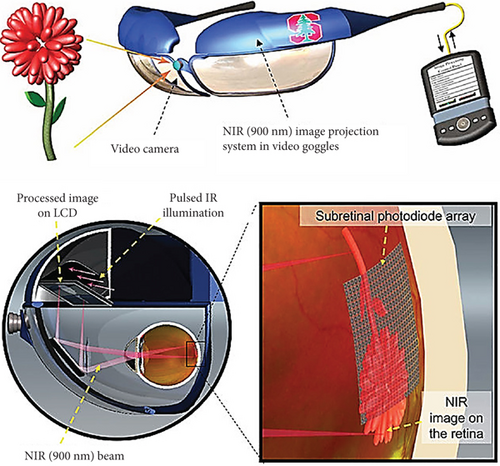
Moreover, this arrangement is useful for improving spatial resolution in a larger visual field [40]. However, some reports demonstrate an issue of visual accuracy limitation due to the array’s configuration. Another study presents evidence of cross-talking among the adjacent neurons. These issues can be fixed by a consecutive triggering of the photodiode pixels [37, 38].
3.1.5. Suprachoroidal Prostheses
Suprachoroidal prosthetic devices prostheses (as shown in Figure 9) are made to locate implants between the choroid and sclera of the visual system to achieve surgical benefit for patients with neurodegenerative disease [39]. It can generate local retinal stimulation that is located in the suprachoroidal space. Since this system does not require transversal surgery, it causes decreased adverse effects in the visual area. Also, this provides the benefit of being easy to maintain and simple to replace when it is needed. However, the clinical trial with these prosthetic devices presents an increasing amount of risk associated with hemorrhage and fibrosis postimplantation. On the other hand, as the system is located away from the neurosensory retina, providing a high amount of stimulation is essential to evoke visual percepts [40].
3.2. Optic Nerve Approaches
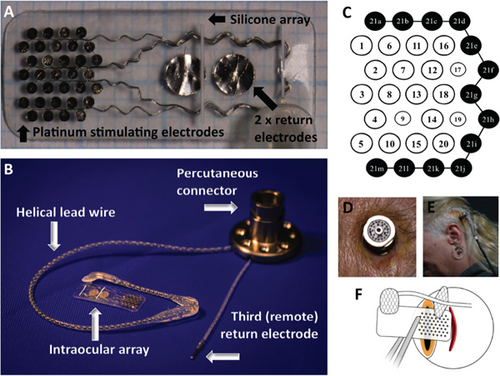
The optic nerve approach possesses a great potential target for the development of visual neuroprosthesis due to the ability to direct activation of nerve fibers, high-level information processing capacity occurring downstream in the visual pathway, and effectiveness in cases of eye trauma [48]. Different types of optic nerve visual prosthetic devices have been used in this approach for the treatment of human blindness problems, which includes a variety of electrodes to stimulate the optic nerves [49]. Based on the interface pattern with the nervous system, these electrodes can broadly be divided into three categories—penetrating, nonpenetrating, and regenerative [50]. Nonpenetrating electrodes are surface electrodes, which wrap around the nerve and couple to the epineurium. On the other hand, penetrating electrodes aid in disrupting the epineurium, and in some cases, the perineurium, to permit closer proximity to the fascicles or the axons. Regenerative electrodes are placed between the two ends of a severed nerve to successfully reestablish functional connections [51]. According to Rijnbeek, Eleveld, and Olthuis Rijnbeek et al. [50], surface electrodes include cuff electrodes, whereas penetrating electrodes include longitudinal intrafascicular electrode (LIFE), transverse intrafascicular multichannel electrode (TIME), and Utah Slanted Electrode Array (USEA). These electrodes have been used to develop neuroprosthetic devises by ensuring the two most potential electrode properties, including extracting meaningful signals over a long period and maximising the number of interfaces between the electrode and nerve fiber [52]. The electrode’s position surrounding optic nerves is presented in Figure 10(a), and Figure 10(b) shows photographs of a typical visual prosthesis used for optic nerve stimulation.

Table 2 summarises various electrodes used for visual neuroprosthetic device construction in the optic nerve approach, and in this section, some of the approaches are discussed.
| Electrodes | Example | Advantage | References |
|---|---|---|---|
| Implanted self-sizing spiral cuff electrode | Cuff electrode with metallic components | Elaborately distributed throughout the visual field and contributing to long-term performance | Christie et al. [54] |
| Multielectrode cuff |
|
Better electrochemical functionality and reduced foreign body response | Rozman et al. [55] and González-González et al. [56] |
| Intrafascicular electrodes | Longitudinal intrafascicular electrode (LIFE) and transverse intrafascicular multichannel electrode (TIME) | Increase targeted stimulation and activation in specific pathways to the brain | Badia et al. [57] and Jung et al. [58] |
| Intraneural electrodes | Self-opening intraneural electrode (SELINE) array and OpticSELINE | Advantage of activation in both internal and external fibers, induce selective optic nerve stimulation, and improve mechanical anchorage to the nerve | Cutrone et al. [59] and Gaillet et al. [48] |
3.2.1. Implanted Self-Sizing Spiral Cuff Electrode
Evidence suggests that a blind person with RP treated with a self-sizing spiral cuff electrode implantation around an optic nerve harbors great potential for restoration of human visual capability. When electrical stimuli are applied to the nerve with the help of these types of electrodes, it produces a localized visual sensation that is elaborately distributed throughout the visual field. In addition, changing the stimulation patterns causes these visual sensations inside the visual fields. Based on these results, visual prosthetics have been suggested to be constructed with self-sizing spiral cuff electrodes to successfully and electrically stimulate the optic nerve. Recently, these cuff electrodes with special architecture have been considered an effective constituent for maintaining the native function of motor and sensory neurons. Moreover, as they contain long-lasting and highly biocompatible properties, these electrodes hold enormous potential to construct neuroprostheses [54].
3.2.2. Multielectrode Cuff
Cuff electrodes are implanted circumferentially on the peripheral nerves, which are made of flexible materials, by maintaining some particular configuration such as cylindrical, helical, spiral, split-cylinder, or folding designs. They are traditionally fabricated in silicone-based components to maintain these cuff electrodes’ softness and chronic stability [54, 60]. However, these cuffs often elicit some adverse effects, including a significant foreign-body immune response that limits nerve stretching and compromises nerve conduction. Moreover, this immune sensitivity includes a fibrotic response, which negatively affects the sensitivity and stimulation thresholds of the electrodes [61]. Several designs of multielectrodes are fabricated with Au and titanium nitride (TiN) to exacerbate these restrictions. These electrodes’ in vitro evaluated performance demonstrates better electrochemical functionality in acute and subchronic recording and stimulation. In addition, immunohistochemical evidence of these multielectrode softening cuff (MSC) devices also showed a reduced foreign body response by the SMP devices compared to silicone nerve cuffs [56]. Recently, an improved method of crafting a multielectrode spiral cuff is suggested, which incorporates platinum-stimulating electrodes and strands of stainless-steel lead wires within the cuff. More importantly, these advanced multielectrode cuffs demonstrate better influence on neurophysiological performance when they are incorporated with visual neuroprosthetic devices focusing on the optic nerve approach [55].
3.2.3. Intrafascicular Electrodes
At present, the extraneural approach is the most widely used approach to interfacing with peripheral nerve fibers by positioning an electrode, or an array of electrodes, and designing a cuff-like structure that wraps around the nerve [54, 62, 63]. Although this approach mitigates the possibility of disruption or damage to the anatomical structures within the nerve, it lacks behind the electrodes to selectively stimulate or record from individual or small populations due to the fibers, the fascicular anatomy of the nerve, and the insulating properties of its connective tissue. This restriction limits the potentiality of neuroprosthetic devices with localized activation with high specificity or recording from small group fibers [58]. Advanced design mechanisms of cuff electrodes have been applied to overcome this shortcoming of limited selectivity of specific fascicles by increasing the nerve’s perimeter and relocating the fascicle’s position near electrode contacts [64]. In addition, neuroprostheses with multiple electrodes around the nerve increase the high specificity of the target by identifying target-specific electrodes [57]. However, these newly reshaped electrodes still have limited selectivity for stimulating or recording specific sites within a fascicle which often causes side effects [58].
Neural interface systems based on intrafascicular electrodes may mitigate these extraneural approach–guided shortcomings. As intrafascicular electrodes broaden the accessing capability to the microstructures and functional microdomains of peripheral nerves, these electrode-based neuroprostheses offer increasing targeted stimulation that enhances controlling activation in specific pathways to the brain, which in turn ameliorates the development of advanced neuroprostheses in the optic nerve approach [57].
3.2.4. Intraneural Electrodes
Neuroprostheses based on the optic nerve approach aim to stimulate the axonal fibers from retinal ganglion cells by incorporating target-specific electrodes. Here, the selection of appropriate target-specific and stable electrodes around the optic nerve contains the challenge of developing successful neuroprostheses. Recently, a self-opening intraneural electrode (SELINE) array has been suggested to improve mechanical stability and high biocompatibility compared with TIME arrays. SELINE is a polyimide-based electrode designed to shape a 3D structure that refers to significantly improved mechanical anchorage to the nerve. In addition, the plastic deformation of polyimide in the SELINE array ensures more stability after implant [59]. Another modified version of this SELINE array has been demonstrated as a visual prosthesis based on optic nerve stimulation called OpticSELINE. This array has been developed using microphotolithography and a silicon wafer as a sacrificial layer. OpticSELINE provides the advantage of activation in both internal and external fibers, and more importantly, it can induce selective optic nerve stimulation and generate spatially organized cortical patterns [48].
3.2.5. C-Sight Project
The main objective of this C-Sight Project is to develop an implantable microelectronic medical device that will restore vision to visually handicraft persons [65]. In vitro exploration of this project is currently investigating an optic nerve prosthesis based on an array of three penetrating metal electrodes to stimulate optic nerves. Additionally, this project applies platinum–iridium electrodes seen in the animal model to explore the stiffness and rigidity of the electrodes [48]. Additionally, this project has developed ring-shaped optic nerve implants for visual neuroprosthesis [66].
3.3. LGN Approaches
The LGN is also called the lateral geniculate body or lateral geniculate complex. This is a visual pathway relay center in the thalamus. A tiny oval ventral projection of the thalamus connects the thalamus to the optic nerve. There are two LGNs in the thalamus: the left LGN and the right LGN. Each LGN has six layers of neurons that alternate with optic fibers in humans [67]. Their six layers and interlaminar structures are responsible for isolating the magno-, parvo-, and koniocellular pathways from both the contralateral and ipsilateral eyes in higher primates. After traveling from the retina to the optic chiasm, the optic nerves are responsible for transporting visual information. Once there, the information is restructured into two visual hemifields. Through the process of optic radiation, these visual hemifields are subsequently projected to the LGN, which then sends its output to the primary visual cortex (Area V1) in order to complete the process [28]. LGN acts as a visual processing stage that shares a basic wiring layout with previous retinal levels of visual processing but adds unique circuit features. In the LGN, a definite core of feedback control excitatory transmission of visual data is tuned spatially and temporally by local inhibitory neurons, much like in the retina [68]. Cells in the LGN are responsive to limited, well-defined regions of visual space known as visual receptive or response fields (RFs). It is possible to think of the normal RF as a spatiotemporal differentiator that reacts most effectively to changes in visual contrast that are highly localized [69].
The LGN is the principal visual information processing center, where the neuronal impulses encoding visual input have not yet been thoroughly processed. The retinal ganglion cells provide data effectively to the LGN, which is sent mostly to the primary visual cortex. Since it has been proven that LGN microstimulation in monkeys yields reliable visual percepts, the LGN serves as a destination for electrical stimulation of artificial visual percepts. As a result, a visual prosthesis depending on LGN stimulation might be useful in helping those who have lost sight due to eye injuries or diseases like glaucoma, RP, or macular degeneration [70]. Moreover, an expanded representation of the fovea, a well-characterized spatial organization that is comparable to that of the retina, and receiving strong feedback from the visual cortex that dynamically influences the activity of LGN neurons to enhance significant perceptual features are some of the significant characteristics of LGN. Through the advancements in deep brain stimulation technologies, it is no longer difficult to gain access to the deep location of the LGN, which is necessary in order to implant thalamic visual prostheses. Due to these properties, LGN is considered a promising candidate target for visual prostheses [71].
A thalamic interface for visual information has a huge impact because it could be used to treat patients with blast-induced eye trauma, glaucoma, or diabetic retinopathy. It would otherwise be unable to benefit from such a prosthesis that provides artificial vision by interacting with the retina. A unique surgical implantation instrument was used to create tissue to obtain LGN deep-brain electrode technology and surgical methods for implanting arrays of such electrodes in the brain [70].
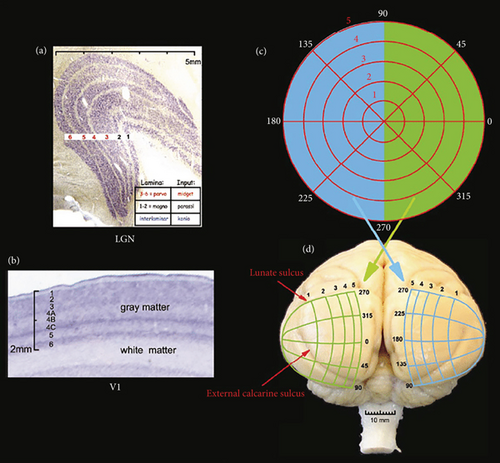
Figure 11 represents the layout of the LGN, including the primary visual cortex (V1), where Figure 11(a) is the cross-section of the LGN, which is Nissl-stained and has six layers in visual presentation. The retinal midget cells input the Top 4 layers (3–6). The parasol cells input the bottom two layers (1–2). The retinal koniocellular cells mostly provide input to intralaminar layers. Figure 11(b) is the cross-section of V1, which is Nissl-stained. The density of neurons and thickness of grey matter are fixed throughout the place. In the grey matter, few layers are entitled. The main input of the parasol cells and midget from the LGN moves to layers 4Cα and 4Cβ. The koniocellular cell inputs put an end in Layers 1 and 2. Figure 11(c) is the layout of the optical field. The left (blue) hemifield programs are at the right half, whereas the right (green) are at the left visual area. Figure 11(d) is the back sight of a monkey’s brain, showing the central visual area design. This area of the monkey is smooth except for the shallow calcarine fissure. The visual area is shown in topographic style, where a large area is allotted for peripheral depiction. The visual area is shown as vertically inverted in the exterior. The top part of the visual area is in the lower portion of V1, and the down visual area is in the top portion of V1 (here, the macaque monkey’s conformal projection system of has been utilized).
3.4. Cortical Approaches
Visual cortical areas consist of the primary visual cortex, secondary visual cortex, and visual association cortices. Upon leaving the LGN, the visual information is transmitted to the primary visual cortex through the use of optic radiations [73]. The information is then transmitted to the secondary visual cortex, and it finally makes its way to the higher level processing areas that are located in the visual association cortex. The primary visual cortex is accountable for the development of fundamental visual functions. However, the secondary visual cortex and the visual association cortices are the ones that are accountable for the generation of more complex visual activities [74]. Many kinds of neuroprosthetics and other devices are now being developed based on the functionality of visual cortical areas. The development of neuroprostheses that access the visual cortex corresponds to exploring new ground in the field of vision restoration [75]. By focusing on the primary visual cortex (V1) and adjacent regions, devices have been developed to bypass damaged or defective components of the eye and optic nerve. These devices utilize complex electrode arrays that are implanted in the primary visual cortex (V1) to transform visual information into brain-readable signals [76].
In visual neuroprosthetics, the cortical approach (Figure 12) significantly impacts the treatment of blind people, particularly those affected by glaucoma, optic atrophy, final-stage RP, or different neurological disorders. This approach aids in penetrating electrodes directly into the occipital cortex area and stimulates the populations of neurons by constructing small dots of light called phosphenes. The process of cortical nerve stimulation is generally defined as intracortical stimulation [78].
The approach of intracortical stimulation in the visual cortex area has evolved from some early experiments conducted by researchers using large surface electrodes. According to the basic theme of the experiments, the more electrical stimulation created by the insertion of electrodes into the cortex area, the larger quantity of perception of light and phosphenes can be induced. To illustrate, each stimulation by a single implanted electrode can induce one point of phosphenes in the cortex area. Consequently, two simultaneous stimulations can create two perceptions of phosphenes in the horizontal or vertical orientation (Figure 13(a)), and three stimulations can induce three perceptions of phosphenes arranged in a triangle form (Figure 13(b)).


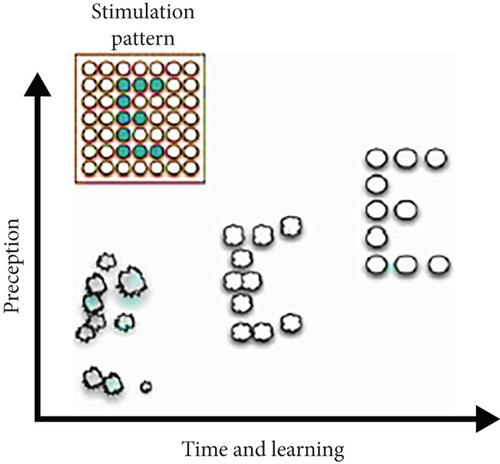
Based on the basic principle of intracortical stimulation, the study suggests that delivering a natural representation of the visual object into the implanted electrodes of the impaired cortical area can successfully enable the brain to create a whole image (Figure 13(c)). This basic idea of stimulation pattern is reviewed by Fernández and Normann [8] in Figures 13(a), 13(b), and 13(c). However, these early studies represent some limitations due to large surface electrodes requiring a relatively high amount of electrical current. On the other hand, as the previous experiment-based studies demonstrate unpredictable spatial patterns of the induced phosphenes, they fail to restore the lost visual ability of patients because of inducing unpredictability [8].
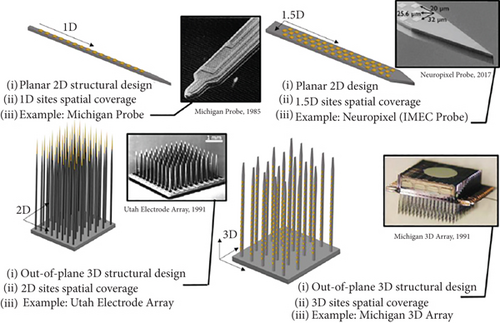
Several initiations have been developed to construct penetrating multielectrode arrays with microdimensions like the targeted cortical neurons to avoid the challenges associated with large surface electrodes. These intracortical microelectrodes are designed with high biocompatibility to ensure stable signal patterns in the cortical area. Flexible polymers or rigid biomaterials are used to fabricate these devices using modern technologies [79]. Utah Electrode Array (UEA) and Michigan array are the two examples of the most popularly used intracortical neural microelectrodes for several years. At the initial stage, Michigan probes are developed where stimulating or recording sites are arranged along a single-layered shank that enables them to target the surrounding neurons in a 1D to 1.5D approach, as illustrated in Figures 13(a) and 13(b). However, it fails to include many electrodes in this single shank because incorporating many electrodes requires an enlarged shank size, which in turn causes tissue damage. A multidimensional shank design has been developed to increase the number of sites without increasing the shank size in a single plane. Based on this fabrication model, 2D probes can be designed and incorporated with an array of electrodes parallel to the surface of the visual cortex, as shown in Figure 13(c) [80].
The UEA is one of the most popular examples of an arrangement that can selectively record and stimulate large cortical neurons [81]. 3D UEA is the only FDA-approved intracortical MEA for long-term human trials. However, this shows some restrictions on device failure due to the heterogeneous integration of different biomaterials inside this multilayer structure. To overcome these limitations, a recent study demonstrates the fabrication of UEA with SIROF electrode coatings and encapsulation with amorphous silicon carbide (a-SiC) [82].
Michigan 3D array (shown in Figure 14) is another intracortical MEA that can stimulate largely distributed areas of the neuronal cortex. By incorporating the defined near-ideal probe, Zardini et al. have suggested a newly developed model that may overcome the limitations of the Michigan 3D array [80].
Apart from the electrical stimulation process in the visual cortex area, a recent study conducted in the mouse model suggests an intracortical magnetic stimulation that offers more effective output than the previous approaches. This model demonstrates implantable microcoils which can magnetically stimulate the cortex [83].
4. Deep Learning–Based Bionic Vision
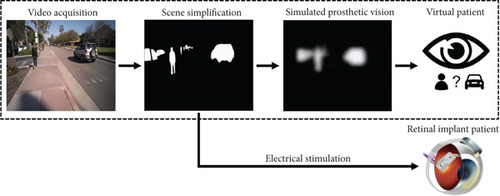
The emergence of current AI technology and new ideas and techniques in the disciplines of artificial vision and neural engineering provides a promising foundation for the further advancement of neurotechnology [84, 85]. Various picture identification challenges have been solved by using deep learning methods. The amazing part about such approaches is that they do not need a sequence of specifically designed models but rather an end-to-end model that could produce descriptions from a snapshot. To describe a picture, computer vision and text classification are required to develop the things in the picture. The most difficult part is figuring out the best location for the camera on the subject’s body. Using the Microsoft Vision API, a camera may take a picture and submit it to a deep learning model, which processes the image and generates a description. Using deep learning for vision processing, outcomes are also affected by the quality of the images, as can be observed in Figure 15. High-resolution photos or videos, for example, are required to achieve reasonable performance in object categorization, increasing data processing, storage, and transport requirements. Image firmness is critical for scenarios where it is vital to identify and categorize items in the distance. To solve this problem, a spectacle with a camera attached to a Raspberry Pi is given. To make the experience more engaging, the user is provided with an on-demand voice assistant, who only answers with a description of the scene in front of them when requested. Amazon Alexa was ideal for this purpose since it enables us to design bespoke skills instead of default skills. To analyze the findings, connect to the internet, and save the model’s produced description in AWS DynamoDB, a database used to store data in the cloud, the Raspberry Pi acts as a computer [86, 87].
Many existing systems concentrate on indoor and outdoor navigation with the help of sensors. In contrast, smaller systems focus on individual activities such as text recognition, alerting during emergencies, and visual explanation with limited mobility [88, 89]. Other systems for the blind and visually handicapped can recognize and avoid obstacles like staircases and potholes in the roadway. Even though they are effective in most circumstances, there are drawbacks to their use, such as their high cost and difficulty in operating. Most of them cannot see warning signs and cautionary boards, making it particularly challenging for the visually impaired [90].
5. Challenges and Future Perspectives
Visual neuroprosthesis development is a rapidly emerging field that encompasses a variety of approaches to restore visual ability in neurodegenerative patients, including the retinal approach, the optic nerve approach, the LGN, and the cortical approach. In recent years, some visual neuroprosthetic devices such as the Argus II retinal system and 3D UEA have received FDA and CE approval for clinical trials. However, these development processes and application approaches harbor multiple challenges that must be overcome for future improvement. Suitable approach selection, appropriate microelectrode design, and prosthetic vision rehabilitation are the major challenges that limit the clinical efficacy of these prosthetics’ devices. To ensure the best therapeutic success from the developed visual neuroprostheses, it is necessary to select a suitable approach before establishing a visual prosthesis model [12]. These approaches’ limitations for prosthesis development must be isolated and refined for future outcomes. For example, retinal approach–based neuroprosthesis development requires sending electrical impulses through implanted electrodes, leading to adverse electrochemical reactions inside the neuronal cell. Due to these improper chemical shifts, some neuronal cells may stop their functionality, which, in turn, proves the inefficacy of the prosthesis devices.
To overcome these challenges, biphasic pulses with a constant charge facility can be provided into the electrodes to restore the chemical imbalance [10]. Moreover, recent research suggests inducing chemical stimulation that may prevent unwanted electrochemical reactions [91]. In the optic nerve approach, even though the electrical stimulation transmitted to the optic nerve produces a series of visual sensations, these are confined within a very restricted area. Some phosphines within this restricted area are not extended. Advanced stimulation strategies are suggested to ensure a wide range of visual sensations that can transfer more visual information quickly [7]. The reduction of optic nerve fiber selectivity due to the electrode attachment is another issue that decreases the potentiality of prosthesis devices.
Providing intraneural stimulation via penetrating electrodes can prevent the reduction of optic nerve fiber selectivity [48]. In the cortical approach–based visual neuroprosthetic development, challenges remain to implant many electrodes over a distributed area, opening the possibility for nerve tissue damage. A multidimensional electrode array may help to overcome these challenges [80]. Because of the easy surgical procedure and compact anatomical structure of the LGN, in recent years, it has become a very important target area for visual neuroprosthetic development [49]. Moreover, a lower requirement for image processing units and being highly adaptive to the CNS turns this approach into the best candidate for visual neuroprosthetic-based sight problem solutions (P [92, 93]).
Designing the target-specific microelectrode is another challenge in visual neuroprosthetic development. These microelectrode fabrication techniques must ensure the implants’ functionality, safety, and longevity. High levels of functionality and longevity can be achieved by carefully inserting the electrodes to ensure the minimum host immune response [94]. In most cases, densely packed electrodes are inserted that adversely affect neighbouring electrodes to provide a large visual information transmission and processing facility. The multilayered organized array may reduce shortcomings [10]. The construction of biocompatible and anticorrosive implants and the design of target-oriented electrodes should be ensured. Despite consequential improvement by incorporating polymers, more research should be conducted to ensure the biocompatibility of these devices [95].
Intracortical stimulation has shown promising results in restoring sensory and motor functions in individuals with neurological disorders [96]. However, there are several challenges that need to be addressed to optimize the effectiveness of this technology. One major challenge is the potential for tissue damage and inflammation caused by the insertion of microelectrodes into the brain. Furthermore, the long-term stability and reliability of the implanted devices need to be improved to ensure sustained therapeutic benefits. Additionally, preclinical animal research is essential for understanding the mechanisms underlying intracortical stimulation and for developing new strategies to enhance its efficacy [97]. Despite these challenges, the future prospects of visual neuroprostheses for the impaired human nervous system are promising, with ongoing advancements in technology and research showing great potential for improving the quality of life for individuals with neurological disorders.
Moreover, proper encapsulation methods should be followed for prosthetic device construction that may prevent adverse effects, including tissue damage. Most experiments are conducted in an animal model or in vitro procedure in a controlled situation that avoids considering unwanted outcomes when implemented in clinical conditions. So, more model validation should be conducted for the new technologies. As visual interference, signal, and image processing components are the basic elements for visual neuroprosthesis development, constructing these elements with appropriate research opens the possibility for a better outcome in the future. A considerable amount of lack has been seen for rehabilitative practice after visual prosthesis device implementation, which has become essential for newly developed technologies. Rehabilitative practice should include outcome measures, device assessments, and training [98].
6. Conclusions
In conclusion, visual neuroprostheses have shown great promise in restoring vision to individuals with visual impairments. Various types of recently developed visual neuroprostheses and their development approaches are discussed including how these devices are constructed, how they perform, and which components are essential for the fabrication. Subsequently, recent achievements, challenges, and future outlooks are introduced to develop highly effective devices for visual neuroprosthetics.
While the technology continues to advance, there are important ethical considerations that must be taken into account, such as ensuring informed consent and privacy protection for patients. Patient perspectives are also crucial in developing and implementing visual neuroprostheses, as their input can provide valuable insights into improving the technology and enhancing the quality of life. Additionally, regulatory frameworks play a key role in ensuring the safety and efficacy of visual neuroprostheses and thus must be carefully considered as the field continues to evolve. Overall, visual neuroprostheses’ state-of-the-art is rapidly advancing, offering hope for a brighter future for individuals with impaired vision.
Nomenclature
-
- AMD
-
- age-related macular degeneration
-
- CNS
-
- central nervous system
-
- GABA
-
- gamma-aminobutyric acid
-
- LGN
-
- lateral geniculate nucleus
-
- MSC
-
- multielectrode softening cuff
-
- MEA
-
- microelectrode array
-
- MPDA
-
- microphotodiode array
-
- RP
-
- retinitis pigmentosa
-
- UEA
-
- Utah Electrode Array
-
- USEA
-
- Utah Slanted Electrode Array
-
- SIROF
-
- sputtered iridium oxide film
-
- SELINE
-
- self-opening intraneural electrode
-
- LIFE
-
- longitudinal intrafascicular electrode
-
- TIME
-
- transverse intrafascicular multichannel electrode
-
- IRIS
-
- intelligent retinal implant system
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No specific fund was received to support this work.
Acknowledgments
The authors would like to thank their respective organizations for providing general support to this work.
Open Research
Data Availability Statement
It is a review article. So, no new data were produced.